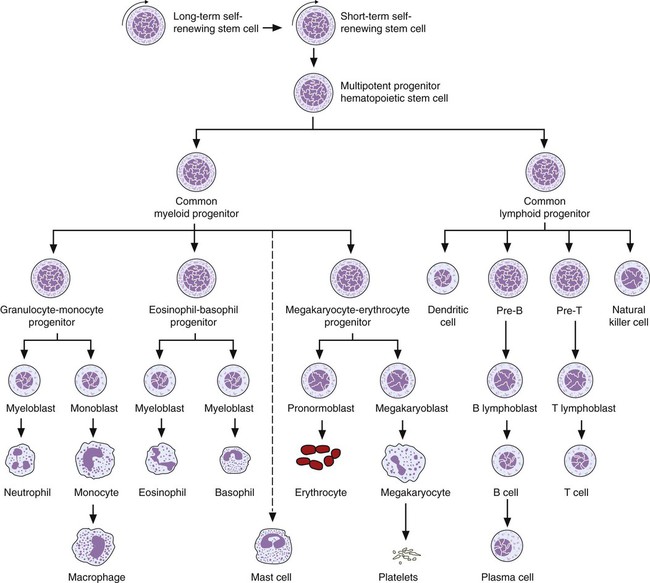
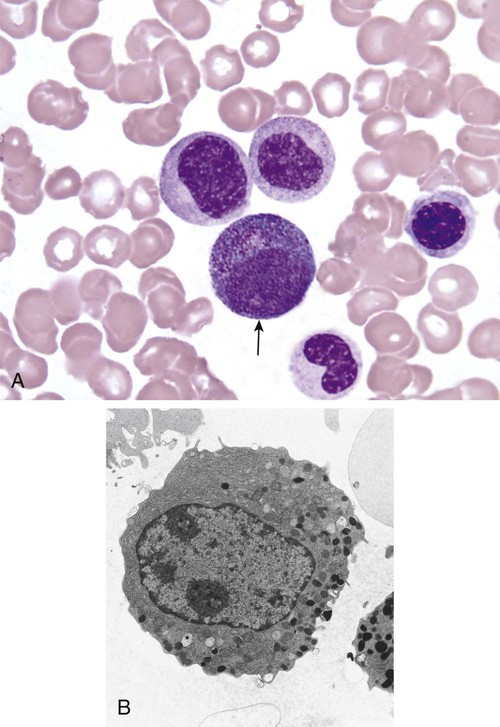
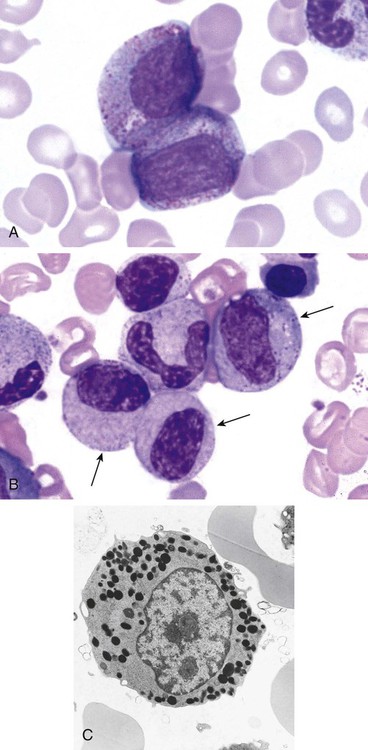
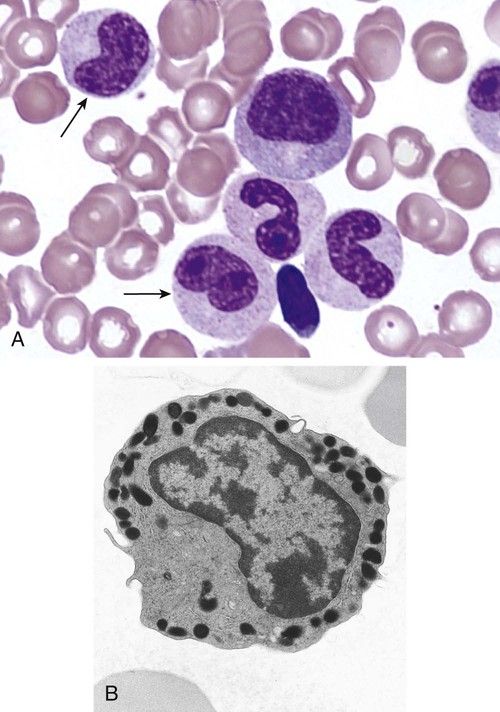
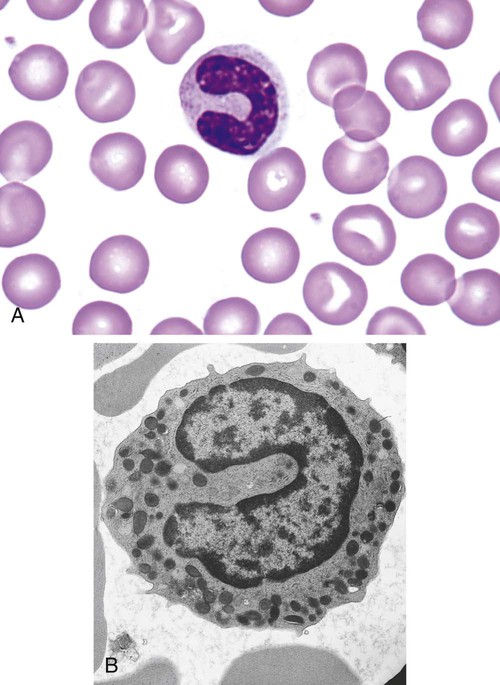
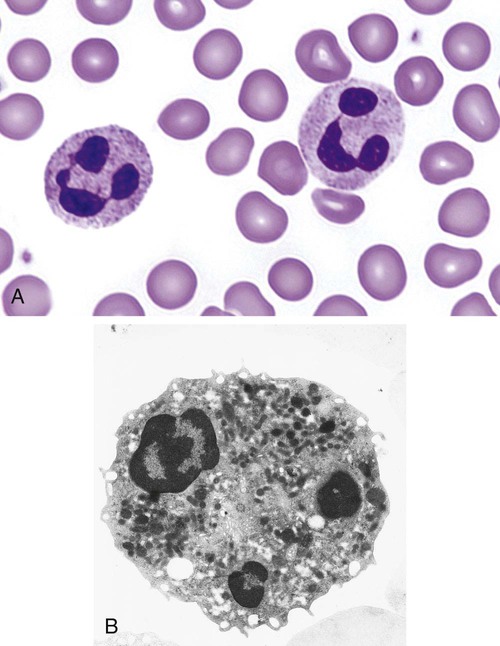
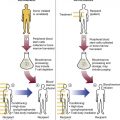
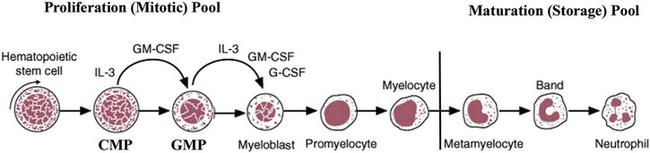
After completion of this chapter, the reader will be able to: 1. Describe the Wright stain morphology of neutrophils at the five stages of maturation and name each stage. 2. Describe the Wright stain morphology of immature and mature eosinophils and basophils. 3. Discuss the various types of granules found in neutrophils, eosinophils, and basophils and list at least three compounds found in the granules of each cell line. 4. Describe in general terms the activity known as phagocytosis. 5. Describe the Wright stain morphology of promonocytes, monocytes, and macrophages. 6. Compare the kinetics of neutrophils and monocytes. 7. Discuss in general terms how the various types of lymphocytes are produced and describe the Wright stain morphology of resting lymphocytes, immature B cells, effector B cells, and cytotoxic T cells or natural killer cells. 8. Define what is meant by the term blastogenesis as related to lymphocyte activation. 9. Describe at least three functions of each type of leukocyte covered in this chapter. In addition to the three granulocytes, there are two other cell types whose cytoplasm may or may not contain visible granules that stain with azures (azurophilic granules) and whose nuclei are not segmented but usually round, oval, indented, or folded. These two types of leukocytes are called mononuclear cells. Mononuclear cells can be subdivided into monocytes and lymphocytes. The manner in which the heterochromatin (see Chapter 6) is clumped is referred to as the chromatin pattern and is relatively unique for some of the leukocytes. This fact aids in their identification, and this is especially true when lymphocytes and monocytes are compared. Leukocytes develop from primitive stem cells in the bone marrow, where most undergo differentiation and maturation (Figure 12-1), and then are released into the circulation. The number of circulating leukocytes varies with sex, age, activity, time of day, and ethnicity; it also differs according to whether or not the leukocytes are reacting to stress, being consumed, or being destroyed, and whether or not they are being produced by the bone marrow in sufficient numbers.1 Reference ranges for total leukocyte counts vary among laboratories depending on the patient population (e.g., a pediatric hospital compared to a geriatric clinic) and the type of instrumentation being used. Generally speaking, the range can be as low as 3.8 × 109/L and as high as 25 to 30 × 109/L for infants or 11.5 × 109/L for adults. Neutrophil development occurs in the bone marrow under normal circumstances. Neutrophils share a common progenitor with monocytes known as the granulocyte-monocyte progenitor (GMP). The