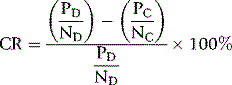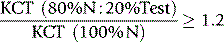Chapter 19 Investigation of a thrombotic tendency
Introduction to thrombophilia
In this chapter, the investigations to detect an acquired thrombotic tendency are presented first, followed by a simplified battery of tests needed to establish the diagnosis of the more important inherited ‘thrombophilias’. Although the number of coagulation factors known to contribute to a thrombotic tendency has increased greatly in the last few years, it remains clear that not all factors have been identified. Hence, the failure to detect one of the traits described does not imply that the individual’s risk of thrombosis is normal. An acquired thrombotic tendency is common and occurs in many conditions but is usually complex, multifactorial and not easily identifiable by a single laboratory test. The large number of traits identified, often with a small associated relative risk, makes their individual utility equally small. Until the interactions of these numerous factors are more completely understood, the clinical history remains a dominant factor in clinical management. The British Committee for Standards in Haematology has published guidelines on the investigation of inherited thrombophilia.1
Tests for the presence of a lupus anticoagulant
The lupus anticoagulant (LAC) is an acquired autoantibody found in various autoimmune disorders and sometimes in otherwise healthy individuals.2 LACs are immunoglobulins that bind to certain proteins when bound to phospholipid. The effective sequestration of phospholipid can then cause prolongation of phospholipid-dependent coagulation tests such as the prothrombin time (PT) or activated partial thromboplastin time (APTT). The name ‘anticoagulant’ is misleading because, despite the in vitro effects, patients do not have a bleeding tendency. Instead, there is a clear association with recurrent venous thromboembolism, cerebrovascular accidents and other arterial events and, in women, with recurrent abortions, fetal loss and other complications of pregnancy.3 Therefore, tests for the presence of the LAC should be carried out in all young individuals with unexplained venous or arterial thrombosis and also in women with recurrent-early or late pregnancy loss.4 Antibodies of this class are members of a larger group called antiphospholipid or anticardiolipin antibodies. (Although not precisely the same these terms are used interchangeably.) Tests for lupus anticoagulant are usually performed in parallel with tests for the presence of antiphospholipid antibodies, usually by ELISA. In general these tests are not performed by haematology laboratories and are therefore not described here. Serological tests for antiphospholipid antibodies are not standardized and agreement between laboratories is poor. A large number of target proteins have been described but the most important, and the only one for which there is evidence of a pathogenic effect, is β2-glycoprotein 1.5 Increasingly, tests specifically for anti-β2-glycoprotein 1 antibodies are performed and possible mechanisms for their prothrombotic activity are being elucidated.6
The presence of a LAC may be detected by the clotting screen and, depending on the reagents and methods used as well as on the potency and avidity of the antibody, either the PT or APTT may be prolonged. However, the sensitivity of both APTT and PT to LAC varies considerably, so that these tests may well be normal and, if clinically suspected, specific tests should always be performed.4 The unmodified test for activated protein C (APC) resistance (see p. 456) is also sensitive to the presence of a LAC.
Patients with a LAC may show other abnormalities, including thrombocytopenia, a positive direct antiglobulin test and a positive antinuclear antibody test. Another frequent target for antiphospholipid antibodies is prothrombin but only rarely are these antibodies sufficient to inhibit or deplete prothrombin activity. Such patients may have a bleeding tendency. A recent international guideline on detection of lupus anticoagulants has been published4 and recommended the following tests:
There are a large number of additional tests which have in the past been successfully used for the detection of LAC, several of which are no longer recommended due to poor reproducibility, technical problems and lack of standardization.4 The kaolin clotting time (KCT) and the dilute thromboplastin inhibition test are retained here because they are still widely used and thought to have some advantages by some authors. Although no single test is sufficiently sensitive to detect all LAC, readers are counselled against performing an excessive (more than two) number of tests because a large number of false positives will be generated. The most recent guidelines for the optimal performance of testing for LAC have been well laid out and are summarized in Box 19.1.4
Box 19.1 Recommendations for the optimal laboratory detection of lupus anticoagulant (LAC)4
(A) Blood collection
(C) Mixing test
(D) Confirmatory test
Dilute Russell’s Viper Venom Time
Principle
Russell’s viper venom (RVV) activates factor X, leading to a fibrin clot in the presence of factor V, prothrombin, phospholipid and calcium ions. A LAC prolongs the clotting time by binding to the phospholipid and preventing the action of RVV. As the following test describes, dilution of the venom and phospholipid makes it particularly sensitive for detecting a LAC.7 Because RVV activates factor X directly, defects of the contact system and factor VIII, IX and XI deficiencies do not influence the test. The DRVVT should be combined with a platelet/phospholipid neutralization procedure to add specificity and this is incorporated into several commercial kits.
Reagents
Platelet Neutralization Test
Reagents for preparation of platelet neutralization reagent
Interpretation
where P is patient’s clotting time and N is the clotting time of normal plasma and D represents the detection procedure and C represents the confirmation (platelet/phospholipid neutralization) procedure.7 A correction of >10% is regarded as positive, but care should be taken to establish a local normal range. Other calculations may also be used such as a simple ratio of PD/PC or percentage correction: [(PD − PC)/PD] × 100%.4
Interpretation of Tests for Lupus Anticoagulant
Detailed instructions for the interpretation of LAC testing have been published.4 No single test detects all lupus-like anticoagulants and, if suspected clinically, then two specific tests should be performed before concluding that a LAC is not present.4,8 Conversely, a single positive test should be repeated 12 weeks later because a transient positive may arise as the result of intercurrent illness or medication. It is crucial to distinguish LAC from specific anti-factor VIII antibodies, which are more typically time dependent but may have some immediate effect as well. Specific factor assays can be useful in discrimination but note that a LAC may result in non-parallelism and spuriously low results in these assays. Similarly, some weak LAC are neutralized by 50:50 mixing with normal plasma and sometimes exhibit a time-dependent effect. Some transient non-specific coagulation inhibitors are not detected by tests for LAC. Tests may be falsely negative while taking warfarin.
