Invasive Bladder Cancer
▪ 23A Radical Cystectomy and Pelvic Lymph Node Dissection
Bernard H. Bochner
Marcus L. Quek
Donald G. Skinner
INTRODUCTION
Invasive bladder cancer is a potentially lethal disease. If left untreated, over 85% of patients will die of the malignancy within 2 years of diagnosis (1). The mainstay of therapy for clinically localized muscle-invasive bladder cancer is a radical cystectomy with a thorough pelvic lymphadenectomy. The surgical approach has evolved as a direct result of our improved understanding of the natural history of invasive bladder cancer, with its propensity for local tumor extension into the perivesical soft tissue and regional lymphatic spread. Thus, the optimal exenterative procedure should include resection of these areas in order to maximize the opportunity for cure.
While clinically localized muscle-invasive disease represents the most common indication for radical cystectomy, select patients with high-risk non-muscle-invasive bladder cancer (2,3,4), regional metastatic involvement (5), and even select patients with advanced stage disease (6,7) may benefit from an aggressive surgical approach. Although the exact role of perioperative systemic chemotherapy continues to be debated, the importance of an appropriately performed radical cystectomy remains paramount to obtaining favorable clinical outcomes (8,9).
The rationale for an aggressive surgical approach for invasive bladder cancer is based on several observations. First, radical cystectomy arguably provides better long-term disease-specific survival and lower local recurrence rates compared to bladder-sparing therapies. Second, removal of the primary bladder tumor and regional lymph nodes provides the most accurate means of staging, thus identifying those that might benefit from adjuvant systemic therapy or inclusion in clinical trials. Last, surgical improvements, such as orthotopic reconstruction and nerve-sparing radical cystectomy, have improved the quality-of-life of patients requiring bladder removal, eliminating the need for urostomy appliances, cutaneous stomas, and the need for catheterization in most instances.
A properly performed radical cystectomy and pelvic lymph node dissection (PLND) can provide long-term disease-free survival for the majority of those with organ-confined and locally-advanced bladder cancer as well as a significant subset of patients with regionally advanced tumors. The surgical technique is critical in order to optimize oncologic efficacy and functional outcomes. Herein, we describe the evolution of the contemporary radical cystectomy, the rationale for a thorough lymphadenectomy, and provide a detailed description of the surgical approach.
HISTORICAL PERSPECTIVE
Early reports of bladder removal for cancer involved a two-stage procedure that included initial urinary diversion (ureteroenterostomies) followed by what would be considered today as a simple cystectomy. This approach was associated with an exceedingly high perioperative mortality rate as high as 30% to 40% (10). As improvements in perioperative care and increased surgical experience emerged, operative mortality for cystectomy decreased and the oncologic efficacy of the procedure was better delineated.
In 1950, Leadbetter and Cooper (10) published a focused review of the literature on the early experience with cystectomy. Of the 429 patients reported between 1939 and 1948, 342 survived the procedure and were evaluable for local and distant disease control. Overall 33% (range: 22.8%-53.3%) of surviving patients died of recurrent disease, typically within the first 1 to 2 years after surgery. Although the patterns of failure were not particularly well documented in these early series, local recurrences were frequent. Local extension of disease into the perivesical tissues that were incompletely excised was likely responsible for many locally recurrent lesions; however, regional metastatic deposits in the pelvic lymphatics that were not controlled at cystectomy also likely represented a source of subsequent local failure.
Orr et al. (11) reviewed the management of over 26,000 cases of bladder cancer, 353 of which were managed with total cystectomy. In this early report, 92 (26%) died within the first year after surgery and only 18 patients (5%) were alive at 5 years. Grossly involved regional nodes were noted intraoperatively in 47% of cases. It must be highlighted that most initial surgical approaches to cystectomy made no attempt at controlling the regional lymphatics.
During this same time, autopsy studies documented the natural pathways of progression of invasive bladder cancer. Several large reports demonstrated that involvement of the regional lymphatics was a frequent finding in patients with invasive bladder cancer and occasionally represented the only site of metastatic disease. Cunningham reported on the autopsy findings of 411 patients with advanced bladder cancer and noted that 24% of patients had evidence of regional lymph node involvement (12). Similarly, Spooner (13) reported on 163 autopsy cases performed in patients with carcinoma of the bladder from the Mayo Clinic between 1914 and 1931, in which 24% had metastatic disease limited to the regional lymph nodes. Colston and Leadbetter (14) reported 98 autopsy cases with over 50% having metastatic disease, of which 25% had involvement of the pelvic or retroperitoneal lymph nodes as their only site of spread. Limitations in the extent of microscopic review of the regional lymph nodes in all of these early reports likely underestimated the extent of regional metastatic disease within these populations.
The relationship between the relative risk of nodal involvement and the depth of invasion of the primary bladder tumor was a landmark association provided by autopsy data from Jewett and Strong (15) in 1946. These investigators reported the autopsy findings of 107 patients with infiltrating bladder tumors in which patients were grouped by the extent of invasion of the primary tumor: Group A consisted of three cases and represented lesions involving the submucosal layer
only; Group B included 15 patients with invasion into the detrussor muscle but confined to the bladder; and Group C included 89 patients with disease extension into the perivesical tissues. No patient in Group A had evidence of metastatic disease; however, 11% of Group B and 52 of the 89 (58%) patients in Group C were found to have evidence of either regional or distant spread. Of the 52 Group C patients with metastatic disease, 33 (63%) had regional lymph node involvement. Six of the fifty-two (12%) had regional pelvic lymph node involvement as their only site of metastatic disease. The relationship between depth of invasion of the primary tumor and risk of regional lymph node involvement has been corroborated in contemporary radical cystectomy series. The risk of regional lymph node involvement ranges from 6% to 10% for organ-confined invasive tumors and 42% to 75% for lesions that involve the perivesical fat or adjacent organs (16,17,18,19).
only; Group B included 15 patients with invasion into the detrussor muscle but confined to the bladder; and Group C included 89 patients with disease extension into the perivesical tissues. No patient in Group A had evidence of metastatic disease; however, 11% of Group B and 52 of the 89 (58%) patients in Group C were found to have evidence of either regional or distant spread. Of the 52 Group C patients with metastatic disease, 33 (63%) had regional lymph node involvement. Six of the fifty-two (12%) had regional pelvic lymph node involvement as their only site of metastatic disease. The relationship between depth of invasion of the primary tumor and risk of regional lymph node involvement has been corroborated in contemporary radical cystectomy series. The risk of regional lymph node involvement ranges from 6% to 10% for organ-confined invasive tumors and 42% to 75% for lesions that involve the perivesical fat or adjacent organs (16,17,18,19).
Based on the disappointing rates of local disease control of the early surgical series and autopsy data that clearly documented that the pathways of progression for invasive disease included involvement of the regional pelvic lymphatics, it became apparent that alterations in the surgical approach toward invasive bladder cancer were needed to improve outcomes. Indeed, in 1950, Marshall and Whitmore (20) concluded after reporting a 38% local failure rate in 100 consecutive patients undergoing cystectomy for bladder cancer that “the implication seems unavoidable that a more radical procedure might be worthy of trial.” The basis of this more radical approach would incorporate wide excision of the bladder and perivesical tissues and a formal attempt at controlling the regional lymphatics via a pelvic lymphadenectomy at the time of cystectomy, thus defining the contemporary radical cystectomy procedure.
DEFINITION
Radical cystectomy implies the en bloc removal of the bladder, urachus, prostate, seminal vesicles, and perivesical fat in men. In women, the term anterior pelvic exenteration is utilized interchangeably since the pelvic organs anterior to the rectum are removed, including the bladder, urachus, ovaries, fallopian tubes, uterus, cervix, and occasionally a portion of the vaginal wall.
PREOPERATIVE EVALUATION
The current clinical staging for bladder cancer involves a combination of physical, laboratory, endoscopic, and radiographic evaluations. A bimanual pelvic examination, preferably under anesthesia, provides a gross assessment of the threedimensional nature of the bladder. A palpable bladder mass or local fixation to the pelvic floor or adjacent viscera implies local extravesical extension, findings of which should lead to consideration of neoadjuvant chemotherapy (21). Cytoscopic evaluation of the urethra and bladder performed at diagnosis should detail the proximity of the tumor to the bladder neck, ureteral orifices, and urethra. The utility of transurethral resection (TUR) of the prostate or bladder neck biopsies to determine candidacy for orthotopic reconstruction remains controversial (22,23).
Imaging of the retroperitoneum and pelvis along with the most common metastatic sites, including lungs, liver, and bone, is often performed with computerized tomography and plain chest radiography. Those with elevated alkaline phosphatase levels with or without bone pain should be further evaluated with nuclear bone scan to rule out osseous involvement. Evaluation for concomitant upper tract urothelial disease or associated hydronephrosis may further alter the surgical plan. Despite improvements in radiographic imaging, accurate preoperative staging remains difficult with clinical understaging noted in 30% to 50% of patients (2,24,25,26,27).
SURGICAL TECHNIQUE OF RADICAL CYSTECTOMY
Preoperative Preparation
The routine use of preoperative mechanical and antibiotic bowel preparation prior to radical cystectomy has recently been challenged (28,29). Concern over alteration of colonic bacterial flora with resulting higher rates of Clostridium difficile colitis due to preoperative oral antibiotic regimens remains controversial but has led many groups to discontinue them from routine use (30). While the majority of patients undergo urinary diversion utilizing small bowel and can probably do without an antibiotic preparation, any patient in whom a colonic diversion is contemplated may benefit from both mechanical and antibiotic bowel cleansing.
Patients are instructed to commence a clear liquid diet starting 1 or 2 days prior to surgery. Simple mechanical bowel preparation with one bottle of magnesium citrate the morning prior (and repeated additional times in the afternoon, if necessary) is well tolerated and obviates the need for enemas. For those that require preoperative antibiotic preparation (previous pelvic radiotherapy, possible urinary diversion with colon), a standard modified Nichols bowel preparation (31) may be used that is initiated the day prior to surgery: 1 g of neomycin orally at 10.00 a.m., 11.00 a.m., 12.00 p.m., 1.00 p.m., 4.00 p.m., 8.00 p.m., and 12.00 a.m., and 1 g of erythromycin base orally at 12.00 p.m., 4.00 p.m., 8.00 p.m., and 12.00 a.m. Patients are then admitted the morning of surgery.
Attention to fluid management is important, especially in the typical elderly bladder cancer patient, particularly on postoperative days 3 and 4 when mobilization of third-space fluid is highest, subsequently necessitating judicious use of diuretics. In addition, intravenous broad-spectrum antibiotics are administered en route to the operating room, providing adequate tissue and circulating levels at the time of incision. Perioperative antibiotics are continued for at least 24 hours.
The use of preoperative anticoagulation has been advocated by some. The possible benefit of anticoagulant use must be weighed against the risk of bleeding which may not be insignificant. We advocate the use of lower extremity intermittent compression devices prior to induction of anesthesia. Preoperative evaluation and counseling by an enterostomal therapy nurse is an absolutely critical component to the success of all patients undergoing cystectomy and urinary diversion. Patients determined to be appropriate candidates for orthotopic reconstruction are instructed as to the possibility that catheterization per urethra may be necessary postoperatively. All patients are site marked for a cutaneous stoma, instructed in the care of a cutaneous diversion (continent or incontinent form), and instructed in proper catheterization techniques should medical, technical, or oncologic factors preclude orthotopic reconstruction. The ideal cutaneous stoma site is determined only after the patient is examined in the supine, sitting, and standing position. Proper stoma site selection is important to patient acceptance should a cutaneous form of diversion be necessary. Incontinent stoma sites are best located higher on the abdominal wall, while stoma sites for continent diversions can be positioned lower on the abdomen (hidden below the belt line) since they do not require an external collecting device. The use of the umbilicus as the site for catheterization may be employed with excellent functional and cosmetic results (32).
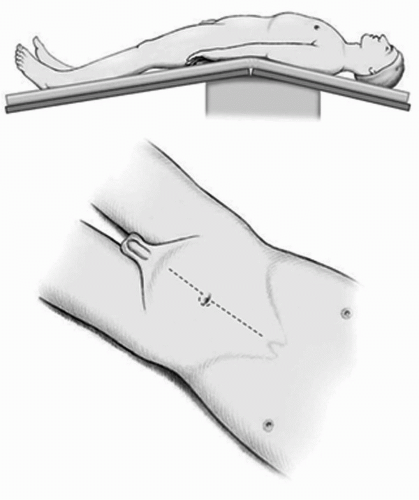 FIGURE 23A.1. Hyperextended supine position for male patients with the superior iliac crest at the fulcrum of the table and legs separated. A vertical midline incision is made to the midepigastrium (may be limited to the umbilicus in many individuals). (Reproduced from Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int 2004;94(1):197-221, with permission.) |
Patient Positioning
The patient should be positioned in the hyperextended supine position with the top of the superior iliac crest located at the break of the operating table (Fig. 23A.1). The legs are slightly abducted to provide access to the rectum if digital examination during surgery is necessary. In the female patient several options for positioning may be used and include the modified frog-legged (Fig. 23A.2), lithotomy position or with the legs placed on spreader bars. All will allow access to the vagina. Care should be taken to ensure that all pressure points are well padded. The abdomen should then be placed parallel to the floor by placing the table in Reverse Trendelenburg position. This will help to keep the bowel in the epigastrium and out of the pelvis.
A nasogastric or orogastric tube is placed, and the patient is prepared from nipples to midthigh. In the female patient, the vagina is fully prepped. After the patient is draped, a catheter is placed in the bladder and drainage is left to gravity.
A nasogastric or orogastric tube is placed, and the patient is prepared from nipples to midthigh. In the female patient, the vagina is fully prepped. After the patient is draped, a catheter is placed in the bladder and drainage is left to gravity.
Incision
A midline incision is made extending from the pubic bone in a cephalad direction. For many patients, the incision may be limited to below or at the umbilicus. This incision allows for proper exposure when performing the extended lymphadenectomy. If extended above the umbilicus, it should be carried lateral on the contralateral side to that used for the cutaneous stoma. If the umbilicus is to be used as the site for a catheterizable stoma, the incision should be carried 2 to 3 cm lateral to the umbilicus. It is important to maintain this lateral location of the incision through the anterior rectus fascia as well to provide an adequate rim of fascia for closure of the abdomen once the procedure is completed. Once the peritoneum and posterior fascia are incised, the urachal remnant (median umbilical ligament) is identified, circumscribed, and removed en bloc with the cystectomy specimen (Fig. 23A.3). Early identification of the urachal remnant prevents early entry into a highriding bladder, and ensures complete removal of all perivesical tissues. In those patients who had previously undergone a cystotomy or a partial cystectomy, the cystotomy tract and prior full thickness of the incision scar should be circumscribed and excised with the specimen.