major cytokine responsible for the stimulation of neutrophil production is granulocyte colony-stimulating factor, or G-CSF.2 Neutrophil development is also under the control of specific transcription factors and apoptotic regulators that are beyond the scope of this chapter. There are two pools of developing neutrophils in the bone marrow (Figure 12-2). The mitotic (or proliferating) pool consists of cells that are dividing by mitosis and includes hematopoietic stem cells (HSCs)3; common myeloid progenitors (CMPs), also known as colony-forming units–granulocyte, erythrocyte, monocyte, and megakaryocyte (CFU-GEMMs); GMP4; myeloblasts; promyelocytes; and myelocytes. The second marrow pool of neutrophils is the storage (or maturation) pool consisting of cells that are no longer dividing but are undergoing nuclear maturation that is visible as nuclear shape changes from round to indented to banded to segmented as well as increased clumping of the nuclear chromatin. The storage pool consists of metamyelocytes, band form neutrophils, and segmented neutrophils. HSCs, CMPs, and GMPs are not distinguishable with the light microscope and Romanowsky staining and may resemble early type I myeloblasts or lymphoid cells. They can, however, be identified through surface antigens and flow cytometry (see Chapter 33). The HSC is CD34+CD38–CD133+Thy-1+; the CMP is CD34+CD38lowIL3RαlowCD45RA−;4 and the GMP is CD34+CD38+CD45RA+.5,6 Myeloblasts make up 1% to 2% of the nucleated cells in the bone marrow. They are frequently subdivided into types I and II. Myeloblasts vary in size depending on the stage of the mitotic cycle (14 to 20 µm). The nucleus is round to oval and usually centrally located. It is homogeneous with delicate euchromatin and two to four visible nucleoli. The cytoplasm is slightly basophilic and the nucleus-to-cytoplasm (N : C) ratio is 1 : 1 to 1 : 0.5 depending on the stage of the mitotic cycle. Type I myeloblasts have no visible granules when stained with Romanowsky stains and viewed with the light microscope. When most of these cells are viewed with the electron microscope or stained for peroxidase enzyme, granules are apparent. Those that do not have detectable granules may represent earlier stem cells such as the CMP. Type II myeloblasts contain up to 20 visible azurophilic granules starting at the Golgi apparatus and spreading throughout the cell (Figure 12-3). These azure granules are referred to variously as primary granules or nonspecific granules because they are the first to appear and they are not unique to any one cell type. Promyelocytes make up 1% to 6% of the nucleated cells in the bone marrow. They vary in size depending on the stage of the mitotic cycle (16 to 25 µm). The nucleus is round to oval and is often eccentric. The cytoplasm is evenly basophilic and full of primary azurophilic granules. These granules are the first in a series of granules to be produced during neutrophil maturation (Box 12-1).7 The nucleus is similar to that described