After completion of this chapter, the reader will be able to: 1. Properly collect and transport hemostasis blood specimens. 2. Reject hemostasis blood specimens due to clots, short draws, or hemolysis. 3. Prepare hemostasis blood specimens for analysis. 4. Describe the principles of platelet aggregometry. 5. Apply appropriate platelet function tests in a variety of conditions and interpret their results. 6. Diagnose von Willebrand disease and monitor its treatment. 7. Analyze plasma markers of platelet activation platelet factor 4 and β-thromboglobulin. 8. Describe the principle of, appropriately select, and correctly interpret the results of clot-based coagulation screening tests, including activated clotting time, prothrombin time, partial thromboplastin time, and the thrombin clotting time. 9. Interpret clot-based screening test results collectively to reach presumptive diagnoses, then recommend and perform confirmatory tests. 10. Perform partial thromboplastin time mixing studies to detect factor deficiencies, lupus anticoagulants, and specific factor inhibitors. 11. Describe the principle of, appropriately select, and correctly interpret coagulation factor assays. 12. Describe the principle of and correctly interpret Bethesda titers for coagulation factor inhibitors. 13. Describe the principle of, appropriately select, and correctly interpret tests of fibrinolysis, including assays for D-dimer, plasminogen, plasminogen activators, and plasminogen activator inhibitors. Most hemostasis laboratory procedures are performed on venous whole blood collected by venipuncture and mixed 9 : 1 with a 3.2% solution of sodium citrate anticoagulant. The specimen is maintained as well-mixed whole blood for platelet function testing or centrifuged to provide platelet-poor plasma (PPP) for other procedures. Phlebotomists, patient care technicians, nurses, medical laboratory scientists, and other health personnel who collect blood specimens must adhere closely to published protocols for specimen collection and management. The nursing or laboratory supervisor is responsible for the current validity of specimen collection and handling protocols and ensures that personnel employ approved techniques.1 Phlebotomists may manage patients using standard protocols for identification, cleansing, tourniquet use, and venipuncture (see Chapter 3). If there is a reason to anticipate excessive bleeding—for instance, if the patient has multiple bruises or mentions a tendency to bleed—the phlebotomist should extend the time for observing the venipuncture site from 1 minute to 5 minutes and should apply a pressure bandage before dismissing the patient. Most hemostasis specimens are collected in plastic blue-stopper (blue-top, blue-closure) sterile evacuated blood collection tubes containing a measured volume of 0.105 to 0.109 mol/L (3.2%) buffered sodium citrate anticoagulant.2 Tubes of uncoated soda-lime glass are unsuitable because their negative surface charge activates platelets and plasma procoagulants. Siliconized (plastic-coated) glass tubes are available, but their use is waning because of concern for potential breakage with consequent risk of exposure to bloodborne pathogens.3 • If the hemostasis specimen is part of a series of tubes to be filled from a single venipuncture site, it must be collected first or immediately after a nonadditive tube. The hemostasis tube may not immediately follow a tube that contains heparin (green stopper), ethylenediamine tetraacetic acid (EDTA, lavender stopper), sodium fluoride (gray stopper), or clot-promoting silica particles such as are contained in plastic red-topped or serum separator (gel) tubes. These additives may become transferred to the hemostasis specimen on the stopper needle and invalidate all hemostasis test results. Nonadditive tubes includes red-topped glass tubes and clear-topped or red and gray marble–topped tubes. If nonadditive tubes are unavailable, the phlebotomist may use and discard a preliminary blue-topped tube.4 • The ratio of whole blood to anticoagulant must be 9 parts blood to 1 part anticoagulant. Evacuated tubes are designed so that the negative internal pressure draws the correct volume of blood from the vein. Collection tube manufacturers indicate the allowable range of collection volume error in package inserts and provide a minimum volume line on each tube. In most cases, the volume of blood collected must be within 90% of the calibrated volume. A short draw—that is, a specimen with a smaller volume than that specified by the manufacturer—generates erroneously prolonged clot-based coagulation test results because the excess anticoagulant relative to blood volume neutralizes test reagent calcium.5 Short-draw specimens are consistently discarded, and a fresh specimen is collected from the patient. Most plastic blue-topped tubes collect 3.0 mL of whole blood; the smaller the collection tube, the narrower the tolerance for short draws. • When specimens are collected using