Abdominal Exploration
A careful, systematic intra-abdominal exploration is performed to determine the extent of disease and to evaluate for any hepatic metastases or gross retroperitoneal lymphadenopathy. The abdominal viscera should be inspected visually and manually to detect any unrelated disease. If no contraindication exists at this time, all adhesions should be incised and freed.
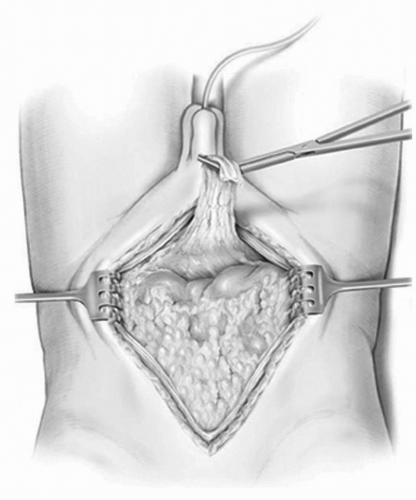 FIGURE 23A.3. The peritoneum is entered and the urachal remnant (median umbilical ligament) is circumscribed and left attached to the bladder for later en bloc removal. (Reproduced from Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int 2004;94(1):197-221, Fig. 3, with permission.) |
Bowel Mobilization
The bowel is mobilized by incising the peritoneal attachments of the right colon. The cecum and the ascending colon are reflected medially by incising the avascular/white line of Toldt. This incision is then carried up the root of the small bowel mesentery to allow full mobilization of the small bowl off its retroperitoneal attachments until the retroperitoneal portion of the duodenum is exposed. This mobilization will facilitate the urethra-neobladder anastomosis if orthotopic diversion is performed and importantly will provide excellent visualization of the cephalad extent of the extended lymph node dissection. The maneuvers of bowel mobilization are carried out along an inverted triangle whose base is formed by the third and the fourth portions of the duodenum, the right edge along lateral to the ascending colon, and left edge along the medial portion of the sigmoid and the descending colonic mesentery (Fig. 23A.4). This mobilization is critical in setting up the operative field for the LN dissection and the radical cystectomy.
The descending colon and the sigmoid mesentery are then mobilized to the region of the lower pole of the left kidney by incising the peritoneum attachments laterally along the avascular/white line of Toldt. The sigmoid mesentery is then elevated off the sacrum promontory and allows for exposure of the presacral region, iliac vessels, and distal aorta up to the origin of the inferior mesenteric artery (Fig. 23A.5). A wide mesenteric window is then opened within the sigmoid mesentery below
the inferior mesenteric vessels. This provides an angulation free-path for the left ureter to pass to the right side of the abdomen for a tension-free ureteroenteric anastomosis.
the inferior mesenteric vessels. This provides an angulation free-path for the left ureter to pass to the right side of the abdomen for a tension-free ureteroenteric anastomosis.
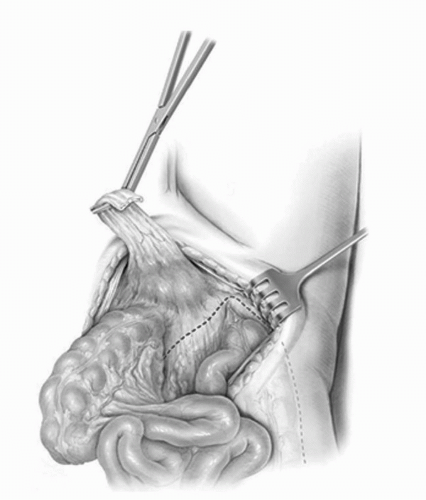 FIGURE 23A.4. Exposure of the retroperitoneum begins by incising along the white line of Toldt, thereby mobilizing the ascending colon medially, and dividing the retroperitoneal attachments to the small bowel mesentery up to the transverse portion of the duodenum. (Reproduced from Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int 2004;94(1):197-221, Fig. 4, with permission.) |
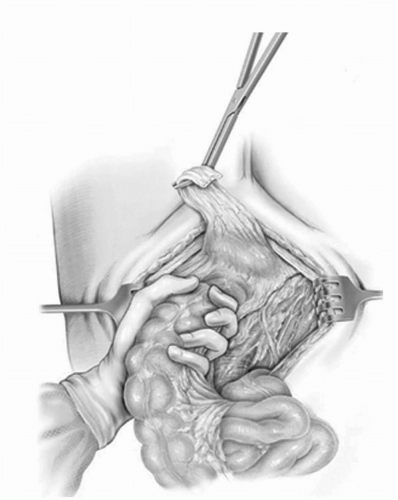 FIGURE 23A.5. The sigmoid mesentery is elevated off the sacrum and mobilized up to the origin of the inferior mesenteric artery thus creating a wide mesenteric window. (Reproduced from Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int 2004;94(1):197-221, Fig. 5, with permission.) |
Following mobilization of the bowel, the bowel contents are carefully packed into the epigastrium. The descending and the sigmoid colon are not packed with the other intestinal segments as they require mobility to properly complete the PLND.
Successful packing of the intestinal contents is a critical step in the procedure as it allows for an unobstructed view of the operative field. Packing begins by sweeping the right colon and small bowel under the surgeon’s left hand along the right sidewall gutter. A moist open laparotomy pad is then swept with the right hand along the palm of the left hand, under the viscera along the retroperitoneum and sidewall gutter. In a similar fashion, the left sidewall gutter is packed ensuring not to incorporate the descending or the sigmoid colon. The central portion of the small bowel is packed with a third laparotomy pad. A moist rolled towel is then positioned horizontally below the laparotomy pads, but cephalad to the bifurcation of the aorta. Occasionally, prior to placement of the first moist pad, a mobile greater omental apron can be used to facilitate packing of the intestinal viscera in a similar fashion to the laparotomy pad. After the bowel has been packed, a wide retractor blade is placed with gentle traction on the previous packing to provide cephalad exposure. Care should be taken to avoid compression of the great vessels by the end of this retractor blade so as not to increase venous pressure within the pelvis and retroperitoneum.
Ureteral Dissection
The ureters are most easily identified in the retroperitoneum above their crossing over the common iliac vessels. The ureters are dissected into the deep pelvis (several centimeters beyond the iliac vessels) and divided between hemoclips. A segment of the distal ureter can be sent for frozen section analysis at this point to ensure the absence of carcinoma in situ or overt tumor based on surgeon preference. The role of frozen section analysis of the distal ureteral margin has recently been questioned (33,34,35,36,37,38); however, some continue to advocate the use of frozen section analysis of the distal ureteral margins to ensure a tumor-free ureteroenteric anastamosis (39,40,41).
The ureter is then minimally mobilized cephalad only enough to allow access to the common iliac vessels and lower aorta and vena cava. It is then packed to prevent inadvertent injury during the extended PLND. Frequently, an arterial branch originating from either the common iliac artery or the lower aorta will need to be secured to properly mobilize the ureter. Care must be taken to maintain the lateral blood supply to the ureter emanating from the gonadal vessels. The gonadal vessels provide an important collateral blood supply to the lower ureter that will be used in the ureteroenteric anastomosis. This is particularly important in patients that have received prior pelvic irradiated. In women, the infundibulopelvic ligaments are ligated and divided at the level of the common iliac vessels.
Extended Lymphadenectomy
We advocate a meticulous, extended lymph node dissection when performing a radical cystectomy. The extent of the PLND may vary depending on patient factors and surgeon preference. When performing a salvage procedure following definitive radiation treatment (>5,000 rads), care should be taken as the lymphadenectomy may be difficult and associated with a significant risk of iliac vessel and obturator nerve injury (42).
The extended lymphadenectomy is initiated at the level of the inferior mesenteric artery. All lymphatic tissues are clipped proximally at this level out to the genitofemoral nerves, which represent the lateral limits of dissection. All lymphatic tissues are split and rolled off the lower aorta and vena cava. The common iliac vessels are then skeletonized, sweeping all tissues distally toward its bifurcation. At this point the distal limits of the dissection are delineated. The parietal peritoneum anterior to the external iliac vessels and medial to the gonadal vessels is divided, and in the process the vas deferens in the male (round ligament in the female) is clipped and divided. This provides exposure to the pelvic nodes (Fig. 23A.6). The medial aspect of the distal external iliac vein is identified. The lymphatics medial to the vein are traced to Cooper’s ligament where the node of Cloquet is identified, clipped, and divided. A row of clips is placed medial to lateral along the external iliac vein and artery at the level of the crossing of the circumflex iliac vein. Care is taken to avoid injury to the genitofemoral nerve fibers lateral to the artery. The lymphatics overlying the external iliac artery and vein are split and rolled to completely free both vessels circumferentially. Psoas branches from both vessels can be found on their lateral aspects near their midpoint, where they should be secured and divided. A small gauze sponge is then passed lateral to the external iliac artery and vein along the psoas muscle and gently pushed distally into the obturator fossa (Fig. 23A.7). This allows for complete clearance of all tissues in the groove between the external iliac artery and pelvic sidewall. The vein is then retracted anteriorly and the obturator nerve freed from all lymphatics (Fig. 23A.8). This allows for isolation of the obturator vessels that typically are secured and divided as they exit the obturator foramen (Fig. 23A.9). Occasionally, the obturator vessels lie in a more lateral location and may be preserved. The remaining lymphatics overlying the hypogastric vessels are then swept medially to completely free the hypogastric branches leading to the anterior pelvic organs. The remaining nodal region that requires clearance is the presacral chain, which can be freed from below the bifurcation of the common iliac vessels and swept off the sacral promontory. Removal of the presacral nodal packet and presciatic nodes can be completed after removal of the bladder specimen. Separate submissions for each nodal region are recommended to optimize the number of nodes reported.
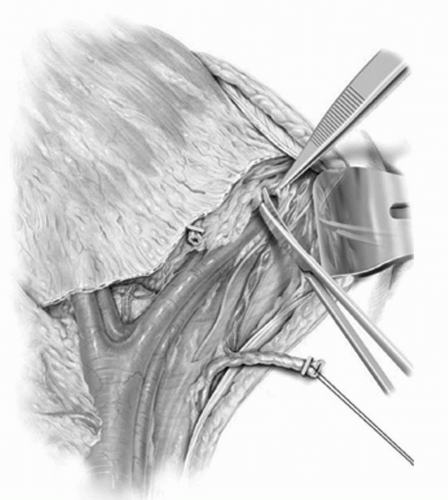 FIGURE 23A.6. All fibrofatty and lymphatic tissues are dissected off the lower aorta, vena cava, and common iliacs. The parietal peritoneum anterior to the external iliac vessels and medial to the gonadal vessels is divided, thus exposing the pelvic lymph nodes. (Reproduced from Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int 2004;94(1):197-221, Fig 6, with permission.) |
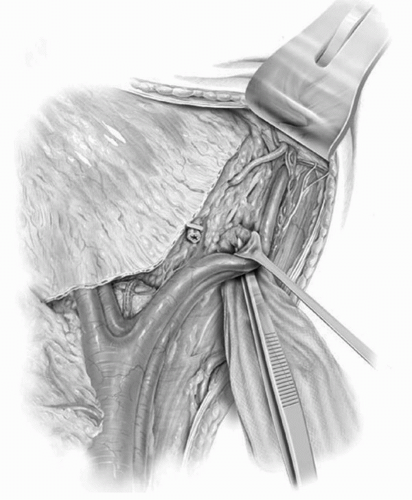 FIGURE 23A.7. Passage of a small gauze sponge lateral to the external iliac vessels, along the pelvic sidewall, and down into the obturator fossa facilitates the complete removal of all lymphatic tissue between the external iliacs and the pelvic sidewall. (Reproduced from Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int 2004;94(1):197-221, Fig. 7, with permission.) |
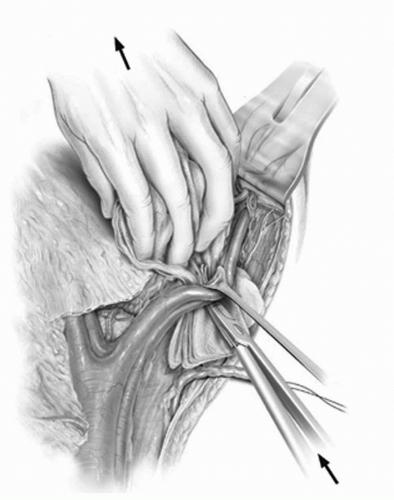 FIGURE 23A.8. The external iliac vessels are retracted laterally and the gauze sponge is carefully withdrawn from the obturator fossa. In doing so, the lymphatics in the obturator-hypogastric space are retrieved while identifying and preserving the obturator nerve. (Reproduced from Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int 2004;94(1):197-221, Fig 8, with permission.) |
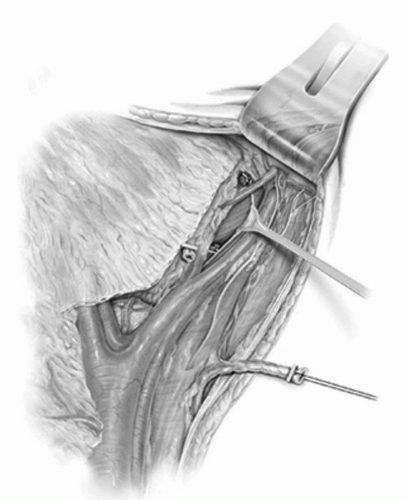 FIGURE 23A.9. The obturator vessels are isolated and secured with clips as they exit the obturator foramen. (Reproduced from Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int 2004;94(1):197-221, Fig. 9, with permission.) |
Ligation of the Lateral Vascular Pedicle to the Bladder
After completion of the lymphadenectomy, the lateral vascular pedicles to the bladder are isolated and divided. Developing the correct plane for isolation of the lateral pedicles is a critical maneuver in performing a safe cystectomy. A finger is passed medial to the hypogastric artery, and posterior to the anterior visceral branches. The index finger is positioned parallel to the sweep of the sacrum. This maneuver defines the two major vascular pedicles that provide supply to the anterior pelvic organs: the lateral pedicle lies anterior to the index finger and the posterior vascular pedicle lies behind the index finger and is composed of the visceral branches located between the bladder and the rectum.