earlier for myeloblasts except that chromatin clumping (heterochromatin) may be visible, especially around the edges of the nucleus. Nucleoli are easily seen (Figure 12-4). Neutrophil myelocytes make up 6% to 17% of the nucleated cells in the bone marrow and are the final stage in which cell division (mitosis) occurs. During this stage, the production of primary granules ceases, and the cell begins to manufacture secondary or specific neutrophil granules. This stage of neutrophil development is sometimes divided into early and late myelocytes. Early myelocytes may look very similar to the promyelocytes described earlier in size and nuclear characteristics except that patches of grainy pale pink cytoplasm representing secondary granules begin to be evident in the area of the Golgi apparatus. This has been referred to as the dawn of neutrophilia. Secondary neutrophilic granules slowly spread through the cell until its cytoplasm is more lavender-pink than blue. As the cell divides, the number of primary granules per cell is decreased, and their membrane chemistry changes so that they are much less visible. Late myelocytes are somewhat smaller than promyelocytes (15 to18 µm), and the nucleus has considerably more heterochromatin. Nucleoli are difficult to see by light microscopy (Figure 12-5). Neutrophil metamyelocytes constitute 20% to 30% of nucleated marrow cells. From this stage forward the cells are no longer capable of division, and the major morphologic change is in the shape of the nucleus. Synthesis of tertiary granules (also known as gelatinase granules) may begin during this stage. The size of the metamyelocyte is a little smaller than that of the myelocyte (14 to 16 µm). The cytoplasm contains very little residual ribonucleic acid (RNA) and therefore little or no basophilia. The nucleus is indented (kidney bean shaped or peanut shaped), and the chromatin is increasingly clumped. Nucleoli are absent (Figure 12-6). Neutrophil band forms make up 9% to 32% of nucleated marrow cells. All evidence of RNA (cytoplasmic basophilia) should be absent, and tertiary granules continue to be formed during this stage. Secretory granules (also known as secretory vesicles) may begin to be formed during this stage. The nucleus is highly clumped, and the nuclear indentation that began in the metamyelocyte stage now exceeds one half the width of the nucleus, but actual segmentation has not yet occurred. Over the past 70 years there has been considerable controversy over the definition of a band form and the differentiation between band forms and segmented forms. There have been up to three schools of thought from the most conservative—holding that a band must have an equal diameter throughout (reference range, 0% to 3% in the peripheral blood)—to the most liberal—requiring that a filament between segments be visible before a band becomes a segmented neutrophil (reference range, 8% to 18% in the peripheral blood). The middle ground states that when doubt exists, the