Kaolin Clotting Time
Principle
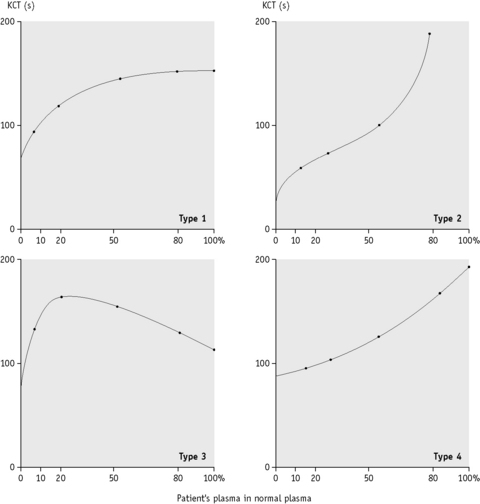
When the APTT is performed in the absence of platelet substitute reagent, it is particularly sensitive to a LAC. If the test is performed on a range of mixtures of normal and patient’s plasma, different patterns of response are obtained, indicating the presence of a LAC, deficiency of one or more of the coagulation factors or the ‘lupus cofactor’ effect.9
Reagents
Results
Plot the clotting times against the proportion of normal to patient’s plasma on linear graph paper as shown in Figure 19.1.
Dilute Thromboplastin Inhibition Test
Principle
When the thromboplastin used for the PT is diluted, the PT becomes prolonged. At a certain point (usually 1:50–1:500 dilution) the concentration of phospholipid is low enough for the test to become sensitive to phospholipid binding antibodies and when a LAC is present the ratio of the test plasma to normal plasma clotting time increases. This test is now considered more useful because some thromboplastin reagents (e.g. Innovin) are more sensitive to LACs. However, it should be noted that diluting thromboplastin makes the system sensitive to low levels of factor VIII as are encountered in mild haemophilia, acquired haemophilia and low levels of factor V or factor VII. Care should be taken that these disorders are not confused. In one study, the test was determined to be positive when the dilute PT ratio (test/mean normal) using Innovin at 1:200 dilution was >1.15.10
Other Acquired Thrombophilic States
There are numerous other disorders that are associated with an increased risk of thrombosis but are not usually diagnosed using coagulation-based tests. Appropriate tests for some of these such as myeloproliferative neoplasms and paroxysmal nocturnal haemoglobinuria are found elsewhere in this book. One of the most important factors precipitating thrombosis is malignancy. However, the value of extensive testing for possible malignancy in patients with thrombosis remains contentious; some studies have shown that a history and examination combined with a few simple tests detect the large majority of malignancy and other systemic disorders. Other studies suggest that more intensive screening including abdominal and pelvic scans are required to achieve this. Large numbers of ‘tumour marker’ analyses result in numerous false positives. Even when tests have been effective in detecting occult malignancy it is not clear there is any improvement in outcome.11,12
Investigation of inherited thrombotic states
Testing for thrombotic syndromes remains frequent, despite doubts about its clinical utility.1 Patients with disorders of pregnancy and those with thrombotic disorders are often referred for investigation. Prior to testing for thrombophilia consideration should be given to the likely benefits including alteration in management that can be achieved. This requires a carefully taken history, noting in particular the circumstances of any previous thrombotic event, a family history of thrombosis and identification of any coexisting disorders. The relevant tests are described below.
Antithrombin (AT)
AT13 (previously known as antithrombin III) is the major physiological inhibitor of thrombin and factors IXa, Xa and XIa. AT deficiency is found in approximately 2% of cases of thrombosis and may be acquired or congenital. Various methods are available for measuring either functional activity or antigenic quantity of AT. The functional methods are based on the reaction with thrombin or factor Xa and can be coagulation based or chromogenic assays. A chromogenic assay is described below.
Antithrombin (AT) Measurement Using a Chromogenic Assay
Interpretation
In an inherited deficiency, the AT concentration is usually <0.7 iu/ml. Most cases are heterozygotes for null mutations (type I deficiency) and have levels of approximately 50% of normal. Be aware that numerous type 2 variants have been described affecting the reactive site, the heparin binding site or having pleiotropic effects, sometimes resulting in assay results that are close to normal. The clinical significance of heterozygous heparin-binding site mutations is probably low. Further tests such as AT antigen, crossed immunoelectrophoresis or mutation analysis may be required to identify variant molecules.14,15 A low level of AT may be acquired as a result of active thrombosis, liver disease, heparin therapy, nephrotic syndrome or asparaginase therapy; very low values are sometimes encountered in fulminant disseminated intravascular coagulation (DIC) or liver failure. Normal newborns have a lower AT concentration (0.60–0.80 iu/ml) than adults. In neonates who are congenitally deficient, very low values (0.30 iu/ml and lower) may be found. It is also important to remember that oral anticoagulant therapy may increase the AT concentration by approximately 0.1 iu/ml in cases of congenital deficiency.