winged-needle butterfly sets, the phlebotomist must compensate for the internal volume of the tubing, which is usually 12 inches long and contains approximately 0.5 mL of air. The phlebotomist must first collect and discard a nonadditive tube or an identical blue-topped tube. This step ensures that the needle set tubing is filled with fresh patient blood before the hemostasis specimen is collected.6 • Clotted specimens are useless for hemostasis testing, even if the clot is small. A few seconds after collection the phlebotomist must gently invert the specimen at least five times to mix the blood with the anticoagulant and prevent clot formation. If possible, the medical laboratory scientist must visually examine for clots just before centrifugation and testing. Many coagulometers are equipped to detect the presence of clots. Clotted specimens are discarded, and a new specimen is collected from the patient. • Excessive specimen agitation causes hemolysis, procoagulant activation, and platelet activation. The phlebotomist must never shake the tube. The test results from visibly hemolyzed specimens are unreliable, and the specimen must be recollected (Table 45-1).7 TABLE 45-1 Hemostasis Specimen Collection Errors That Require Collection of a New Specimen • Excess manipulation of the needle may promote the release of procoagulant substances from the skin and connective tissue, which contaminate the specimen and cause clotting factor activation. Consequently, test results from specimens collected during a traumatic venipuncture may be falsely shortened and unreliable.8 • During blood collection, the phlebotomist must remove the tourniquet within 1 minute of its application to avoid stasis.9 Stasis is a condition in which venous flow is slowed. Stasis results in the local accumulation of coagulation factor VIII and von Willebrand factor (VWF), which may result in false shortening of clot-based coagulation test results. Although syringes are impractical for high-volume hemostasis screening, they are occasionally used in place of evacuated blood collection units. Syringes, joined with winged-needle sets, are especially useful for collecting specimens from patients whose veins are small, fragile, or scarred by repeated venipunctures. The use of syringes presents additional needle stick risk to the phlebotomist, so careful training and handling are essential.10 The phlebotomist selects sterile syringes of 20-mL capacity or less with nonthreaded Luer-slip hubs. The phlebotomist assembles syringes, a winged needle set (Figure 45-1), a tubing clamp, and standard venipuncture materials. The phlebotomist then uses the following protocol: 1. Use standard patient identification and standard blood specimen management precautions (see Chapter 3) 2. Most syringes are delivered with the plunger withdrawn about 1 mm from the end of the barrel. Move the plunger outward and inward within the barrel. Expel all air from the barrel and affix the needle set to the Luer-slip hub. 3. Optional: draw precisely measured anticoagulant into the syringe prior to collection. 4. Cleanse the venipuncture site, affix the tourniquet, and insert the winged needle. Immobilize the needle set by loosely taping the tube to the arm about 2 inches from the needle. 5. Fill the syringe using a gentle, even pressure. 6. Place the syringe on a clean surface and clamp the tubing with a hemostat near the needle hub. 7. Attach a second syringe if needed; release the clamp and fill the second syringe. 8. Replace the clamp, remove the needle set, and immediately activate the needle cover. Whether evacuated collection tubes or syringes are used, the bore of the needle should be sufficient to prevent hemolysis and activation of platelets and plasma procoagulants. If the overall specimen is 25 mL, a 20- or 21-gauge thin-walled needle is used (Table 45-2). For a larger specimen, a 19-gauge needle is required. A 23-gauge needle is acceptable for pediatric patients or patients whose veins are small, but the negative collection pressure must be reduced. All needles provide safety closures that either cover or blunt the needle immediately after completion of the venipuncture. TABLE 45-2 Selection of Needles for Hemostasis Specimens Blood specimens may be drawn from heparin or saline locks, ports in intravenous lines, peripherally inserted central catheters (PICC tubes), central venous catheters, or dialysis catheters. Vascular access device management requires strict adherence to protocol to ensure sterility, prevent emboli, and prevent damage to the device. Personnel must be trained and must recognize the signs of complications and take appropriate action. Institutional protocol may limit vascular access device blood collection to physicians and nurses. Before blood is collected for hemostasis testing, the line must be flushed with 5 mL of saline, and the first 5 mL of blood, or six times the volume of the tube, must be collected and discarded. The phlebotomist must not flush with