The lateral pedicle is entrapped between the left index and the middle fingers, and firm traction is applied vertically and caudally. This maneuver will facilitate the identification of the individual anterior vascular branches originating from the anterior portion of the hypogastric artery (Fig. 23A.10). The posterior branches of the hypogastric artery including the superior gluteal, iliolumbar, and lateral sacral arteries are preserved to avoid gluteal claudication. If necessary, the hypogastric artery may be ligated distal to the takeoff of the posterior divisions; however, this is only required for very large pelvic masses that otherwise obscure the view of the anterior hypogastric branches. The initial anterior branch to the bladder, the superior vesical artery, is identified and defines the cephalad border of the pedicle. Once isolated, the lateral pedicle is then divided between hemoclips or with a roticulating vascular stapler down to the endopelvic fascia, or as far as is technically possible. The inferior vesicle vein serves as an important landmark as it is located just cephalad to the endopelvic fascia. Incision of the endopelvic fascia just lateral to the prostate should be performed at this point to help identify the distal limit of the lateral pedicle.
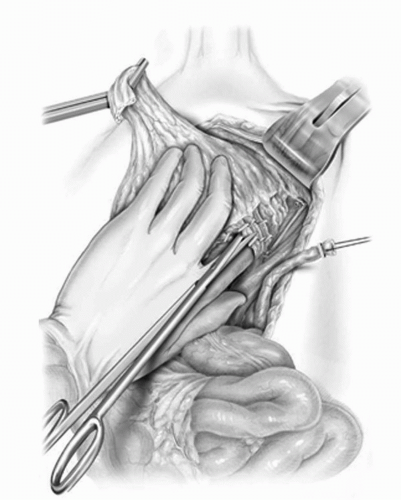 FIGURE 23A.10. The lateral vascular pedicle to the bladder is isolated and divided. (Reproduced from Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int 2004;94(1):197-221, Fig. 10, with permission.) |
Ligation of the Posterior Pedicle to the Bladder
Following division of the lateral pedicles, the bladder specimen is retracted anteriorly exposing the rectovesical cul-de-sac (pouch of Douglas). The surgeon elevates the bladder anteriorly while the assistant retracts on the peritoneum of the rectosigmoid colon in a posterior and cephalad direction. This will provide exposure to the deep regions of the cul-de-sac, and places the peritoneal reflection on traction, which will facilitate its division. The peritoneum lateral and then anteriorly to the rectum is incised across the cul-de-sac to join the incision on the contralateral side (Fig. 23A.11). The anterior and posterior peritoneal reflections converge to form Denonvilliers’ fascia within the cul-de-sac. Denovilliers provides an anatomic separation in the male as between the prostate and the seminal vesicles anteriorly from the rectum posteriorly. The prostate and seminal vesicles are separated from the rectum by incising through the posterior sheath of Denonvilliers’ (Denonvilliers’ space) allowing one to develop the plane between Denovilliers and the rectum. This separation should develop easily with blunt and sharp dissection. Incision of the peritoneal reflection in the cul-de-sac slightly on the rectal side rather than the bladder side will facilitate entering the proper plane of dissection (Fig. 23A.12). A posterior sweeping motion of the fingers with pressure maintained on the
back of the prostate and seminal vesicles will allow the rectum to be swept off the seminal vesicles, prostate, and bladder in men, and off the posterior vaginal wall in women. Sharp dissection may be required to dissect the anterior rectal fibers off the prostate and/or bladder and should be employed at any point during this dissection to prevent rectal injury.
back of the prostate and seminal vesicles will allow the rectum to be swept off the seminal vesicles, prostate, and bladder in men, and off the posterior vaginal wall in women. Sharp dissection may be required to dissect the anterior rectal fibers off the prostate and/or bladder and should be employed at any point during this dissection to prevent rectal injury.
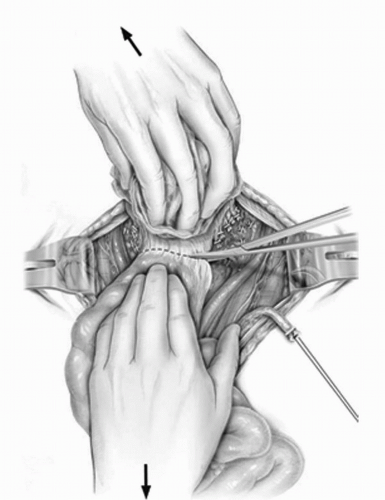 FIGURE 23A.11. The peritoneum in the cul-de-sac between the bladder and the rectum is incised. (Reproduced from Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int 2004;94(1):197-221, Fig. 11, with permission.) |
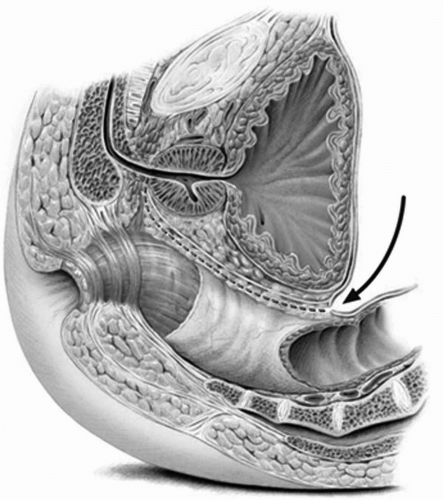 FIGURE 23A.12. Development of the space between the anterior rectal wall and the posterior sheath of Denonvilliers’ fascia. (Reproduced from Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int 2004;94(1):197-221, Fig. 12, with permission.) |
Several situations may impede the proper development of the posterior plane, most commonly, when the incision in the cul-de-sac is made too far anteriorly and proper entry into Denonvilliers’ space is prevented. Additionally, posterior tumor infiltration, prior prostatectomy, prior low anterior resection of the colon, or previous high-dose pelvic irradiation can obliterate the plane making the posterior dissection difficult. To prevent injury to the rectum in these situations, only sharp dissection should be performed under direct vision to reduce the potential for rectal injury. If a rectotomy occurs, a two-layer or three-layer closure is recommended. A diverting proximal colostomy is not routinely required unless gross contamination occurs or if the patient has received previous pelvic radiation therapy. If orthotopic or vaginal reconstruction is planned, an omental interposition is recommended to prevent fistulization between suture lines.
Use of a roticulating vascular stapler may also be used to facilitate division of the posterior pedicles (Fig. 23A.13). The endopelvic fascia is incised lateral to the prostatic apex, medial to the levator ani muscles. Opening the endopelvic fascia facilitates the apical dissection of prostate and urethra. In the female patient, the posterior pedicles including the cardinal ligaments are divided 4 to 5 cm beyond the cervix. A sponge stick placed into the vagina will help identify the vaginal apex so that the posterior vaginal wall may be properly incised just distal to the cervix. The vagina is then circumscribed anteriorly with the cervix attached to the cystectomy specimen (Fig. 23A.14). If there is concern about an adequate surgical margin at the posterior or base of the bladder, then the anterior, and if necessary, lateral vaginal wall should be removed en bloc with the bladder specimen with subsequent vaginal reconstruction if sexual
function is desired. It is our preference to spare the anterior vaginal wall if orthotopic diversion is planned. This eliminates the need for vaginal reconstruction, which helps maintain the complex musculofascial support system and helps prevent injury to the pudendal innervation to the rhabdosphincter and proximal urethra, both important components to the continence mechanism in women. The anterior vaginal wall is then sharply dissected off the posterior bladder down to the region of the bladder neck (vesicourethral junction) which is identified by palpating the Foley catheter balloon (Fig. 23A.15). At this point, the specimen remains attached only at the apex in men and vesicourethral junction in women.
function is desired. It is our preference to spare the anterior vaginal wall if orthotopic diversion is planned. This eliminates the need for vaginal reconstruction, which helps maintain the complex musculofascial support system and helps prevent injury to the pudendal innervation to the rhabdosphincter and proximal urethra, both important components to the continence mechanism in women. The anterior vaginal wall is then sharply dissected off the posterior bladder down to the region of the bladder neck (vesicourethral junction) which is identified by palpating the Foley catheter balloon (Fig. 23A.15). At this point, the specimen remains attached only at the apex in men and vesicourethral junction in women.
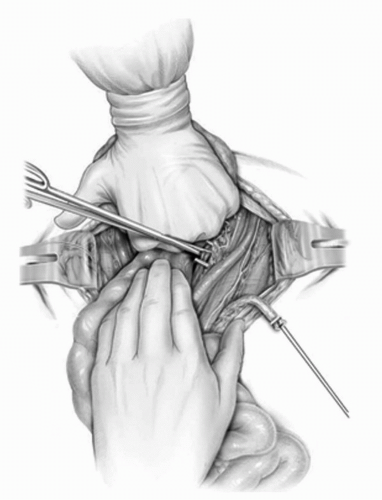 FIGURE 23A.13. The posterior pedicle to the bladder is developed and then divided down to the endopelvic fascia. (Reproduced from Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int 2004;94(1):197-221, Fig. 13, with permission.) |
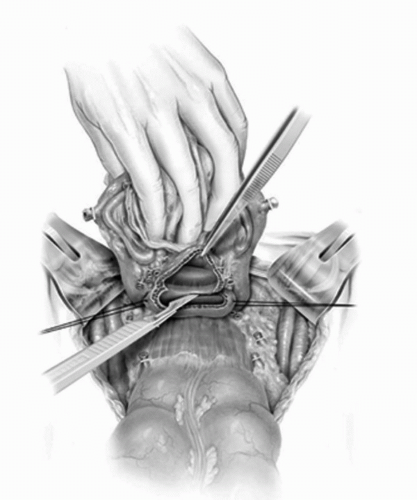 FIGURE 23A.14. The vagina is incised just distal to the cervix and then carried anteriorly along the lateral and the anterior vaginal wall. (Reproduced from Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int 2004;94(1):197-221, Fig. 17, with permission.) |
Anterior Apical Dissection in the Male Patient
Once the cystectomy specimen is completely free and mobile posteriorly, attention may be directed to the anterior dissection. The opened endopelvic fascia lateral to the prostate is extended both anteriorly and posteriorly. The levator muscles are carefully swept off the lateral and apical portions of the prostate. Care should be taken in avoiding any extensive dissection in the region anterior to the urethra along the pelvic floor. Incision of the puboprostatic ligaments is limited to the portion that is directly attached to the apex of the prostate and should not be extended to the portion connected to the proximal urethra.
The dorsal venous complex is divided just distal to the prostatic apex and controlled with suture that reapposes the cut edges of the complex only. Excessive incorporation of levator muscle in this suture may inhibit proper function of the rhabdosphincter. The urethra is then incised 270° just beyond the apex of the prostate, and a series of 2-0 Monocryl sutures are placed in the anterior urethra, carefully incorporating only the mucosa and the submucosa of the striated urethral sphincter muscle anteriorly. Posteriorly, the rectourethralis muscle or the caudal extent of Denonvilliers’ fascia may be included in the urethral suture. After the posterior urethra is divided, the specimen is removed and a frozen section analysis of the distal urethral margin of the cystectomy specimen is performed to exclude tumor involvement.
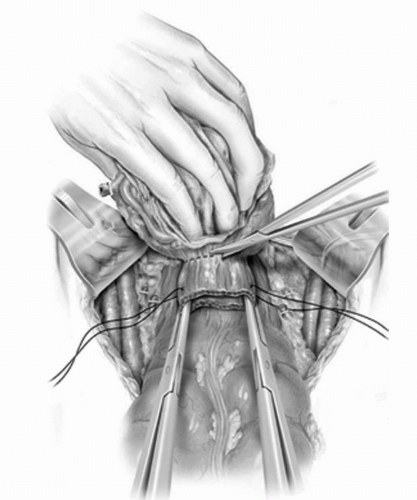 FIGURE 23A.15. The anterior vaginal wall is sharply dissected off the posterior bladder down to the vesicourethral junction. (Reproduced from Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int 2004;94(1):197-221, Fig. 18, with permission.) |
If a cutaneous form of urinary diversion is planned, the urethra is stretched above the urogenital diaphragm, and divided at the level of the pelvic floor to excise all visible pelvic portions of the urethra.
Nerve-Sparing Approach in the Male
When properly performed, a standard radical cystoprostatectomy should render men impotent in virtually all cases. Several modifications in the technique of radical cystectomy may allow preservation of the neurovascular bundles and hence spontaneous erectile function. Based on the neuroanatomic studies of Walsh and Donker (43), the relationship of the pelvic nerve plexus to the seminal vesicles and bladder was defined. In 1987, Schlegel and Walsh (44) modified the standard radical cystoprostatectomy to preserve the autonomic innervations arising from the pelvic plexus. Using the seminal vesicles as an intraoperative landmark, the pelvic plexus, which is located lateral and posterior to the seminal vesicles, may be spared during the ligation of the posterior pedicle.
Patient selection is absolutely critical when considering a nerve-sparing radical cystoprostatectomy (45). Patients who are impotent preoperatively and those in whom tumor involvement of the posterior pedicle of the bladder is suspected at the time of cystectomy are not candidates for a nerve-sparing approach. When performed correctly and applied to appropriately selected patients, oncologic efficacy does not appear to be compromised and erections adequate for intercourse postoperatively are achieved in approximately 45% of patients (46).
Posterior/Anterior Dissection in the Female
If anterior vaginal wall preservation is planned, the posterior vagina is incised at the apex just distal to the cervix (Fig. 23A.14). This incision is carried anteriorly along the lateral and anterior vaginal wall forming a circumferential incision. The anterior-lateral vaginal wall is then grasped with curved Kocher clamps to provide countertraction that facilitates the dissection between the anterior vaginal wall and the posterior bladder (Fig. 23A.15). Development of the posterior plane and vascular pedicle is best performed sharply and carried just distal to the vesicourethral junction which can be identified by palpation of the Foley catheter balloon (Fig. 23A.16).