cell should be called a segmented neutrophil (reference range, 5% to 10% in the peripheral blood) (Figure 12-7). In the past decade, an increasing number of laboratories are no longer distinguishing between band forms and segmented forms, because the clinical utility of this differentiation has been called into question.8 PMN neutrophils (segmented neutrophils) make up 7% to 30% of nucleated cells in the bone marrow. Secretory granules continue to be formed during this stage. The only morphologic difference between this cell and the band form is the presence of between three and four nuclear segments connected by threadlike filaments (Figure 12-8). Neutrophil kinetics involves the movement of neutrophils between five areas known as pools: the mitotic pool in the bone marrow, the storage pool in the bone marrow, the circulating pool in the peripheral blood, the marginated pool in the peripheral blood, and the tissue pool. Neutrophil production has been calculated to be on the order of between 0.9 and 1.0 × 109 cells/kg per day.9 The mitotic pool contains approximately 2.11 × 109 cells/kg, whereas the storage pool contains roughly 5.6 × 109 cells/kg or a 5-day supply. The transit time from the HSC to the myeloblast has not been measured. The transit time from myeloblast through myelocyte has been estimated to be roughly 6 days, and the transit time through the storage pool is roughly 6 to 7 days.9 Granulocyte release from the bone marrow is stimulated by G-CSF.2 Once in the peripheral blood, neutrophils are divided randomly into a circulating neutrophil pool (CNP) and a marginal neutrophil pool (MNP). The ratio of these two pools is roughly 50 : 50 overall10; however, marginated neutrophils in the capillaries of the lungs make up a considerably larger portion of peripheral neutrophils.11 There do not appear to be any functional differences between neutrophils of either the CNP or the MNP, and cells move freely between the two peripheral pools.12 The half-life of neutrophils in the blood is relatively short at 6 to 8 hours.8 Integrins and selectins are of significant importance in allowing neutrophils to marginate as well as exit the blood and enter the tissues by a process known as diapedesis.13 Those neutrophils that do not migrate into the tissues eventually undergo programmed cell death or apoptosis and are removed by macrophages in the spleen.14 Once neutrophils are in the tissues, their life span is variable depending on whether or not they are responding to infectious or inflammatory agents. In the absence of infectious or inflammatory agents, the neutrophil’s life span is measured in hours. Some products of inflammation and infection tend to prolong the neutrophil’s life span through antiapoptotic signals, whereas others such as MAC-1 trigger the death and phagocytosis of neutrophils that have “reached the end of their useful lifespan.”13 Neutrophil recruitment to an inflammatory site begins when chemotactic agents bind to neutrophil receptors. Chemotactic