heparin. Blood is collected into a syringe and transferred to an evacuated tube as described in the prior section on hemostasis specimen collection with syringes and winged needle sets.11 Several near-patient testing (point-of-care) coagulometers (see Chapter 47) generate PT results from a specimen consisting of 10 to 50 mcL of whole blood. These instruments are designed to test either anticoagulated venous whole blood or capillary (finger-stick) blood and represent a significant convenience to patients and to anticoagulation clinics.12 Many are designed for patient self-testing, and pediatric or neonatal testing, and laboratory scientists are often charged with training patients in proper capillary puncture technique.13 Capillary specimen punctures are made using sterile spring-loaded lancets designed to make a cut of standard depth and width while avoiding injury (see Chapter 3). The phlebotomist or patient selects and cleanses the middle or fourth (ring) finger and activates the device so that it produces a puncture that is just off center of the fingertip and perpendicular to the fingerprint lines. After wiping away the first drop of blood, which is likely to be contaminated by tissue fluid, the phlebotomist places the collection device directly adjacent to the free-flowing blood and allows the device to fill. The phlebotomist wipes excess blood from the outside of the device and introduces it to the coagulometer to complete the assay. The phlebotomist then presses a gauze pad to the wound and instructs the patient to maintain pressure until bleeding ceases. The key to accurate measurement of PT is a free-flowing puncture. Often it is necessary for the phlebotomist to warm the patient’s hand to increase blood flow to the fingertips. Blood collection device distributors provide dry disposable warming devices for this purpose. The phlebotomist avoids squeezing (“milking”) the finger, because this renders the blood specimen inaccurate by raising the concentration of tissue fluid relative to blood cells.14 The anticoagulant used for hemostasis testing is buffered 3.2% (0.105 to 0.109 mol/L) sodium citrate, Na3C6H5O7•2H2O, molecular weight 294.10. Sodium citrate binds calcium ions to prevent coagulation, and the buffer stabilizes specimen pH as long as the tube stopper remains in place.15 The anticoagulant solution is mixed with blood to produce a 9 : 1 ratio; 9 parts whole blood to 1 part anticoagulant. In most cases, 0.3 mL of anticoagulant is mixed with 2.7 mL of whole blood, which are the volumes in the most commonly used evacuated plastic collection tubes, but any volumes are valid, provided that the 9 : 1 ratio is maintained. The ratio yields a final citrate concentration of 10.5 to 10.9 mmol/L of anticoagulant in whole blood.16 Some laboratory scientists prepare specimen tubes locally for special hemostasis testing. The 9 : 1 blood-to-anticoagulant ratio is effective provided the patient’s hematocrit is 55% or less. In polycythemia, the decrease in plasma volume relative to whole blood unacceptably raises the anticoagulant-to-plasma ratio, which causes falsely prolonged results on clot-based coagulation tests. The phlebotomist must provide tubes with relatively reduced anticoagulant volumes for collection of blood from a patient whose hematocrit is known to be 55% or higher. The amount of anticoagulant needed may be computed for a 5-mL total specimen volume by using the graph in Figure 45-2 or the following formula, which is valid for any total volume: where C is the volume of sodium citrate in milliliters, V is volume of whole blood–sodium citrate solution in milliliters, and H is the hematocrit in percent. Few data support the use of EDTA-anticoagulated specimens for coagulation testing, because calcium ion chelation interferes with coagulation assays.17 EDTA is the anticoagulant used in collecting specimens for complete blood counts, including platelet counts. EDTA may be required for specimens used for molecular diagnostic testing, such as testing for factor V Leiden mutation or the prothrombin G20210A mutation. Likewise, acid citrate dextrose (ACD, yellow stopper) and dipotassium EDTA (K2EDTA) with gel (white stopper) tubes may be used for molecular diagnosis, as specified by institutional protocol. Heparinized specimens have never been validated for use in plasma coagulation testing but may be necessary in cases of platelet satellitosis (satellitism) as a substitute for specimens collected in EDTA or sodium citrate. Citrate theophylline adenosine dipyridamole (CTAD, blue stopper) tubes are used to halt in vitro platelet or coagulation activation for specialty assays such as those for the platelet activation markers platelet factor 4 (PF4) and platelet surface membrane P-selectin (measured by flow cytometry) or the coagulation activation markers prothrombin fragment 1+2 and thrombin-antithrombin. Sodium citrate–anticoagulated whole-blood specimens are placed in a rack and allowed to stand in a vertical position