The anterior vaginal wall should be removed en bloc with the cystectomy specimen when posterior invasive tumors are present to ensure that a complete resection is achieved. If the anterior wall is excised a 1-cm length of proximal urethra is mobilized while the remaining distal urethra is left intact with the anterior vaginal wall. Vaginal reconstruction may be accomplished by folding the posterior wall over or by closing the lateral walls side to side. Formal techniques for complete reconstruction of the vagina have been extensively described and can be fashioned from myocutaneous flaps (gracilus or rectus muscles), tabularized or detubularized bowel segments, or a peritoneal or an omental flap.
If an orthotopic reconstruction is planned in a female, it is critical that no dissection be performed anterior to the urethra along the pelvic floor. The endopelvic fascia should remain undisturbed to prevent injury to the innervation of the rhabdosphincter, which is critical in maintaining continence. Branches of the pudendal nerve course along the pelvic floor posterior to the levator muscles in women and supply the rhabdoid sphinteric complex (47,48,49). It is important to avoid
any dissection anterior to the urethra as this may injure the pudendal nerve branches and adversely affect continence.
any dissection anterior to the urethra as this may injure the pudendal nerve branches and adversely affect continence.
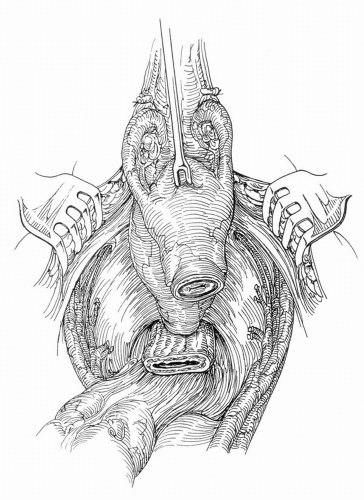 FIGURE 23A.16. Female cystectomy specimen attached at the vesicourethral junction. (Reproduced from Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int 2004;94(1):197-221, Fig. 14, with permission.) |
Several anatomic factors in the female facilitate urethral suture placement including a more anteriorly positioned urethra and a wider pelvic inlet (Fig. 23A.17). An adequate number of sutures, usually totaling 8 to 12, should be used within the urethra depending on its size. Frozen section analysis is performed on the distal urethral margin of the cystectomy specimen to exclude tumor. Once the vaginal cuff is closed it is then anchored via a colposacralpexy using a strut of nonabsorbable material (Marlex, Prolene, or Goretex mesh) to the sacral promontory. Fixation of the vagina is performed with care to avoid angulation or undue tension. The peritoneal edge of the sigmoid mesentery can be used to cover the mesh anteriorly. A well-vascularized omental pedicle graft based on the left gastroepiploic arcade is placed between the reconstructed vagina and the neobladder prior to completion of the urethralneobladder anastomosis to separate the suture lines and prevent fistulization (Fig. 23A.18).
If a cutaneous diversion is planned in the female patient, the entire urethra is resected from either an abdominal or a vaginal approach.
POSTOPERATIVE CARE
A detailed, team-oriented approach to the care of these generally elderly patients undergoing radical cystectomy helps reduce perioperative morbidity and mortality. Routine established clinical care pathways have been developed to facilitate discharge planning (50). Routine monitoring in the surgical intensive care unit may not be necessary depending on the institutional resources and patient’s comorbidities (51). Careful attention to fluid management is imperative as third-space fluid loss in these patients can be significant. Patients with compromised cardiac or pulmonary function may require invasive central venous pressure or cardiac output monitoring. Prophylaxis against stress ulcer is initiated with an H2-blocker. Intravenous broad-spectrum antibiotics are continued for at least 24 hours in all patients. Aggressive pulmonary toilet is encouraged with incentive spirometry and early ambulation.
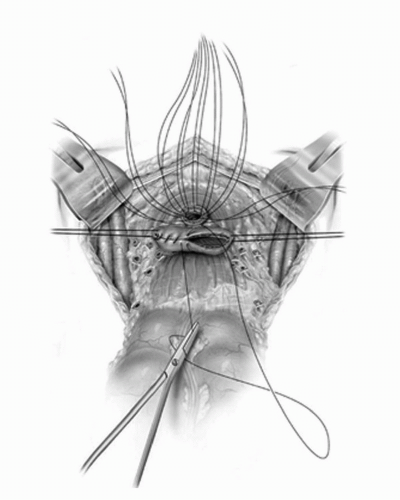 FIGURE 23A.17. Urethral sutures are placed and the vaginal cuff is closed in two layers. (Reproduced from Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int 2004;94(1):197-221, Fig. 19, with permission.) |
Prophylaxis against deep vein thrombosis is important in all patients undergoing extensive pelvic dissection for malignancy. Various anticoagulation protocols have been proposed for prophylaxis, including subcutaneous heparin or lowmolecular-weight heparnoids, oral warfarin, and mechanical intermittent compression devices (52). Pain control by either epidural anesthesia or a patient-controlled systemic analgesic system provides comfort and enhances deep breathing and early ambulation. Limitation of postoperative narcotics and use of nonsteroidal anti-inflammatory analgesics (such as ketorolac) may help facilitate return of bowel motility. The catheter and drain management is specific to the form of urinary diversion. Postoperative care pathways have been developed that have been shown to decrease hospital length of stay and standardize care (50,53).
OUTCOMES OF RADICAL CYSTECTOMY
The oncologic goals of radical cystectomy for bladder cancer are to provide complete local and regional disease control, minimize the risk of distant recurrence, and optimize patient survival. The surgical approach, and indeed the overall
management strategy to invasive bladder cancer, has evolved as a direct result of an appreciation for the aggressive nature of the disease process. The incorporation of perioperative systemic chemotherapy has emerged as an important addition to the management of invasive bladder cancer (21,54,55,56,57) and is discussed in detail in another chapter. The early results of radical cystectomy were disappointing primarily due to the unacceptably high perioperative morbidity related to the urinary diversion. Perioperative mortality rates have decreased to 2% to 3% in most contemporary surgical series (58,59,60,61). Neoadjuvant therapy and form of urinary diversion do not appear to alter the mortality rate of radical cystectomy. Routine use of neoadjuvant radiation therapy has shown no added benefit and may be considered only in those patients who have had a previous partial cystectomy or an extravesical tumor spill at the time of endoscopic resection of the primary bladder tumor (62).
management strategy to invasive bladder cancer, has evolved as a direct result of an appreciation for the aggressive nature of the disease process. The incorporation of perioperative systemic chemotherapy has emerged as an important addition to the management of invasive bladder cancer (21,54,55,56,57) and is discussed in detail in another chapter. The early results of radical cystectomy were disappointing primarily due to the unacceptably high perioperative morbidity related to the urinary diversion. Perioperative mortality rates have decreased to 2% to 3% in most contemporary surgical series (58,59,60,61). Neoadjuvant therapy and form of urinary diversion do not appear to alter the mortality rate of radical cystectomy. Routine use of neoadjuvant radiation therapy has shown no added benefit and may be considered only in those patients who have had a previous partial cystectomy or an extravesical tumor spill at the time of endoscopic resection of the primary bladder tumor (62).
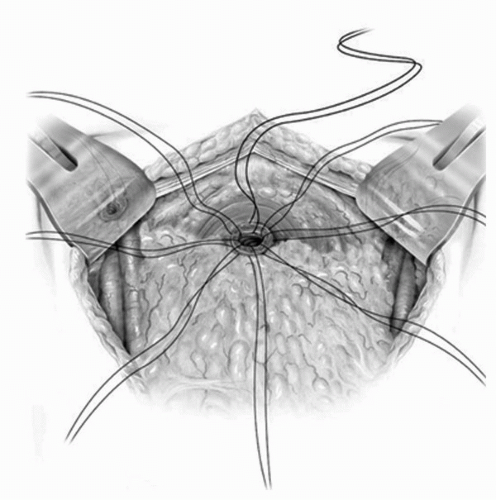 FIGURE 23A.18. A well-vascularized omental pedicle graft based on the left gastroepiploic arcade is placed between the vagina and the neobladder to prevent fistulization. (Reproduced from Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int 2004;94(1):197-221, Fig. 20, with permission.) |
Outcomes for Organ-Confined Bladder Cancer
The pathologic stage of the primary bladder tumor and the presence of lymph node metastases are perhaps the most important survival determinants in patients undergoing cystectomy for bladder cancer (17). Histopathologic assessment allows for risk stratification and provides important prognostic information that may direct subsequent treatment. The rate of organ-confined disease varies from 17% to 57% (Table 23A.1) (16,17,18,63,64,65). Organ-confined lesions include pTis, pTa, pT1, and pT2 lesions. Overall, the recurrence-free survival in this pathologic subgroup of organ-confined, lymph node-negative bladder tumors is as high as 85% at 5 years and 82% at 10 years (17). Noninvasive lesions, as expected, have a better recurrence-free survival compared to invasive disease (pT1-2) (26,66,67,68,69). There is evidence to suggest delays in treatment result in more advanced disease and poorer overall survival in patients with muscle-invasive bladder cancer (70,71,72,73). Furthermore, caution should be exercised when delaying definitive surgical therapy for patients with high-risk non-muscle-invasive tumors that do not readily respond to intravesical treatments (2,4).
For those with muscle-invasive node-negative disease, the prognostic significance of pathologic substaging based on the depth of invasion into the muscle layer remains controversial, with some reporting no difference in clinical outcomes between superficial (pT2a) and deep (pT2b) invasion into the muscularis propria (74,75,76) and others showing some prognostic stratification (77).
Outcomes for Locally Advanced Bladder Cancer
Non-organ-confined (extravesical), lymph node-negative tumors are reported in approximately 18% to 65% of patients undergoing cystectomy (Table 23A.1) (16,17,18,63,64,65). These include pT3a, pT3b, pT4a, and pT4b N0 lesions. There does not appear to be any difference in survival between those with microscopic and gross extravesical extension (pT3a vs. pT3b) (78,79,80). In an analysis of 129 patients with pathologic T3 disease managed with cystectomy alone, the 10-year recurrence-free survival was 54%. Lymph node metastasis was observed in 43 (33%) of the 129 patients and was associated with a lower 10-year recurrence-free survival rate (32%) than was lymph node-negative status in patients with extravesical extension (60%). The majority of recurrences occurred at a distant site, highlighting the excellent local control provided by radical cystectomy even in patients with locally extensive extravesical disease (78).
Direct tumor extension into contiguous organs such as the prostate and rectum in men and the vagina in women (pT4) portends a worse prognosis. In a large series, 5- and 10-year recurrence-free survival rates for surgically treated patients with pT4 disease were 50% and 45%, respectively (17). Many of these patients may benefit from neoadjuvant chemotherapy prior to radical cystectomy. Despite the overall poor prognosis for this group, a subset of patients with locally advanced disease
continues to benefit from radical cystectomy, and certain pathologic findings may permit prognostic stratification. Current staging designates pT4a disease as contiguous invasion into the prostate. Other forms of prostate involvement including CIS of the urethra or ducts and lamina propria invasion of the periurethral tissues may have an improved prognosis compared to invasive lesions into the prostatic stroma (81,82). Overall survival at 5 years for patients with carcinoma in situ of the prostatic urethra or ductal tumors is 71%, but only 36% for those with prostatic stromal invasion (83). Similarly, Pagano et al. (84) demonstrated that the 5-year overall survival for patients whose tumors invaded the prostate through the urethra was 46% compared with 7% for those whose tumor extended through the bladder wall to involve the prostate. Additionally, prostatic involvement has been noted to be an independent risk factor for subsequent urethral recurrences (85,86).
continues to benefit from radical cystectomy, and certain pathologic findings may permit prognostic stratification. Current staging designates pT4a disease as contiguous invasion into the prostate. Other forms of prostate involvement including CIS of the urethra or ducts and lamina propria invasion of the periurethral tissues may have an improved prognosis compared to invasive lesions into the prostatic stroma (81,82). Overall survival at 5 years for patients with carcinoma in situ of the prostatic urethra or ductal tumors is 71%, but only 36% for those with prostatic stromal invasion (83). Similarly, Pagano et al. (84) demonstrated that the 5-year overall survival for patients whose tumors invaded the prostate through the urethra was 46% compared with 7% for those whose tumor extended through the bladder wall to involve the prostate. Additionally, prostatic involvement has been noted to be an independent risk factor for subsequent urethral recurrences (85,86).
TABLE 23A.1 INCIDENCE OF ORGAN-CONFINED, EXTRAVESICAL, AND LYMPH NODE-POSITIVE DISEASE FOLLOWING RADICAL CYSTECTOMY | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Local Recurrences Following Radical Cystectomy
Radical cystectomy provides the best local (pelvic) control of the disease. An overall local pelvic recurrence rate of 7% was observed in the USC cystectomy series (17). Patients with organ-confined, lymph node-negative tumors demonstrated only a 6% local recurrence rate, compared to a 13% local recurrence rate in those with non-organ-confined, lymph node-negative tumors. Even those at highest risk of a local recurrence (lymph node-positive disease) had only a 13% local recurrence rate following cystectomy. Nearly all patients suffering a pelvic recurrence following cystectomy will die of their disease despite additional therapeutic efforts.
LYMPH NODE-POSITIVE BLADDER CANCER AND THE ROLE OF THE PELVIC LYMPHADENECTOMY
The routine use of an extended PLND in bladder cancer is a debated topic despite reports to suggest improved clinical outcomes. Although there exists an abundance of retrospective reviews that have correlated the extent of lymphadenectomy with patient survival, there are currently no prospective, randomized trials to assess this issue in regards to cancer-specific and overall survival.
Lymphatic Drainage of the Bladder—Anatomic and Historical Considerations
Anatomic studies performed around the turn of the 19th century, both in Europe and the United States, provided detailed descriptions of the lymphatic drainage of the urinary bladder. The classic descriptions of the lymphatic pathways within the bladder include a rich subepithelial lymphatic plexus that provides drainage through the detrusor musculature to the exterior of the bladder. Efferent lymphatic channels drain into perivesical lymph nodes that lie on the anterior, posterior, and lateral aspects of the bladder and the perivesical tissues. Larger lymphatic channels then pass to nodal basins located along the external iliac vessels, hypogastric vessels, and lateral sacral/sacral promontory. These basins form a ring of pelvic nodes and constitute the primary drainage sites. All primary landing sites subsequently drain into the more proximal common iliac node chain and then on to the lower retroperitoneal nodal groups.