agents may be produced by microorganisms, by damaged cells, or by other leukocytes such as lymphocytes or other phagocytes. The first neutrophil response is to roll along endothelial cells using stronger adhesive molecules than those used by nonstimulated marginated neutrophils. Rolling consists of transient adhesive contacts between neutrophil selectins and adhesive molecules on the surface of endothelial cells. At the same time, secretory granules containing additional adhesive molecules are fused to the neutrophil’s plasma membrane. β2 integrins such as CD11b/CD18 from secretory granules contribute to tight stationary binding between neutrophils and endothelial cells. This is followed by diapedesis or transmigration of neutrophils either between or through endothelial cells—a process that is also mediated by integrins and integrin-associated proteins. Tertiary granules containing gelatinase and collagenase are released by transmigrating neutrophils. Gelatinase degrades denatured collagen as well as types IV and V collagen and activates chemokines such as interleukin-8 (IL-8).15 Neutrophils then migrate in a directional manner toward the area of greatest concentration of chemotactic agents. Once at the site of infection or inflammation, neutrophils begin the process of phagocytosis (Box 12-2). They utilize their enormous inventory of surface receptors either to directly recognize the pathogen, apoptotic cell, or particle or to recognize opsonic molecules attached to the foreign particle such as antibodies or complement components. With recognition comes attachment and engulfment, in which cytoplasmic pseudopodia surround the particle, forming a phagosome within the neutrophil cytoplasm.16 Formation of the phagosome allows the reduced nicotinamide adenine dinucleotide (NADH) oxidase complex within the phagosome membrane to assemble; this leads to the generation of reactive oxygen species such as hydrogen peroxide, which is converted to hypochlorite by myeloperoxidase. Likewise, a series of metabolic changes culminate in the fusion of primary and/or secondary granules to the phagosome and the release of numerous bacteriocidal molecules into the phagosome.17 This combination of reactive oxygen species and non–oxygen-dependent mechanisms is generally able to destroy most pathogens. A second function of neutrophils is the generation of neutrophil extracellular traps or NETs.18 NETs are extracellular threadlike structures believed to represent chains of nucleosomes from unfolded nuclear chromatin material (DNA). These structures have enzymes from neutrophil granules attached to them and have been shown to be able to trap and kill gram-positive and gram-negative bacteria as well as fungi. NETs are generated at the time that neutrophils die as a result of antibacterial activity.
Leukocyte Development, Kinetics, and Functions
Granulocytes
Neutrophils
Neutrophil Development

Neutrophil Kinetics
Neutrophil Functions
Leukocyte Development, Kinetics, and Functions