with the stopper intact and uppermost. The pH remains constant as long as the specimen is sealed. Specimens are maintained at 18° C to 24° C (room temperature), never at refrigerator temperatures (Table 45-3). Storage at 1° C to 6° C activates factor VII, activates platelets, and causes the cryoprecipitation of large VWF multimers.18,19 Also, specimens should never be stored at temperatures greater than 24° C, because heat causes deterioration of coagulation factor VIII. TABLE 45-3 Hemostasis Specimen Storage Times and Temperatures Specimens collected for PT testing may be held at 18° C to 24° C and tested within 24 hours of the time of collection. Specimens collected for partial thromboplastin time (PTT) testing also may be held at 18° C to 24° C, but must be tested within 4 hours of the time of collection, provided that the specimen does not contain unfractionated heparin anticoagulant. If a patient is receiving unfractionated heparin therapy, specimens for PTT testing must be centrifuged within 1 hour of the time of collection, and the plasma, which should be PPP, must tested within 4 hours of the time of collection.20 Blood for whole-blood platelet aggregometry or lumiaggregometry must be collected with 3.2% sodium citrate and held at 18° C to 24° C until testing. Chilling destroys platelet activity. Aggregometry may be started immediately and must be completed within 3 hours of specimen collection. The scientist mixes the specimen by gentle inversion, checks for clots just before testing, and rejects specimens with clots. Most specimens for whole-blood aggregometry are mixed 1 : 1 with normal saline before testing, although if the platelet count is less than 100,000/mcL the specimen is tested undiluted.21 Clot-based plasma coagulation tests require PPP–plasma with a platelet count of less than 10,000/mcL.22 Sodium citrate–anticoagulated whole blood is centrifuged at 1500 xg for 15 minutes in a swinging bucket centrifuge to produce supernatant PPP. Alternatively, the angle-head StatSpin Express 2 (Iris Sample Processing, Inc., Westwood, Mass.) generates 4400 xg and can produce PPP within 3 minutes. Both make it possible for automated coagulometers to sample from the supernatant plasma of the primary tube. The advantage of the slower swinging bucket centrifuge head is that it produces a straight, level plasma–blood cell interface, whereas angle-head centrifuge heads cause platelets to adhere to the side of the tube. If the tube is allowed to stand, the platelets drift back into the plasma and release granule contents. Each hemostasis laboratory manager establishes the correct centrifugation speed and times for the local laboratory, and centrifugation must yield PPP from specimens with high initial platelet counts. The presence of greater than 10,000 platelets/mcL in plasma affects clot-based test results. Platelets are likely to become activated in vitro and release the membrane phospholipid phosphatidylserine, which triggers plasma coagulation and neutralizes LA if present, interfering with LA testing. Platelets also secrete fibrinogen, factors V and VIII, and VWF (see Chapter 13). These may desensitize PT and PTT assays and interfere with clot-based coagulation assays. In addition, platelets release PF4, a protein that binds and neutralizes therapeutic heparin in vitro, falsely shortening the PTT and interfering with heparin management. Laboratory scientists inspect hemostasis plasmas for hemolysis (red), lipemia (cloudy, milky), and icterus (golden yellow from bilirubin). Visible hemolysis implies platelet or coagulation pathway activation. Visibly hemolyzed specimens are rejected, and new specimens must be obtained. Lipemia and icterus may affect the end-point results of optical coagulation instruments. The hemostasis laboratory manager may choose to maintain a separate mechanical end-point coagulometer to substitute for the optical instrument if the specimen is too cloudy for optical determinations. Conversely, some optical instruments detect and compensate for lipemia and icterus via spectrophotometric analysis.23 Platelet function tests are designed to detect qualitative (functional) platelet abnormalities in patients who are experiencing the symptoms of mucocutaneous bleeding (see Chapter 44). A platelet count is performed and the blood film is reviewed before platelet function tests are begun, because thrombocytopenia is a common cause of hemorrhage (see Chapter 43).24 Qualitative platelet abnormalities are suspected only when bleeding symptoms are present and the platelet count exceeds 50,000/mcL. Although hereditary platelet function disorders are rare, acquired defects are common.25 Acquired platelet defects are associated with liver disease, renal disease, myeloproliferative neoplasms, myelodysplastic syndromes, myeloma, uremia, autoimmune disorders, anemias, and drug therapy. Platelet morphology is often a clue; for instance, in Bernard-Soulier syndrome the blood film reveals mild thrombocytopenia and