Following the identification of the primary and secondary lymphatic drainage basins of the bladder within the pelvis, it remained to be determined whether a therapeutic advantage could be gained through their excision at the time of cystectomy. Experience with a similar radical surgical approach for cervical cancer, in which a therapeutic pelvic lymphadenectomy was combined with a total exenterative procedure, not only demonstrated that it could safely be executed but that it also potentially improved outcome (87). Early experience with radical cystectomy and regional lymphadenectomy for patients with nodal metastases suggested that despite a more aggressive surgical approach, most node-positive patients experienced an exceedingly poor long-term outcome. In 1956, Whitmore and Marshall (88) published their experience of 100 consecutive bladder cancer patients treated with radical cystectomy and a regional pelvic lymphadenectomy. In 32 patients with node-positive disease, only 22% were reported alive at 1 year. Three patients (9%), the majority of whom developed metastatic disease, survived 2 years and only one (3%) was alive at 3 years. While these data represented an overall improvement in outcome in node-positive patients, the poor rate of survival among patients with regionally advanced disease led many to question whether radical cystectomy was indicated in the presence of positive regional nodes.
Supported by reports of success with the Wertheim technique for cervical cancer, Leadbetter and Cooper (10) proposed inclusion of a thorough PLND that included the hypogastric, external iliac, presacral, and common iliac lymphatics at cystectomy. Subsequently Skinner (8) championed the benefits of a thorough lymph node dissection at the time of cystectomy and presented evidence supporting its therapeutic benefits. Contemporary experience with patients whose bladder cancer has involved the regional pelvic lymph nodes establishes a significantly more favorable outcome following radical cystectomy and pelvic lymphadenectomy compared with early published experiences. Current reports note that approximately 25% to 33% of all patients with invasive bladder cancer involving the regional lymph nodes will be rendered disease free following radical cystectomy and a thorough PLND (16,17,63,64,89,90). Stein et al. (91) reported the 5- and 10-year recurrence-free
survival to be 35% and 34%, respectively, for a series of 246 patients with node-positive bladder cancer following radical cystectomy and an extended pelvic lymphadenectomy that included all nodes from the aortic bifurcation distally to the inguinal ligament. The Memorial Sloan-Kettering experience with 193 contemporary node-positive bladder cancer patients that received radical cystectomy and a more limited PLND (proximal limit at the bifurcation of the common iliac vessels) reported a 31% disease-specific survival and 25% overall survival at 5 years (90). Ghoneim et al. found 188 node-positive patients out of 1,026 (18%) patients with bladder cancer. A 23.4% 5-year survival was reported in the 188 node-positive patients who received a lymphadenectomy at cystectomy that included the nodes up to the distal common iliac vessels (16). Mills et al. (89) reported a 29% overall survival in 83 node-positive patients who underwent cystectomy and a pelvic lymphadenectomy that included nodes from the bifurcation of the common iliac distally. Other groups have similarly documented that overall approximately one quarter to one third of all patients with regionally metastatic bladder cancer can be expected to survive 5 years following radical cystectomy and pelvic lymphadenectomy (63).
survival to be 35% and 34%, respectively, for a series of 246 patients with node-positive bladder cancer following radical cystectomy and an extended pelvic lymphadenectomy that included all nodes from the aortic bifurcation distally to the inguinal ligament. The Memorial Sloan-Kettering experience with 193 contemporary node-positive bladder cancer patients that received radical cystectomy and a more limited PLND (proximal limit at the bifurcation of the common iliac vessels) reported a 31% disease-specific survival and 25% overall survival at 5 years (90). Ghoneim et al. found 188 node-positive patients out of 1,026 (18%) patients with bladder cancer. A 23.4% 5-year survival was reported in the 188 node-positive patients who received a lymphadenectomy at cystectomy that included the nodes up to the distal common iliac vessels (16). Mills et al. (89) reported a 29% overall survival in 83 node-positive patients who underwent cystectomy and a pelvic lymphadenectomy that included nodes from the bifurcation of the common iliac distally. Other groups have similarly documented that overall approximately one quarter to one third of all patients with regionally metastatic bladder cancer can be expected to survive 5 years following radical cystectomy and pelvic lymphadenectomy (63).
Subgroups of patients with regionally metastatic disease may actually demonstrate a significantly better prognosis and can be identified by having a lower volume of disease both at the level of the primary tumor and the regional lymph nodes. The survival for node-positive patients whose primary tumors are confined to the bladder is significantly better than that observed in node-positive patients with more invasive primary tumors. Vieweg et al. (64) reported a 51% 5-year overall survival in 44 P0-P3a, LN+ patients compared to a 17% 5-year survival in 149 patients with P3b-P4, N+ disease. The USC group reported a 46% versus 30% 5-year recurrence-free survival in node-positive patients with organ-confined versus non-organ-confined primary tumors, respectively (91). A similar stratification of outcome can be observed when considering the volume of disease at the level of the regional lymph nodes. If the number of positive nodes or size of the involved node(s) is considered as a measure of the extent of tumor involvement of the regional nodes, patients with fewer involved nodes or smaller tumor-bearing nodes have been found to have an improved outcome. Vieweg et al. reported the 5-year disease-specific survival of 193 node-positive patients that underwent cystectomy based on TNM node staging (1987 system) as 44%, 27%, and 0% for N1, N2, and N3 patients, respectively. The median survival for these three groups was 3.1, 1.9, and 0.9 years, respectively (p = 0.0006). The number of involved lymph nodes has been reported as an independent prognostic indicator following radical cystectomy (90). Lerner et al. (92) noted that patients with fewer than six involved lymph nodes exhibited a significantly improved 5-year survival compared to patients with greater than six positive nodes. An update of this series found that eight or fewer nodes involved was an optimized cutoff in that patients with eight or fewer involved nodes (n = 193) demonstrated a 41% 5-year recurrence-free survival and 37% overall 5-year survival compared with 10% and 4%, respectively, in patients with greater than eight involved nodes (n = 51, p < 0.001) (91). Other series have confirmed similar differences in outcome, as noted by Mills et al. (89) in which patients with fewer than five involved lymph nodes did significantly better compared with those with more involved nodes (5-year survival of approximately 50% vs. 10%).
Additional measurements of the burden of disease, such as the size of the involved nodes and the presence of extracapsular lymph node extension by tumor within the regional lymph nodes, may also prove to be prognostically important. Mills et al. reported that patients with involved nodes >0.5 cm or the presence of extracapsular extension within the involved nodes demonstrated a lower survival. Multivariate analysis of the relative importance of the number of positive nodes, size of involved nodes, or the presence of extracapsular lymph node perforation demonstrated that only the presence of extracapsular perforation remained independently predictive of outcome, with a hazard ratio of 2.6 (89).
While the number of lymph nodes involved with disease contains important prognostic information, simultaneous consideration of the extent of the lymphadenectomy performed appears to enhance the prognostic significance of such data. For example, consider two patients, both with four positive lymph nodes identified after cystectomy. Patient one had a total of 10 lymph nodes evaluated pathologically, while patient two had 40 lymph nodes analyzed. Should both patients be considered similarly staged? Would both patients have a similar anticipated outcome? Data from both University of Southern California and Memorial Sloan-Kettering Cancer Center (MSKCC) would suggest that evaluation of the number of involved nodes in the context of the total number of lymph nodes removed provides a more accurate means to identify higher risk node-positive patients (91,93). Using information obtained from the ratio of positive lymph nodes to the total number of lymph nodes evaluated (density of positive lymph nodes), both institutions have independently demonstrated an improved stratification of node-positive patients into differing risk groups. Using 20% as a cutoff for the percentage of involved lymph nodes, a 44% versus 17% 5-year recurrence-free survival was observed for patients with less than compared with >20% of their total nodes involved with disease, respectively. An even greater difference in outcome was reported using a ratio-based analysis in a series of 162 node-positive patients who had undergone cystectomy and lymphadenectomy. The 5-year disease-specific survival of patients with <20% of total nodes involved was approximately 65% compared with 5% for patients with >20% of evaluated lymph nodes involved with tumor. Positive-node ratio provided improved prognostic information compared to 1997 TNM nodal staging criteria or total number of involved lymph nodes.
Importance of Extent of Lymphadenectomy
Given the importance in staging information provided by the lymphadenectomy, the question of the extent of the dissection required for adequate staging or therapeutic value remained to be clarified. An established set of necessary anatomic boundaries and extent (number of lymph nodes) of the lymph node dissection that would provide optimal staging and therapeutic efficacy for bladder cancer is presently not available. A lack of prospectively validated studies has led to ongoing controversy regarding the necessary extent of dissection (94).
Wishnow et al. (95) attempted to determine the rate of involvement of the pelvic lymph nodes within the common iliac chain or more distally in the obturator, hypogastric, and external iliac nodes in bladder cancer patients undergoing radical cystectomy (95). In a series of 130 patients with grossly negative lymph nodes at the time of cystectomy, 88% of whom had common iliac nodes resected, 14% of patients were identified with microscopically involved nodes. Seventeen of the eighteen patients with microscopically involved lymph nodes had one or two positive nodes. None of the 17 patients with one or two microscopically involved nodes had common iliac or lateral external iliac lymph nodes involved. Based on these findings, the authors recommended limiting the proximal limit of the lymph node dissection to the bifurcation of the common iliac vessels for patients with no evidence of grossly positive lymph nodes.
More recently at MSKCC, a series of 144 bladder cancer patients undergoing radical cystectomy were prospectively evaluated to determine the site of regional lymph node involvement. Eighteen patients (14%) were found to have
microscopically involved regional lymph nodes including four with disease involving the common iliac nodes. In this series all but one patient with common iliac nodes had simultaneous involvement of a more distal lymph node region (hypogastric, obturator, or external iliac) suggesting that excision of the common iliac nodes would benefit a subset of microscopically LN-positive patients.
microscopically involved regional lymph nodes including four with disease involving the common iliac nodes. In this series all but one patient with common iliac nodes had simultaneous involvement of a more distal lymph node region (hypogastric, obturator, or external iliac) suggesting that excision of the common iliac nodes would benefit a subset of microscopically LN-positive patients.
Additional information on the distribution of positive pelvic nodes is provided by a multicenter, prospective trial in which all patients underwent an extended PLND (proximal limits of dissection at or above the bifurcation of the aorta) (96). Of the 290 patients evaluated in this series, 81 (27.9%) demonstrated evidence of tumor involvement in 599 pelvic nodes. Involved nodes above the bifurcation of the common iliac vessels comprised 35% of all positive nodes. A total of 20 patients (6.9%) demonstrated involvement of the common iliac nodes without evidence of disease within the more distal nodal regions (obturator, hypogastric, or external iliac). While exact information on the nature of the nodes (gross or microscopically enlarged) was not available, in the 29 patients with only a single lymph node metastasis, 10% were located above the bifurcation of the common iliac vessels providing further strong support for the need to extend the dissection to minimally include the common iliac chain.
Outcomes Based on Extent of Dissection
Data describing the relationship between the extent of the node dissection and outcome are provided by single-institution, retrospective reviews. Poulsen and Krarup (97) reported a comparative analysis of two consecutive series of bladder cancer patients undergoing either a limited (proximal limit at the bifurcation of the common iliac vessels) or an extended (including the nodes up to the level of the bifurcation of the aorta) lymphadenectomy performed at the time of radical cystectomy. Previously untreated bladder cancer patients were included in the study in which 126 underwent an extended (between 1993 and 1997) and 68 received a limited (between 1990 and 1993) LN dissection. The two groups were well matched demographically, with a slightly higher percentage of extravesical tumors in the extended lymphadenectomy group. As anticipated, the extended node dissection yielded a greater number of lymph nodes compared to the more limited excision (25 vs. 14, p < 0.001). Both groups had a similar proportion of node-positive patients, 27% in the extended and 24% in the limited LN groups. Despite the increased number of more advanced tumors in the extended dissection group, a similar 5-year recurrence-free survival, risk of local recurrence, and risk of distant metastasis was observed. In the subgroup of patients with organ-confined primary tumors, however, patients who underwent an extended dissection benefited with an improved 5-year recurrence-free survival (85% vs. 64%, p < 0.02).
Leissner et al. presented their analysis of 302 patients who received an extended lymph node dissection at the time of radical cystectomy for bladder cancer. They noted that patients with a greater number of lymph nodes reported in their pathology report had an improved disease-free and overall survival as well as improved local tumor control. At 5 years, 51% of patients with ≤15 lymph nodes removed were alive and disease free compared to 65% for those with ≥16 lymph nodes evaluated. The improvement in local control was also significant, with pelvic recurrences identified in 27% compared to 17% of patients with ≤15 and ≥16 lymph nodes evaluated, respectively (p < 0.01) (19).
Herr et al. reported a similar improved outcome in 322 patients, in which those that underwent a more extensive node dissection, as represented by a greater number of lymph nodes reported, exhibited an improved overall survival. For patients with node-positive disease, approximately 50% versus 20% of patients with greater or less than 11 lymph nodes removed were alive at 5 years, respectively (98). Additional supportive data obtained from a national cohort of patients are provided by a multivariate analysis of Surveillance, Epidemiology, and End Results (SEER) registry data on 1,923 radical cystectomy patients. This retrospective study found that the number of lymph nodes examined was positively associated with an improved survival, particularly in patients with higher stage disease. Patients with at least four lymph nodes evaluated demonstrated an improved outcome. However, patients with 10 to 14 nodes reported exhibited the greatest improvement in overall survival (99).
While suggestive of a therapeutic role, these data are retrospective in nature and do not include factors that may bias surgical selection in their analysis. Koppie et al. performed a sensitivity analysis that sought to take into consideration important factors that may have both biased selection for a particular extent of dissection and effected outcome (i.e., survival). Their cohort included 1,121 radical cystectomy patients in which age and comorbidity was considered when evaluating the potential therapeutic importance of the PLND. Their data demonstrated that older sicker patients were more likely to undergo a less aggressive PLND compared to younger, healthier patients. However despite correcting for age, comorbidity, tumor stage and other factors, they still observed an improved survival in patients undergoing a more extensive PLND (100).
While no prospective randomized data are currently available comparing a limited to more extended PLND, comparative data from different institutions performing either limited or more extensive dissections do exist. Dhar et al. reported a 13-year comparative study evaluating disease-specific outcomes in 336 patients who underwent radical cystectomy with a limited PLND to 322 patients that underwent a more extensive PLND at RC. Patients who underwent a more extensive PLND were more likely to be LN positive (improved staging) and demonstrated improved recurrence-free survival. Five-year disease-free survival for lymph node-positive patients undergoing an extended PLND was 35% versus only 7% in patients that received a limited PLND (101).