large gray platelets (see Figure 44-1). Similarly, the presence of large platelets on the blood film associated with elevated mean platelet volume often indicates rapid platelet turnover, such as occurs in immune thrombocytopenic purpura or thrombotic thrombocytopenic purpura. Giant or dysplastic platelets are seen in myeloproliferative neoplasms, acute leukemia, and myelodysplastic syndromes. The bleeding time test was the original test of platelet function, although it is now largely replaced by near-patient analysis of platelet function using the PFA-100 (Siemens Healthcare Diagnostics, Inc., Deerfield, Ill.), the Ultegra (Accumetrics, San Diego, Calif.), or platelet aggregometry.26 To perform the test, the phlebotomist uses a lancet to make a small controlled puncture wound and records the duration of bleeding, comparing the results with the universally accepted reference interval of 2 to 9 minutes. The bleeding time test was first described by Duke27 in 1912 and modified by Ivy28 in 1941. In 1978 some standardization was attempted: a blood pressure cuff was inflated to 40 mm Hg, a calibrated spring-loaded lancet (Surgicutt Bleeding Time Device; International Technidyne Corporation, Edison, N.J.) was triggered on the volar surface of the forearm a few inches distal to the antecubital crease, and the resulting wound was blotted every 30 seconds with filter paper until bleeding stopped.29,30 Functional platelets adhere to subendothelial collagen, aggregate with each other, and secrete the contents of their α-granules and δ-granules (see Chapter 13). Normal adhesion requires intact platelet membranes and functional plasma VWF. Normal aggregation requires that platelet membranes and platelet activation pathways are intact, that the plasma fibrinogen concentration is normal, and that normal secretions are release from platelet granules. Platelet adhesion, aggregation, and secretion are assessed using in vitro platelet aggregometry. PRP aggregometry is performed using a specialized photometer called a light-transmittance aggregometer (PAP-8E Platelet Aggregation Profiler; Bio/Data Corporation, Horsham, Pa.).31 After calibrating the instrument in accordance with manufacturer instructions, the operator pipettes the PRP to instrument-compatible cuvettes, usually 500 mcL; drops in one clean plasticized stir bar per sample; places the cuvettes in incubation wells; and allows the samples to warm to 37° C for 5 minutes. The operator then transfers the first cuvette, containing specimen and stir bar, to the instrument’s reaction well and starts the stirring device and the recording computer. The stirring device turns the stir bar at 800 to 1200 rpm, a gentle speed that keeps the platelets in suspension. The instrument directs focused light through the sample cuvette to a photodetector (Figure 45-3). As the PRP is stirred, the recorder tracing first stabilizes to generate a baseline, near 0% light transmission. After a few seconds, the operator forcibly pipettes an agonist (aggregating agent) into the sample to trigger aggregation. In a normal specimen, after the agonist is added, the shape of the suspended platelets changes from discoid to spherical, and the intensity of light transmission increases in proportion to the degree of shape change. Percent transmittance is monitored continuously and recorded (Figure 45-4). As platelet aggregates form, more light passes through the PRP, and the tracing begins to move toward 100% light transmission. Platelet deficiencies are reflected in diminished or absent aggregation; many laboratory directors choose 40% aggregation as the lower limit of normal. In whole-blood platelet aggregometry, platelet aggregation is measured by electrical impedance using a 1 : 1 saline–whole blood suspension (Model 700 Whole Blood/Optical Lumi-Aggregometer; Chrono-log Corporation, Havertown, Pa.).32 The operator pipettes aliquots of properly mixed whole blood to cuvettes and adds equal volumes of physiologic saline. Suspension volume may be 300 to 500 mcL. The operator drops in one stir bar per cuvette and places the cuvettes in 37° C incubation wells for 5 minutes. The operator transfers the first cuvette to a reaction well, forcibly pipettes in an agonist, and suspends a pair of low-voltage cartridge-mounted disposable direct current (DC) electrodes in the mixture. As aggregation occurs, platelets collect on the electrodes, impeding the DC current (Figure 45-5). The rise in impedance is directly proportional to platelet aggregation and is amplified and recorded by instrument circuitry. A whole-blood aggregometry tracing closely resembles a PRP-based light-transmittance aggregometry tracing as shown in Figure 45-4. The Whole Blood/Optical Lumi-Aggregometer may be used for simultaneous measurement of platelet aggregation and the secretion of adenosine triphosphate (ATP) from activated platelet δ-granules.33 The procedure for lumiaggregometry differs little from that for conventional aggregometry and simplifies the diagnosis of platelet dysfunction.34 As ATP is released it oxidizes a firefly-derived luciferin-luciferase reagent (Chrono-lume; Chrono-log Corporation) to generate cold chemiluminescence proportional to the ATP concentration. A photodetector amplifies the luminescence, which is recorded as a second tracing on the aggregation report.35 Lumiaggregometry may be performed using whole blood or PRP.36 To perform lumiaggregometry, the operator adds an ATP standard to the first sample, then adds luciferin-luciferase and tests for full luminescence. The operator then adds luciferin-luciferase and an agonist to the second sample; the instrument monitors for aggregation and secretion simultaneously. Thrombin is typically the first agonist used, because thrombin induces full secretion. The luminescence induced by thrombin is measured, recorded, and used for comparison with the luminescence produced by the additional agonists. Normal secretion induced by agonists other than thrombin produces luminescence at a level of about 50% of that resulting from thrombin. Figure 45-6 depicts simultaneous aggregation and secretion responses to thrombin; Figure 45-7 is a scanning electron micrograph of resting and activated platelets. The agonists used most frequently in clinical practice are thrombin or synthetic thrombin receptor–activating peptide (TRAP), adenosine diphosphate (ADP), epinephrine, collagen, arachidonic acid, and ristocetin. Table 45-4 lists representative concentrations and platelet activation pathways tested by each agonist. Small volumes (2 to 5 mcL) of concentrated agonist are used so that they have little dilutional effect in the reaction system.37 TABLE 45-4 Platelet Aggregometry Agonists, Reaction Concentrations, and Platelet Receptors Thrombin (or TRAP) cleaves two platelet membrane protease-activatable receptors (PARs), PAR1 and PAR2, both members of the seven-transmembrane repeat receptor family (see Chapter 13). Thrombin or TRAP also cleaves glycoprotein (GP) 1bα and GP V. Internal platelet activation is effected by membrane-associated G proteins and both the eicosanoid and the diacylglycerol pathways. Thrombin-induced activation results in full secretion and aggregation. In lumiaggregometry, the operator ordinarily begins with 1 unit/mL of thrombin or TRAP to induce the release of 1 to 2 nmol/L of ATP, detected by the firefly luciferin-luciferase luminescence assay. Other agonists—for instance, 5 mcg/mL of collagen—induce the release of at most 0.5 to 1.0 nmol/L of ATP. Thrombin-induced secretion may be diminished to less than 1 nmol/L in storage pool deficiencies (see Chapter 44), but is relatively unaffected by membrane disorders or pathway enzyme deficiencies. ADP is the most commonly used agonist, particularly in aggregometry systems that measure only aggregation and not luminescence. The operator adjusts the ADP concentration to between 1 mcmol/L and 10 mcmol/L to induce “biphasic” aggregation (see Figure 45-4). At ADP concentrations near 1 mcmol/L, platelets achieve only primary aggregation, followed by disaggregation. The recording deflects from the baseline for 1 to 2 minutes and then returns to baseline. Primary aggregation involves shape change with formation of microaggregates, both reversible. Secondary aggregation is the formation of full platelet aggregates after release of platelet δ-granule ADP. At agonist ADP concentrations near 10 mcmol/L, there is simultaneous irreversible shape change, secretion, and formation of aggregates, resulting in a monophasic curve and full deflection of the tracing. ADP concentrations between 1 and 10 mcmol/L induce a biphasic curve: primary aggregation followed by a brief flattening of the curve called lag phase and then secondary aggregation.
Laboratory Evaluation of Hemostasis
Hemostasis Specimen Collection
Patient Management During Hemostasis Specimen Collection
Hemostasis Specimen Collection Tubes
Hemostasis Specimen Collection Rules
Error
Comments
Short draw
Whole-blood volume less than 90% of required volume or less than manufacturer-specified minimum
Clot in specimen
Each specimen must be visually inspected prior to centrifugation; the presence of even a small clot requires that the specimen be recollected.
Visible hemolysis
Hemolysis, pink or red plasma, indicates in vitro activation of platelets and coagulation, and results will be unreliable.
Lipemia or icterus
Optical instruments may not measure clots in cloudy or highly colored specimens. The scientist automatically shifts to the use of a mechanical instrument.
Prolonged tourniquet application
Stasis elevates the concentration of von Willebrand factor and factor VIII and shortens the measured time on clot-based tests.
Specimen storage at 1° C to 6° C
Storage at refrigerator temperatures causes precipitation of large von Willebrand factor multimers and destroys platelet integrity.
Specimen storage at more than 25° C
Storage at above standard room temperature causes coagulation factor VIII deterioration.