The ability to limit the dissection to the ipsilateral side of the pelvis in patients with tumors located on one side of the bladder has also been proposed. It is apparent, however, that despite a clear laterality in the location of the primary bladder tumor, contralateral nodal involvement will frequently be identified. Data from Leissner et al. (96) found that of 32 node-positive patients with primary tumors located specifically to one side of the bladder, the risk of contralateral lymph node involvement was only slightly less than that found for the ipsilateral nodes. Sentinal node studies have also confirmed that the initial node region involved with disease may be located in the contralateral side of the primary tumor (102).
SURGICAL STANDARDS FOR BLADDER CANCER AND CURRENT PRACTICE
The establishment of the minimum number of lymph nodes needed for adequate staging, prognosis, or improved outcome would provide a surgical standard that could be widely applied. Much work to establish such standards for the surgical management of colorectal, breast, and gastric cancers has been completed (103,104,105). To date no such standard has been established for the lymphadenectomy for bladder cancer. Major difficulties in establishing such a standard include the wide variation in reported node yields following either limited or extended dissections and the lack of prospective studies
validating any such “standard.” The variable extent of surgical dissection and the differing techniques used for pathologic review contribute to the differences in reported median node number as well as individual anatomic variation in the number of nodes present, surgeon, patient age, and pretreatment. While increasing data support that surgeon experience is related to outcome following major surgical procedures (106), Leissner et al. (19) demonstrated that node yield following radical cystectomy was not related to surgical experience. In this series, some surgeons with the highest surgical volume reported the lowest node yields. Recent data from MSKCC evaluating the factors associated with node yield variability in a series of 144 consecutive radical cystectomies demonstrated that only the extent of the dissection was associated with overall node yield within a group of four experienced surgeons. Patient age, neoadjuvant systemic chemotherapy, the time from TUR, or prior use of Bacillus Calmette-Guerin use did not exhibit a statistically significant association with node count (107). In contrast, others have found that increasing patient age is associated with lower node yields after an extended dissection (96). The way in which the nodes are submitted to pathology also affects the number of nodes reported. By sending separate nodal packets from the different anatomic node regions as opposed to an en bloc submission with the main specimen, Bochner et al. (108) reported a 3.5-fold increase in the number of reported nodes for a standard dissection and a 1.6-fold increase in node yield for an extended dissection (108). Similar increases in node counts have been reported by others as well (109).
validating any such “standard.” The variable extent of surgical dissection and the differing techniques used for pathologic review contribute to the differences in reported median node number as well as individual anatomic variation in the number of nodes present, surgeon, patient age, and pretreatment. While increasing data support that surgeon experience is related to outcome following major surgical procedures (106), Leissner et al. (19) demonstrated that node yield following radical cystectomy was not related to surgical experience. In this series, some surgeons with the highest surgical volume reported the lowest node yields. Recent data from MSKCC evaluating the factors associated with node yield variability in a series of 144 consecutive radical cystectomies demonstrated that only the extent of the dissection was associated with overall node yield within a group of four experienced surgeons. Patient age, neoadjuvant systemic chemotherapy, the time from TUR, or prior use of Bacillus Calmette-Guerin use did not exhibit a statistically significant association with node count (107). In contrast, others have found that increasing patient age is associated with lower node yields after an extended dissection (96). The way in which the nodes are submitted to pathology also affects the number of nodes reported. By sending separate nodal packets from the different anatomic node regions as opposed to an en bloc submission with the main specimen, Bochner et al. (108) reported a 3.5-fold increase in the number of reported nodes for a standard dissection and a 1.6-fold increase in node yield for an extended dissection (108). Similar increases in node counts have been reported by others as well (109).
Despite evidence supporting the importance of the lymphadenectomy for both improved prognosis and possible therapeutic benefit, national data on surgical practice suggest that many surgeons limit or avoid the node dissection at cystectomy. SEER registry data that included 1,923 patients who underwent cystectomy in the United States between 1988 and 1996 indicated that 53% of reported cystectomies had three or fewer lymph nodes reviewed in the final pathologic report (99). Improving trends however have been reported suggesting that there is increasing use of more extensive PLND at the time of radical cystectomy.
CONCLUSION
Anatomic and clinical experience with invasive bladder cancer has established the natural pathways of disease progression. Decades of experience with radical surgery for the management of muscle-invasive bladder cancer clearly emphasizes the role that surgical quality may play in patient outcome. Future advances in establishing surgical standards for the treatment of bladder cancer will require well-controlled prospective trials that directly compare varying extents of surgery with their ability to provide local and distant disease control as well as disease-specific survival. This will set the stage for clear benchmarks that can then be broadly applied to clinical practice.
Acknowledgment
This chapter is dedicated to the memory of John Stein, MD, for his outstanding contributions in the field of bladder cancer.
References
1. Prout GR, Marshall VF. The prognosis with untreated bladder tumors. Cancer 1956;9(3):551-558.
2. Stein JP. Indications for early cystectomy. Urology 2003;62(4):591-595.
3. Chang SS, Cookson MS. Radical cystectomy for bladder cancer: the case for early intervention. Urol Clin North Am 2005;32(2):147-155.
4. Montgomery JS, Weizer AZ, Montie JE. T1 bladder cancer: advocating early cystectomy to improve oncologic control. Urol Oncol 2010;28(5):466-468.
5. Herr HW, Donat SM. Outcome of patients with grossly node positive bladder cancer after pelvic lymph node dissection and radical cystectomy. J Urol 2001;165(1):62-64; discussion 64.
6. Sweeney P, Millikan R, Donat M, et al. Is there a therapeutic role for post-chemotherapy retroperitoneal lymph node dissection in metastatic transitional cell carcinoma of the bladder? J Urol 2003;169(6):2113-2117.
7. Dodd PM, McCaffrey JA, Herr H, et al. Outcome of postchemotherapy surgery after treatment with methotrexate, vinblastine, doxorubicin, and cisplatin in patients with unresectable or metastatic transitional cell carcinoma. J Clin Oncol 1999;17(8):2546-2552.
8. Skinner DG. Management of invasive bladder cancer: a meticulous pelvic node dissection can make a difference. J Urol 1982;128(1):34-36.
9. Herr HW. Surgical factors in bladder cancer: more (nodes) + more (pathology) = less (mortality). BJU Int 2003;92(3):187-188.
10. Leadbetter WF, Cooper JF. Regional gland dissection for carcinoma of the bladder: a technique for one-stage cystectomy, gland dissection, and bilateral uretero-enterostomy. J Urol 1950;63(2):242-260.
11. Orr LM, Carson RB, Novak WF. A statistical study of present-day methods used in the treatment of tumors of the bladder. J Urol 1939;42:778-799.
12. Cunningham JH. Tumors of the bladder. J Urol 1931;25:559.
13. Spooner AD. Metastasis in epithelioma of the urinary bladder. Trans Am Assoc Genitourin Surg 1934;27:81.
14. Colston JAC, Leadbetter WF. Infiltrating carcinoma of the bladder. J Urol 1936;36:669.
15. Jewett HJ, Strong GH. Infiltrating carcinoma of the bladder: relation of depth of penetration of the bladder wall to incidence of local extension and metastases. J Urol 1946;55:366-372.
16. Ghoneim MA, el-Mekresh MM, el-Baz MA, et al. Radical cystectomy for carcinoma of the bladder: critical evaluation of the results in 1,026 cases. J Urol 1997;158(2):393-399.
17. Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 2001;19(3):666-675.
18. Madersbacher S, Hochreiter W, Burkhard F, et al. Radical cystectomy for bladder cancer today—a homogeneous series without neoadjuvant therapy. J Clin Oncol 2003;21(4):690-696.
19. Leissner J, Hohenfellner R, Thuroff JW, et al. Lymphadenectomy in patients with transitional cell carcinoma of the urinary bladder; significance for staging and prognosis. BJU Int 2000;85(7):817-823.
20. Marshall VF, Whitmore WF Jr. Simple cystectomy for cancer of the urinary bladder; 100 consecutive cases; 2 years later. J Urol 1950;63(2):232-241.
21. Black PC, Brown GA, Grossman HB, et al. Neoadjuvant chemotherapy for bladder cancer. World J Urol 2006;24(5):531-542.
22. Lerner SP, Shen S. Pathologic assessment and clinical significance of prostatic involvement by transitional cell carcinoma and prostate cancer. Urol Oncol 2008;26(5):481-485.
23. Kassouf W, Spiess PE, Brown GA, et al. Prostatic urethral biopsy has limited usefulness in counseling patients regarding final urethral margin status during orthotopic neobladder reconstruction. J Urol 2008;180(1):164-167; discussion 167.
24. Voges GE, Tauschke E, Stockle M, et al. Computerized tomography: an unreliable method for accurate staging of bladder tumors in patients who are candidates for radical cystectomy. J Urol 1989;142(4):972-974.
25. Pagano F, Bassi P, Galetti TP, et al. Results of contemporary radical cystectomy for invasive bladder cancer: a clinicopathological study with an emphasis on the inadequacy of the tumor, nodes and metastases classification. J Urol 1991;145(1): 45-50.
26. Amling CL, Thrasher JB, Frazier HA, et al. Radical cystectomy for stages Ta, Tis and T1 transitional cell carcinoma of the bladder. J Urol 1994;151(1):31-35; discussion 35-36.
27. Frazier HA, Robertson JE, Dodge RK, et al. The value of pathologic factors in predicting cancer-specific survival among patients treated with radical cystectomy for transitional cell carcinoma of the bladder and prostate. Cancer 1993;71(12):3993-4001.
28. Xu R, Zhao X, Zhong Z, et al. No advantage is gained by preoperative bowel preparation in radical cystectomy and ileal conduit: a randomized controlled trial of 86 patients. Int Urol Nephrol 2010;42(4);947-950.
29. Maffezzini M, Campodonico F, Canepa G, et al. Current perioperative management of radical cystectomy with intestinal urinary reconstruction for muscle-invasive bladder cancer and reduction of the incidence of postoperative ileus. Surg Oncol 2008;17(1):41-48.
30. Wren SM, Ahmed N, Jamal A, et al. Preoperative oral antibiotics in colorectal surgery increase the rate of Clostridium difficile colitis. Arch Surg 2005;140(8):752-756.
31. Nichols RL, Broido P, Condon RE, et al. Effect of preoperative neomycin-erythromycin intestinal preparation on the incidence of infectious complications following colon surgery. Ann Surg 1973;178(4):453-462.
32. Stein JP, Daneshmand S, Dunn M, et al. Continent right colon reservoir using a cutaneous appendicostomy. Urology 2004;63(3):577-580; discussion 580-571.
33. Donat SM. Argument against frozen section analysis of distal ureters in transitional cell bladder cancer. Nat Clin Pract Urol 2008;5(10):538-539.
34. Johnson DE, Wishnow KI, Tenney D. Are frozen-section examinations of ureteral margins required for all patients undergoing radical cystectomy for bladder cancer? Urology 1989;33(6):451-454.
35. Lee SE, Byun SS, Hong SK, et al. Significance of cancer involvement at the ureteral margin detected on routine frozen section analysis during radical cystectomy. Urol Int 2006;77(1):13-17.
36. Raj GV, Tal R, Vickers A, et al. Significance of intraoperative ureteral evaluation at radical cystectomy for urothelial cancer. Cancer 2006;107(9):2167-2172.
37. Schoenberg MP, Carter HB, Epstein JI. Ureteral frozen section analysis during cystectomy: a reassessment. J Urol 1996;155(4):1218-1220.
38. Schumacher MC, Scholz M, Weise ES, et al. Is there an indication for frozen section examination of the ureteral margins during cystectomy for transitional cell carcinoma of the bladder? J Urol 2006;176(6 Pt 1):2409-2413; discussion 2413.
39. Stein JP. Should frozen section examination of the ureteral margins be routinely performed during cystectomy? Nat Clin Pract Urol 2007;4(10):536-537.
40. Osman Y, El-Tabey N, Abdel-Latif M, et al. The value of frozen-section analysis of ureteric margins on surgical decision-making in patients undergoing radical cystectomy for bladder cancer. BJU Int 2007;99(1):81-84.
41. Tollefson MK, Blute ML, Farmer SA, et al. Significance of distal ureteral margin at radical cystectomy for urothelial carcinoma. J Urol 2010;183(1):81-86.
42. Crawford ED, Skinner DG. Salvage cystectomy after irradiation failure. J Urol 1980;123(1):32-34.
43. Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol 1982;128(3):492-497.
44. Schlegel PN, Walsh PC. Neuroanatomical approach to radical cystoprostatectomy with preservation of sexual function. J Urol 1987;138(6):1402-1406.
45. Kessler TM, Burkhard FC, Studer UE. Clinical indications and outcomes with nerve-sparing cystectomy in patients with bladder cancer. Urol Clin North Am 2005;32(2):165-175.
46. Schoenberg MP, Walsh PC, Breazeale DR, et al. Local recurrence and survival following nerve sparing radical cystoprostatectomy for bladder cancer: 10-year followup. J Urol 1996;155(2):490-494.
47. Colleselli K, Stenzl A, Eder R, et al. The female urethral sphincter: a morphological and topographical study. J Urol 1998;160(1):49-54.
48. Grossfeld GD, Stein JP, Bennett CJ, et al. Lower urinary tract reconstruction in the female using the Kock ileal reservoir with bilateral ureteroileal urethrostomy: update of continence results and fluorourodynamic findings. Urology 1996;48(3):383-388.
49. Bhatta Dhar N, Kessler TM, Mills RD, et al. Nerve-sparing radical cystectomy and orthotopic bladder replacement in female patients. Eur Urol 2007;52(4):1006-1014.
50. Chang SS, Cookson MS, Baumgartner RG, et al. Analysis of early complications after radical cystectomy: results of a collaborative care pathway. J Urol 2002;167(5):2012-2016.
51. Chang SS, Cookson MS, Hassan JM, et al. Routine postoperative intensive care monitoring is not necessary after radical cystectomy. J Urol 2002;167(3):1321-1324.
52. Forrest JB, Clemens JQ, Finamore P, et al. AUA Best Practice Statement for the prevention of deep vein thrombosis in patients undergoing urologic surgery. J Urol 2009;181(3):1170-1177.