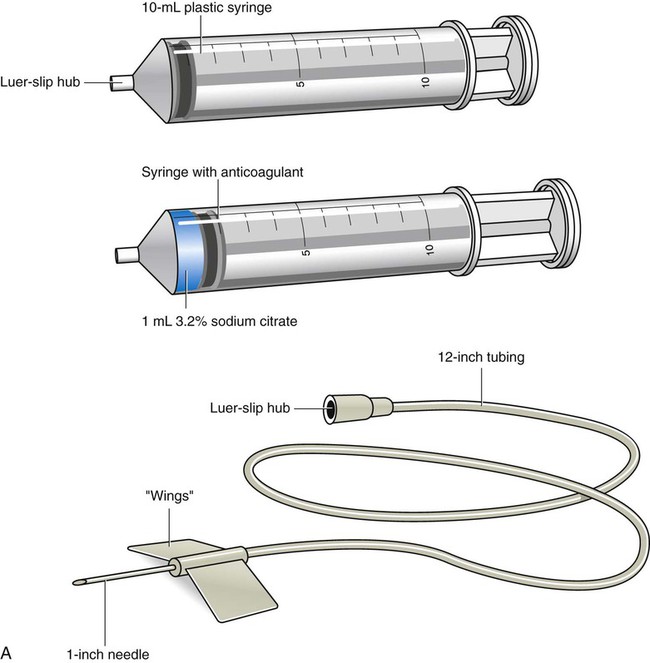
Specimen Collection Using Syringes and Winged-Needle Sets
Selection of Needles for Hemostasis Specimens
Application
Preferred Needle Gauge and Length
Adult with good veins, specimen ≤25 mL
20 or 21 gauge, thin walled, 1.0 or 1.25 inches long
Adult with good veins, specimen ≥25 mL
19 gauge, 1.0 or 1.25 inches long
Child or adult with small, friable, or hardened veins
23 gauge, winged-needle set; apply minimal negative pressure
Transfer of blood from syringe to tube
19 gauge
Syringe with winged-needle set
20 or 21 gauge, thin walled; use only for small, friable, or hardened veins or special coagulation testing
Collection of Specimens from Vascular Access Devices
Specimen Collection Using Capillary Puncture
Anticoagulants Used for Hemostasis Specimens
Sodium Citrate (Primary Hemostasis Anticoagulant)
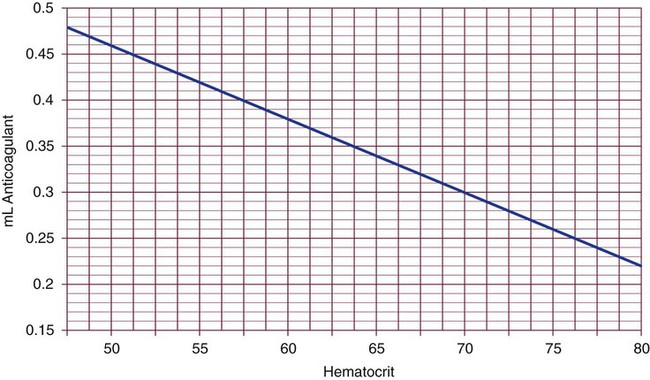
Adjustment of Sodium Citrate Volume for Elevated Hematocrits
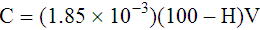
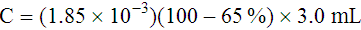
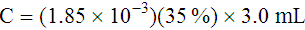
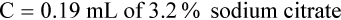
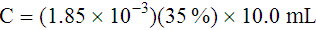
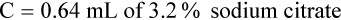
Other Anticoagulants Used for Hemostasis Specimens
Hemostasis Specimen Management
Hemostasis Specimen Storage Temperature
Application
Temperature
Time
PT with no unfractionated heparin present in specimen
18°-24° C
24 hr
PTT with no unfractionated heparin present in specimen
18°-24° C
4 hr
PTT for monitoring unfractionated heparin therapy
18°-24° C
Separate within 1 hr, test within 4 hr
PT when unfractionated heparin is present in specimen
18°-24° C
Separate within 1 hr, test within 4 hr
Factor assays
18°-24° C
4 hr
Optical platelet aggregometry using platelet-rich plasma
18°-24° C
Wait 30 min after centrifugation, test within 3 hr of collection
Whole-blood aggregometry
18°-24° C
Test within 3 hr of collection
Storage in household freezer
−20° C
2 wk
Storage for 6 mo
−70° C
6 mo
Hemostasis Specimen Storage Time
Preparation of Hemostasis Specimens for Assay
Whole-Blood Specimens Used for Platelet Aggregometry
Platelet-Poor Plasma Required for Clot-Based Testing
Platelet Function Tests
Bleeding Time Test for Platelet Function
Platelet Aggregometry and Lumiaggregometry
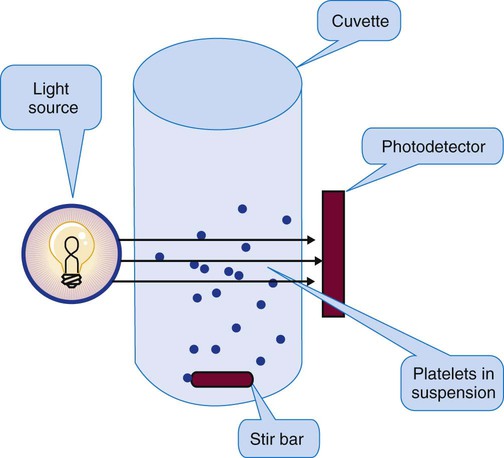
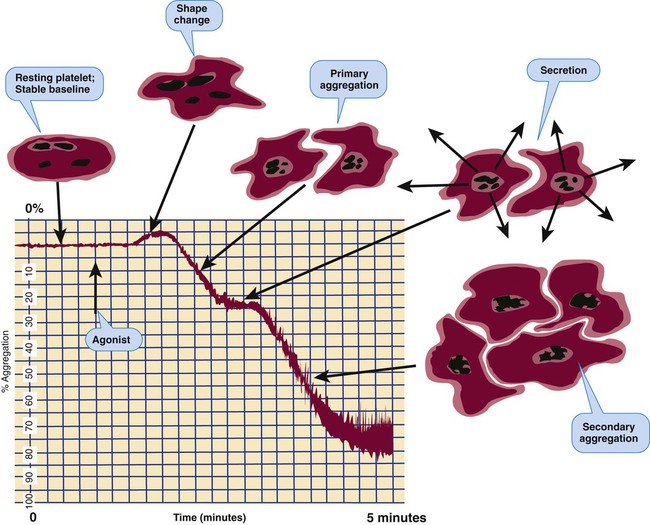
Platelet Aggregometry Using Platelet-Rich Plasma
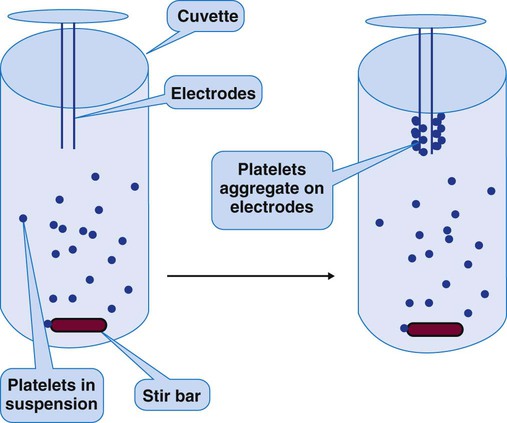
Whole-Blood Platelet Aggregometry
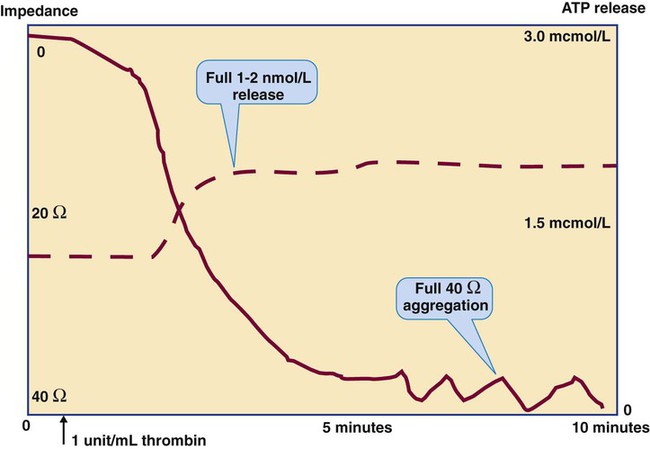
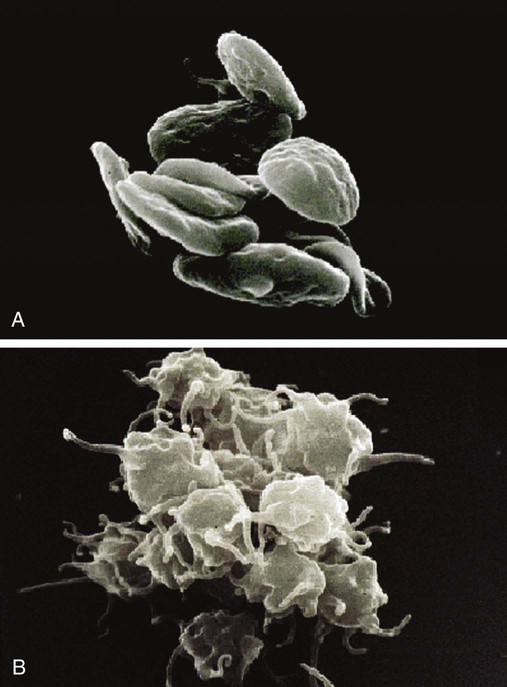
Platelet Lumiaggregometry
Platelet Agonists (Activating Agents) Used in Aggregometry
Agonist
Typical Concentration
Receptors
Thrombin
1 unit/mL
PAR1 and PAR4; GP Ibα and GP V
ADP
1-10 mcmol/L
P2Y1, P2Y12
Epinephrine
2-10 mcmol/L
α2-Adrenergic receptor
Collagen
5 mcg/mL
GP Ia/IIa, GP VI
Arachidonic acid
500 mcmol/L
TPα, TPβ
Ristocetin
10 mg/mL
GP Ib/V/IX in association with von Willebrand factor
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Laboratory Evaluation of Hemostasis