53. Pruthi RS, Chun J, Richman M. Reducing time to oral diet and hospital discharge in patients undergoing radical cystectomy using a perioperative care plan. Urology 2003;62(4):661-665; discussion 665-666.
54. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349(9):859-866.
55. Calabro F, Sternberg CN. Neoadjuvant and adjuvant chemotherapy in muscle-invasive bladder cancer. Eur Urol 2009;55(2):348-358.
56. Donat SM. Integrating perioperative chemotherapy into the treatment of muscle-invasive bladder cancer: strategy versus reality. J Natl Compr Canc Netw 2009;7(1):40-47.
57. Raghavan D, Quinn D, Skinner DG, et al. Surgery and adjunctive chemotherapy for invasive bladder cancer. Surg Oncol 2002;11 (1-2):55-63.
58. Quek ML, Stein JP, Daneshmand S, et al. A critical analysis of perioperative mortality from radical cystectomy. J Urol 2006;175(3 Pt 1):886-889; discussion 889-890.
59. Isbarn H, Jeldres C, Zini L, et al. A population based assessment of perioperative mortality after cystectomy for bladder cancer. J Urol 2009;182(1):70-77.
60. Hollenbeck BK, Wei Y, Birkmeyer JD. Volume, process of care, and operative mortality for cystectomy for bladder cancer. Urology 2007;69(5):871-875.
61. Rosario DJ, Becker M, Anderson JB. The changing pattern of mortality and morbidity from radical cystectomy. BJU Int 2000;85(4):427-430.
62. Skinner DG, Lieskovsky G. Contemporary cystectomy with pelvic node dissection compared to preoperative radiation therapy plus cystectomy in management of invasive bladder cancer. J Urol 1984;131(6):1069-1072.
63. Bochner BH, Kattan MW, Vora KC. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol 2006;24(24):3967-3972.
64. Vieweg J, Gschwend JE, Herr HW, et al. The impact of primary stage on survival in patients with lymph node positive bladder cancer. J Urol 1999;161(1):72-76.
65. Poulsen AL, Horn T, Steven K. Radical cystectomy: extending the limits of pelvic lymph node dissection improves survival for patients with bladder cancer confined to the bladder wall. J Urol 1998;160(6 Pt 1):2015-2019; discussion 2020.
66. Tilki D, Reich O, Svatek RS, et al. Characteristics and outcomes of patients with clinical carcinoma in situ only treated with radical cystectomy: an international study of 243 patients. J Urol 2010;183(5):1757-1763.
67. Chade DC, Shariat SF, Godoy G, et al. Clinical outcomes of primary bladder carcinoma in situ in a contemporary series. J Urol 2010;184(1):74-80.
68. Huang GJ, Kim PH, Skinner DG, et al. Outcomes of patients with clinical CIS-only disease treated with radical cystectomy. World J Urol 2009; 27(1):21-25.
69. Freeman JA, Esrig D, Stein JP, et al. Radical cystectomy for high risk patients with superficial bladder cancer in the era of orthotopic urinary reconstruction. Cancer 1995;76(5):833-839.
70. Gore JL, Lai J, Setodji CM, et al. Mortality increases when radical cystectomy is delayed more than 12 weeks: results from a Surveillance, Epidemiology, and End Results-Medicare analysis. Cancer 2009;115(5):988-996.
71. Kulkarni GS, Urbach DR, Austin PC, et al. Longer wait times increase overall mortality in patients with bladder cancer. J Urol 2009;182(4):1318-1324.
72. Lee CT, Madii R, Daignault S, et al. Cystectomy delay more than 3 months from initial bladder cancer diagnosis results in decreased disease specific and overall survival. J Urol 2006;175(4):1262-1267; discussion 1267.
73. Sanchez-Ortiz RF, Huang WC, Mick R, et al. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol 2003;169(1):110-115; discussion 115.
74. Yu RJ, Stein JP, Cai J, et al. Superficial (pT2a) and deep (pT2b) muscle invasion in pathological staging of bladder cancer following radical cystectomy. J Urol 2006;176(2):493-498; discussion 498-499.
75. Boudreaux KJ Jr, Clark PE, Lowrance WT, et al. Comparison of american joint committee on cancer pathological stage T2a versus T2b urothelial carcinoma: analysis of patient outcomes in organ confined bladder cancer. J Urol 2009;181(2):540-545; discussion 546.
76. Cheng L, Neumann RM, Scherer BG, et al. Tumor size predicts the survival of patients with pathologic stage T2 bladder carcinoma: a critical evaluation of the depth of muscle invasion. Cancer 1999;85(12):2638-2647.
77. Tilki D, Reich O, Karakiewicz PI, et al. Validation of the AJCC TNM substaging of pT2 bladder cancer: deep muscle invasion is associated with significantly worse outcome. Eur Urol 2010;58(1):112-117.
78. Quek ML, Stein JP, Clark PE, et al. Natural history of surgically treated bladder carcinoma with extravesical tumor extension. Cancer 2003;98(5):955-961.
79. Quek ML, Stein JP, Clark PE, et al. Microscopic and gross extravesical extension in pathological staging of bladder cancer. J Urol 2004;171(2 Pt 1):640-645.
80. Boudreaux KJ Jr, Chang SS, Lowrance WT, et al. Comparison of American Joint Committee on Cancer pathologic stage T3a versus T3b urothelial carcinoma: analysis of patient outcomes. Cancer 2009;115(4):770-775.
81. Ayyathurai R, Gomez P, Luongo T, et al. Prostatic involvement by urothelial carcinoma of the bladder: clinicopathological features and outcome after radical cystectomy. BJU Int 2007;100(5):1021-1025.
82. Lerner SP, Colen J, Shen S. Prostatic biology, histologic patterns and clinical consequences of transitional cell carcinoma. Curr Opin Urol 2008;18(5):508-512.
83. Esrig D, Freeman JA, Elmajian DA, et al. Transitional cell carcinoma involving the prostate with a proposed staging classification for stromal invasion. J Urol 1996;156(3):1071-1076.
84. Pagano F, Bassi P, Ferrante GL, et al. Is stage pT4a (D1) reliable in assessing transitional cell carcinoma involvement of the prostate in patients with a concurrent bladder cancer? A necessary distinction for contiguous or noncontiguous involvement. J Urol 1996;155(1):244-247.
85. Stein JP, Clark P, Miranda G, et al. Urethral tumor recurrence following cystectomy and urinary diversion: clinical and pathological characteristics in 768 male patients. J Urol 2005;173(4):1163-1168.
86. Freeman JA, Tarter TA, Esrig D, et al. Urethral recurrence in patients with orthotopic ileal neobladders. J Urol 1996;156(5):1615-1619.
87. Brunschwig A. Complete excision of pelvic viscera for advanced carcinoma: a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer 1948;1(2): 177-183.
88. Whitmore WF Jr, Marshall VF. Radical surgery for carcinoma of the urinary bladder: one hundred consecutive cases four years later. Cancer 1956;9(3):596-608.
89. Mills RD, Turner WH, Fleischmann A, et al. Pelvic lymph node metastases from bladder cancer: outcome in 83 patients after radical cystectomy and pelvic lymphadenectomy. J Urol 2001;166(1):19-23.
90. Vieweg J, Gschwend JE, Herr HW, et al. Pelvic lymph node dissection can be curative in patients with node positive bladder cancer. J Urol 1999;161(2):449-454.
91. Stein JP, Cai J, Groshen S, et al. Risk factors for patients with pelvic lymph node metastases following radical cystectomy with en bloc pelvic lymphadenectomy: concept of lymph node density. J Urol 2003;170(1):35-41.
92. Lerner SP, Skinner DG, Lieskovsky G, et al. The rationale for en bloc pelvic lymph node dissection for bladder cancer patients with nodal metastases: long-term results. J Urol 1993;149(4):758-764; discussion 764-755.
93. Herr HW. Superiority of ratio based lymph node staging for bladder cancer. J Urol 2003;169(3):943-945.
94. Stein JP. Lymphadenectomy in bladder cancer: how high is “high enough”? Urol Oncol 2006;24(4):349-355.
95. Wishnow KI, Johnson DE, Ro JY, et al. Incidence, extent and location of unsuspected pelvic lymph node metastasis in patients undergoing radical cystectomy for bladder cancer. J Urol 1987;137(3):408-410.
96. Leissner J, Ghoneim MA, Abol-Enein H, et al. Extended radical lymphadenectomy in patients with urothelial bladder cancer: results of a prospective multicenter study. J Urol 2004; 171(1):139-144.
97. Poulsen J, Krarup T. Pelvic lymphadenectomy (staging) in patients with bladder cancer laparoscopic versus open approach. Scand J Urol Nephrol Suppl 1995;172:19-21.
98. Herr HW, Bochner BH, Dalbagni G, et al. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol 2002;167(3):1295-1298.
99. Konety BR, Joslyn SA, O’Donnell MA. Extent of pelvic lymphadenectomy and its impact on outcome in patients diagnosed with bladder cancer: analysis of data from the surveillance, epidemiology and end results program data base. J Urol 2003;169(3):946-950.
100. Koppie TM, Serio AM, Vickers AJ, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer 2008;112(11):2384-2392.
101. Dhar NB, Klein EA, Reuther AM, et al. Outcome after radical cystectomy with limited or extended pelvic lymph node dissection. J Urol 2008;179(3):873-878; discussion 878.
102. Sherif A, De La Torre M, Malmstrom PU, et al. Lymphatic mapping and detection of sentinel nodes in patients with bladder cancer. J Urol 2001;166(3):812-815.
103. Mathiesen O, Carl J, Bonderup O, et al. Axillary sampling and the risk of erroneous staging of breast cancer. An analysis of 960 consecutive patients. Acta Oncol 1990;29(6):721-725.
104. Caplin S, Cerottini JP, Bosman FT, et al. For patients with Dukes’ B (TNM Stage II) colorectal carcinoma, examination of six or fewer lymph nodes is related to poor prognosis. Cancer 1998;83(4):666-672.
105. Siewert JR, Bottcher K, Stein HJ, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 1998;228(4):449-461.
106. Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med 2003;349(22): 2117-2127.
107. Bochner BH, Cho D, Herr HW, et al. Prospectively packaged lymph node dissections with radical cystectomy: evaluation of node count variability and node mapping. J Urol 2004;172(4 Pt 1):1286-1290.
108. Bochner BH, Herr HW, Reuter VE. Impact of separate versus en bloc pelvic lymph node dissection on the number of lymph nodes retrieved in cystectomy specimens. J Urol 2001;166(6):2295-2296.
109. Stein JP, Penson DF, Cai J, et al. Radical cystectomy with extended lymphadenectomy: evaluating separate package versus en bloc submission for node positive bladder cancer. J Urol 2007;177(3):876-881; discussion 881-872.
▪ 23B Role of Radical Cystectomy in Patients with Advanced Bladder Cancer
S. Machele Donat
Harry W. Herr
INTRODUCTION
Radical cystectomy is the mainstay of treatment for invasive bladder cancer. The curative intent of radical cystectomy is to remove all cancer in the bladder, pelvis, and regional lymph nodes. Radical cystectomy with a pelvic lymphadenectomy cures the majority of patients with invasive tumors confined to the bladder (stage pT1-2), about half with microscopic tumor spread outside the bladder (stage pT3a), and a significant minority with low-volume positive (N1) pelvic lymph nodes (1,2,3). Radical cystectomy alone rarely cures locally advanced (stage pT3b-4), extensive node-positive (N2-3), or metastatic (M+) bladder cancer (4). The role of surgery as an adjunct to chemotherapy, even in patients with locally advanced bulky pelvic tumors, grossly positive lymph nodes, unresectable bladder tumors, or distant metastases, is currently evolving coincident with developments of newer, more effective chemotherapy regimens.
DEFINITION OF ADVANCED BLADDER CANCER
We define advanced bladder cancer as significant pelvic tumor invasion outside the bladder or metastasis to regional or distant sites. Such tumors include bulky extravesical pelvic tumor (stage pT3b-4), grossly positive pelvic and/or regional lymph nodes (N2-3), unresectable bladder cancer (fixed bladder mass), and distant nodal (retroperitoneal), soft tissue, or visceral metastases. Radical surgery plays a role in each of these clinical scenarios representing increasing tumor volume and burden. Radical cystectomy may provide local control for locally advanced pelvic and regional disease, and postchemotherapy surgery may salvage some patients with unresectable or metastatic bladder cancer.
RADICAL CYSTECTOMY FOR ADVANCED EXTRAVESICAL (PT3B-4) BLADDER CANCER
Locally advanced (stage pT3b-4) nonmetastatic bladder cancer is rare and rarely cured by surgery alone. Radical cystectomy cures in less than half the patients with measurable extravesical tumor spread, owing to high-volume pelvic disease, positive lymph nodes in 40% or more, and preexisting distant metastases, collectively accounting for tumor relapse and surgical failure. Table 23B.1 (1,2,3,4,5,6,7,8,9) shows the outcomes from contemporary cystectomy series in which the 5-year survival rates vary between 26% and 44% after radical cystectomy, with higher survival noted among patients with resectable pelvic masses but negative lymph nodes. Patients with pathologic stage pT3b-4 tumors associated with microscopic positive nodes have a 20% 5-year survival rate, but fewer than 5% with high-volume nodal metastasis survive (10).
RADICAL CYSTECTOMY FOR GROSSLY POSITIVE LYMPH NODES
The possibility of surgical cures among patients with high-volume nodal metastasis is low, ranging only between 13% and 29% in contemporary series (Table 23B.1). Due to the inaccuracies of clinical staging, the nodal status and pathologic stage of a bladder cancer are unknown at the time of laparotomy and thus provides little help to the surgeon faced with deciding whether or not to proceed with surgery when palpable, grossly positive lymph nodes are encountered.
We addressed the surgeon’s dilemma from this perspective by analyzing the long-term outcome of patients undergoing cystectomy and resection of extensive nodal disease (7). Of a total of 84 patients with gross positive nodes discovered at cystectomy, 20 (24%) survived 10 years after surgery alone (Fig. 23B.1). Of 53 patients with clinical stage T2 (organconfined) primary tumors, 17 (32%) survived versus 3 of 31 (10%) with stage T3 (extravesical) tumors. The University of Southern California group has shown a similar 27% 10-year survival rate among 84 patients with five or more positive nodes (1), indicating that some patients with grossly positive but resectable lymph nodes can be cured with surgery. However, our earlier experience demonstrated that of the subset of patients with locally advanced (pT3b-4) node-positive bladder cancer, fewer than 20% survived 5 years (11). Collectively, the data suggest that few patients with bulky pelvic tumors and positive lymph nodes are cured with surgery alone, and argue for a multimodality therapy combining a systemic chemotherapeutic intervention with radical cystectomy and extended pelvic lymphadenectomy.
We addressed the surgeon’s dilemma from this perspective by analyzing the long-term outcome of patients undergoing cystectomy and resection of extensive nodal disease (7). Of a total of 84 patients with gross positive nodes discovered at cystectomy, 20 (24%) survived 10 years after surgery alone (Fig. 23B.1). Of 53 patients with clinical stage T2 (organconfined) primary tumors, 17 (32%) survived versus 3 of 31 (10%) with stage T3 (extravesical) tumors. The University of Southern California group has shown a similar 27% 10-year survival rate among 84 patients with five or more positive nodes (1), indicating that some patients with grossly positive but resectable lymph nodes can be cured with surgery. However, our earlier experience demonstrated that of the subset of patients with locally advanced (pT3b-4) node-positive bladder cancer, fewer than 20% survived 5 years (11). Collectively, the data suggest that few patients with bulky pelvic tumors and positive lymph nodes are cured with surgery alone, and argue for a multimodality therapy combining a systemic chemotherapeutic intervention with radical cystectomy and extended pelvic lymphadenectomy.
TABLE 23B.1 SURVIVAL AFTER RADICAL CYSTECTOMY ALONE FOR LOCALLY ADVANCED (MACROSCOPIC EXTRAVESICAL TUMOR) AND REGIONALLY METASTATIC (GROSSLY NODE POSITIVE) BLADDER CANCER | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
NEOADJUVANT CHEMOTHERAPY FOR LOCALLY ADVANCED BLADDER CANCER
Following radical cystectomy for locally advanced bladder cancer, there is a significant rate of tumor recurrence, most commonly as distant metastases but also pelvic recurrence. Most patients who suffer tumor relapse die of bladder cancer. For this reason, systemic chemotherapy has been explored in both neoadjuvant (preoperative) and adjuvant (postoperative) settings. Recent studies show that neoadjuvant chemotherapy improves the survival of patients who are potentially curable by cystectomy. These encouraging results also suggest that therapeutic chemotherapy may improve survival after radical cystectomy, even for more advanced bladder cancer.
A cooperative, group, randomized study [Southwest Oncology Group (SWOG) 8,710, Intergroup-0080] of 307 patients with muscle-invasive bladder cancer (stage cT2-T4a) found a significant and clinically meaningful improvement in survival among patients who received neoadjuvant chemotherapy over cystectomy alone (12). With more than 8 years follow-up, the median survival time was 46 months among patients in the cystectomy group versus 77 months of the combination group (p = 0.04). At 5 years, 57% of the patients in the chemotherapy plus cystectomy group were alive, as compared with 43% of those in the cystectomy group (p = 0.06). There were 77 deaths from bladder cancer in the cystectomy group and 54 deaths in the combination therapy group (p = 0.002), translating into a 14% reduction in absolute mortality and a 5% improvement in 5-year survival rate. In both groups, the survival benefit of neoadjuvant methotrexate, vinblastine, doxorubicin, and cisplatin (M-VAC) was strongly related to downstaging of the tumor to pT0. In the neoadjuvant M-VAC patients, 38% had no evidence of cancer at cystectomy (pT0), as compared with 15% of the patients in the cystectomy-alone group (p < 0.001). Overall, the patients who were pT0 at surgery demonstrated an 85% 5-year survival rate.
Of particular relevance to treatment strategies for advanced bladder tumor, patients entered on the SWOG trial with extravesical disease (stage T3 or T4) derived significant benefit from neoadjuvant MVAC chemotherapy. Figure 23B.2 shows a median survival time of 65 months among 92 locally advanced disease patients after MVAC + cystectomy compared with 24 months in 93 patients after cystectomy alone (p = 0.04). Among advanced-disease patients, there was a 10% reduction in mortality in the combination therapy group compared with the cystectomy alone group. For patients with clinical T2 tumors, the 5-year survival was improved by only 5%, whereas patients with stage T3b-T4 tumors had a 20% improvement in 5-year survival.
The M.D. Anderson Cancer Center also addressed combined chemotherapy and surgery in patients suspected to have pathologic extravesical tumor extension and nodal involvement (13). A total of 140 patients (including 94 with T3b-4 tumors) were randomly assigned to receive either two courses of neoadjuvant M-VAC followed by cystectomy plus three additional cycles of chemotherapy, or, alternatively, to
have initial cystectomy followed by five cycles of adjuvant chemotherapy. Although there was no difference in outcome between the two groups by intent to treat, 81 patients (58%) remained disease free, with a median follow-up of 6.8 years. Of particular relevance was the finding of a nearly 40% cure rate among patients with pathologically proven lymph node metastasis, better than any reported outcome with surgery alone in a similar cohort. Of note, all patients in this study with pathologically confirmed extravesical extension also had nodal involvement. These findings support an improved cure fraction among patients with locally advanced bladder cancer by a combination of multiagent chemotherapy and surgery.
have initial cystectomy followed by five cycles of adjuvant chemotherapy. Although there was no difference in outcome between the two groups by intent to treat, 81 patients (58%) remained disease free, with a median follow-up of 6.8 years. Of particular relevance was the finding of a nearly 40% cure rate among patients with pathologically proven lymph node metastasis, better than any reported outcome with surgery alone in a similar cohort. Of note, all patients in this study with pathologically confirmed extravesical extension also had nodal involvement. These findings support an improved cure fraction among patients with locally advanced bladder cancer by a combination of multiagent chemotherapy and surgery.
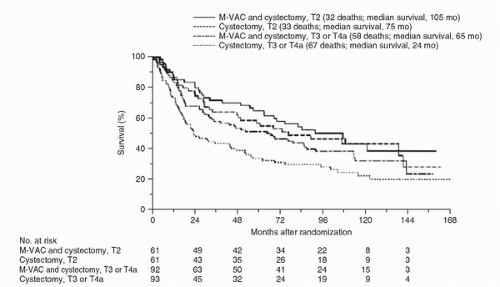 FIGURE 23B.2. Survival according to treatment group and whether patients had muscle invasion (stage T2) or more advanced disease (stage T3 or T4a). |
Meta-analyses of all randomized trials in more than 3,000 patients confirm that neoadjuvant chemotherapy improves survival of patients with muscle-invasive bladder cancer treated by cystectomy (14,15). Platinum-based combination chemotherapy has a 5% absolute survival benefit at 5 years and a 13% reduction in risk of death from bladder cancer among patients undergoing cystectomy. These collective results, as well as the dismal outcome associated with cystectomy alone, argue strongly for integrating systemic chemotherapy and cystectomy, especially in patients with locally advanced and metastatic bladder cancer.
Although radical cystectomy is usually advised after combination chemotherapy for most invasive bladder cancers (16), selected patients may be treated by partial cystectomy if they achieve a major reduction in tumor size and a complete cystoscopic response to neoadjuvant chemotherapy. Of 26 patients with cT2-T4N0M0 disease who underwent a partial cystectomy after M-VAC, 17 (65%) survived beyond 5 years, including 14 (54%) with an intact functioning bladder (17). Patients with no tumor (pT0) in their surgical specimens had a 5-year survival rate of 87% (14 of 16) compared with 30% (3 of 10) among patients with residual invasive cancer. Twelve patients (46%) developed bladder recurrences, which were invasive in five (18%) and superficial in seven (26%). Neoadjuvant chemotherapy may permit bladder sparing in highly selected patients who have significant downsizing of their tumors located in sites favorable to partial cystectomy. The bladder remains at risk for new tumors, but the majority of local recurrences can be treated successfully by local therapy or salvage cystectomy.
We believe that therapeutic platinum-based combined chemotherapy (at least four cycles) should be given before cystectomy in patients who have clinical evidence of extravesical pelvic tumor (palpable mass, hydronephrosis) and a high likelihood of nodal or silent distant metastases. (Hydronephrosis is not so much an indicator of extravesical disease as one of muscle-invasive disease. Unlike palpable mass, hydronephrosis has not been used by most as an indicator for neoadjuvant chemotherapy.) Additional efforts at improving our ability to accurately identify locally advanced and regional/pelvic nodal disease are needed to allow for better selection of patients for preoperative chemotherapy.
RATIONALE FOR POSTCHEMOTHERAPY SURGERY
Platinum-based chemotherapy is the primary treatment of metastatic bladder cancer (18). Various combined regimens have yielded major response proportions ranging from 39% to 72%, including complete pathologic remission in 20% to 36% of cases (19). However, the median survival time from the identification of metastatic disease remains at about 1 year, with only 3% to 7% of patients surviving longer than 5 years (20). Lymph node metastases and pelvic soft-tissue tumors respond more favorably than tumors involving the lung, liver, or bone (19). Longer survival is seen in patients with lymph node and soft tissue disease, and a substantially worse prognosis is found in those with visceral involvement. Tumor relapse in the bladder, pelvis, and distant sites is common, even after a complete clinical response to chemotherapy (21). Based on these findings, the primary tumor and regional or
retroperitoneal nodes would appear to be most amenable to surgical cure with extended lymph node dissection and radical cystectomy. Selected tumors in the lung, liver, and even bone may also be amenable for complete postchemotherapy surgical resection. Lastly, a complete response to chemotherapy alone or chemotherapy plus surgery is the best correlate of prolonged survival and is a prerequisite for cure in advanced bladder cancer (19). These observations provide a rationale for postchemotherapy surgery that may benefit selected patients with locally advanced, initially unresectable, or inoperable primary or metastatic bladder cancers.
retroperitoneal nodes would appear to be most amenable to surgical cure with extended lymph node dissection and radical cystectomy. Selected tumors in the lung, liver, and even bone may also be amenable for complete postchemotherapy surgical resection. Lastly, a complete response to chemotherapy alone or chemotherapy plus surgery is the best correlate of prolonged survival and is a prerequisite for cure in advanced bladder cancer (19). These observations provide a rationale for postchemotherapy surgery that may benefit selected patients with locally advanced, initially unresectable, or inoperable primary or metastatic bladder cancers.
POSTCHEMOTHERAPY SURGERY FOR UNRESECTABLE AND NODE-POSITIVE BLADDER CANCER
When the M-VAC regimen became established in 1983 as effective chemotherapy against metastatic bladder cancer (22), oncologic logic persuaded us to combine chemotherapy and surgery for patients with locally advanced but inoperable disease. We initially treated 41 consecutive patients with unresectable or extensive node-positive bladder cancer (stages pT4b, N2-3, M0) with M-VAC followed by an attempt at postchemotherapy radical cystectomy (23). Of the 41 patients, 29 underwent surgical exploration and cystectomy was accomplished in 24. Cystectomy was not performed in 17 patients due to lack of response to chemotherapy or patient refusal. After a minimum follow-up of 4 years (range, 4-7 years), nine patients (22%) survived, including seven with no cancer (pT0) left in the bladder (complete response to chemotherapy) and two after resection of residual viable bladder cancer (complete response to M-VAC plus surgery) (Fig. 23B.3A). Overall survival according to whether surgery was performed showed 8 of 24 patients alive after cystectomy versus only 1 of 17 without cystectomy (Fig. 23B.3B, p = 0.009). We concluded from this initial experience that patients with advanced unresectable bladder cancer who did not have a surgical option initially may benefit from combination chemotherapy (M-VAC) when downstaging allows potentially curative surgery to be performed as combined modality strategy.
 FIGURE 23B.3. (A) Survival of CR to chemotherapy. (B) Overall survival according to postchemotherapy surgery versus no surgery. |
An update of our experience with postchemotherapy surgery from 1984 to 1999 (24) showed that of the 207 patients with unresectable and regionally metastatic bladder cancer treated with cisplatin-based chemotherapy regimens, 80 (39%) underwent surgery, 12 (6%) refused surgery although clinically deemed resectable, and 115 (55%) did not undergo cystectomy secondary to either tumor progression or poor performance status (24). Postchemotherapy surgery was designed to resect all pelvic and metastatic nodal sites of disease documented before chemotherapy with a radical cystectomy and a bilateral pelvic and retroperitoneal lymph dissection. En bloc resection of extravesical soft tissue pelvic masses was included with the bladder and adjacent contiguous organs. Response to chemotherapy was documented as shrinkage of measurable tumor in the bladder and nodes on follow-up computed tomography (CT) scans, and evaluation under anesthesia and transurethral resection (TUR) biopsies of tumor sites showing downstaging of local tumor invasion or no evidence of residual tumor.
Of the 80 operated patients, 34 (42%) survived 9 months to 5 years, including 20 of 49 (41%) with resection of residual viable disease (Table 23B.2). Of these, 60 patients who had received M-VAC and had mature follow-up of 5 years or
more, 19 (32%) survived 5 years, including 10 of 34 (29%) after resection of persistent tumor. Only 1 of 12 patients (8%) who refused surgery survived longer than 1 year, and died of disease at 3 years post chemotherapy. Of the patients who failed to achieve a major clinical response to chemotherapy, none achieved any survival benefit from postchemotherapy surgery. In addition, although technically challenging, there were no operative mortalities or surgical morbidity requiring reoperation, indicating that postchemotherapy surgery may be performed safely in experienced hands. Our experience demonstrates that postchemotherapy surgery may benefit a subset of patients with unresectable or regionally metastatic bladder cancer who achieve a major response to chemotherapy.
more, 19 (32%) survived 5 years, including 10 of 34 (29%) after resection of persistent tumor. Only 1 of 12 patients (8%) who refused surgery survived longer than 1 year, and died of disease at 3 years post chemotherapy. Of the patients who failed to achieve a major clinical response to chemotherapy, none achieved any survival benefit from postchemotherapy surgery. In addition, although technically challenging, there were no operative mortalities or surgical morbidity requiring reoperation, indicating that postchemotherapy surgery may be performed safely in experienced hands. Our experience demonstrates that postchemotherapy surgery may benefit a subset of patients with unresectable or regionally metastatic bladder cancer who achieve a major response to chemotherapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree