Jeffrey I. Cohen *
Introduction to Herpesviridae
Although there are hundreds of herpesviruses that infect nearly all animals, eight herpesviruses naturally infect humans (Table 137-1). A ninth herpesvirus, herpes B virus, which naturally infects macaques, can cause fatal encephalitis in humans. This chapter is an overview of herpesviruses that infect humans. Individual herpesviruses are discussed in subsequent chapters (see Chapters 138 to 144).
TABLE 137-1
Biologic Features of Herpesviruses That Infect Humans
| VIRUS | GENOME | SITES OF LATENCY | ||
| Subfamily* | Size (kbp) | Receptor(s) | ||
| Human Virus | ||||
| HSV-1 (HHV-1) | Alpha | 152 | Nectin-1, nectin-2 TNFRSF14, 3-OS-HS | Sensory and cranial nerve ganglia |
| HSV-2 (HHV-2) | Alpha | 152 | Nectin-1, nectin-2 TNFRSF14 | Sensory and cranial nerve ganglia |
| Varicella-zoster virus (HHV-3) | Alpha | 125 | Sensory and cranial nerve ganglia | |
| Cytomegalovirus (HHV-5) | Beta | 229 | PDGFR α, EGFR Integrin α2β1, α6β1, αvβ3 | Monocytes, macrophages, CD34+ cells |
| HHV-6 | Beta | 165 | CD46, CD134 | CD34+ cells, monocytes, macrophages |
| HHV-7 | Beta | 145 | CD4 cells | |
| Epstein-Barr virus (HHV-4) | Gamma | 172 | CD21, MHC class II, Integrins αvβ6, αvβ8 | Memory B cells |
| Kaposi sarcoma–associated herpesvirus (HHV-8) | Gamma | 165 | Integrin αvβ3 αvβ5, αvβ1, XCT, DC-SIGN, EphA2 | B cells |
| Simian Virus | ||||
| Herpes B virus (cercopithecine herpesvirus 1) | Alpha | 150 | Nectin-1 | Sensory and cranial nerve ganglia |
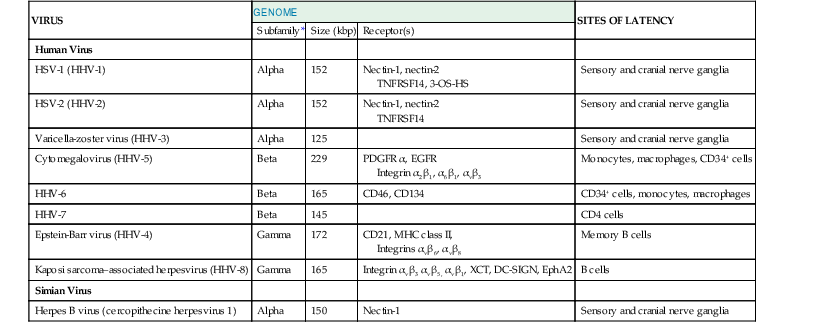
* Alpha, Beta, and Gamma refer to subfamilies Alphaherpesvirinae, Betaherpesvirinae, and Gammaherpesvirinae, respectively.
DC-SIGN, dendritic cell-specific ICAM-3 grabbing nonintegrin; EGFR, epidermal growth factor receptor; EphA2, ephrin receptor tyrosine kinase A2; HHV, human herpesvirus; HSV, herpes simplex virus; MHC, major histocompatibility complex; PDGFR, platelet-derived growth factor receptor; 3-OS-HS, 3-O-sulfotransferases; TNFRSFI4, tumor necrosis factor receptor superfamily, member 14; XCT, light chain of the human cystine/glutamate transporter system.
Modified from Straus SE. Introduction to Herpesviridae. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Churchill Livingstone; 2005:1756-1762.
Classification
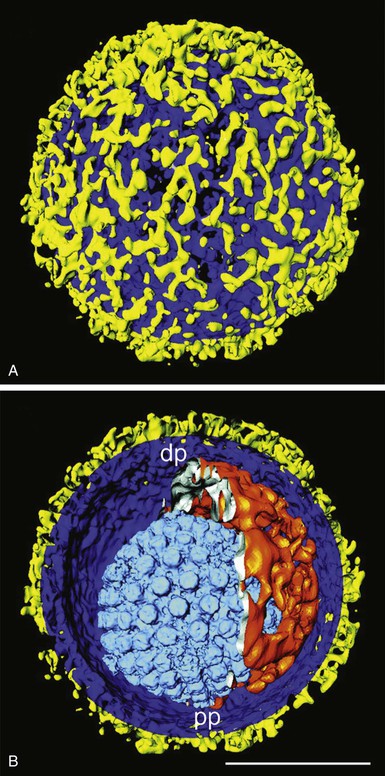
Members of the Herpesviridae are distinguished from other virus families by their structure and genome. Herpesviruses contain double-stranded DNA surrounded by an icosahedral nucleocapsid, which is wrapped inside a tegument consisting of several viral and cellular proteins and then surrounded by an envelope studded with viral glycoproteins (Fig. 137-1).1 The envelope is derived from host cell membranes. Herpesviruses range from 120 to 260 nm in diameter. Virions contain a characteristic set of viral proteins as well as some host cell proteins.
Human herpesviruses are subdivided into three subfamilies (see Table 137-1). The Alphaherpesvirinae include herpes simplex virus (HSV) types 1 and 2, varicella-zoster virus (VZV), and herpes B virus. These viruses are latent in neurons of sensory ganglia, and infection of cultured cells leads to rapid destruction of the cells. They usually cause mucocutaneous infections in healthy individuals. The Betaherpesvirinae include cytomegalovirus (CMV), human herpesvirus 6 (HHV-6), and human herpesvirus 7 (HHV-7). These viruses have a more limited host range, replicate slowly in cell culture, and establish latency in mononuclear cells. The Gammaherpesvirinae include Epstein-Barr virus (EBV) and Kaposi sarcoma–associated herpesvirus (KSHV, also known as HHV-8). These viruses establish latency in lymphoid cells and cause lytic infection in epithelial cells or fibroblasts. The Betaherpesvirinae and Gammaherpesvirinae can cause lymphoproliferation with mononucleosis.
EBV and HHV-6 are present as one of two types, referred to as types A and B. EBV types can differ depending on the geographic location of the infected individual. Although HSV-1 and HSV-2 share 50% or more sequence identity, different types of EBV and HHV-6 are much more closely related.
Genome Structure and Proteins
The human herpesviruses consist of 125,000 to 229,000 bp of double-stranded DNA (see Table 137-1), and their overall guanosine and cytosine content varies from 36% (for HHV-7) to 70% (for HSV-2). The genomes consist of long unique regions and short repeated regions. These repeats, as well as single nucleotide polymorphisms, are often useful for molecular epidemiologic studies because restriction endonuclease digestion results in different-sized fragments that can help to indicate whether different persons are infected with different strains of virus. Thus, after transplantation, the sizes of the repeated regions can help to indicate whether the viruses originate from the donor or the recipient.2 EBV has terminal repeats at the ends of its genome. The number of terminal repeats is fixed in cells that were infected with the same clone of virus but varies in number if the cells were infected with different viral clones.3 The viral genome is linear inside the virion but circularizes in infected cells. Portions of many herpesvirus genes overlap in the genome; most herpesvirus genes are not spliced.
HHVs encode 71 to several hundred genes.4 Herpesviruses encode a core set of approximately 40 proteins that are conserved among all the herpesvirus species and include proteins involved in nucleic acid synthesis (e.g., viral DNA polymerase), nucleic acid metabolism (e.g., ribonucleotide reductase), protein modification (e.g., protein kinases), and virion structure (major capsid protein, glycoproteins B, H, and L). In addition, members of the three subfamilies of the Herpesviridae each contain a conserved set of genes unique to each subfamily, such as genes encoding proteins important for virus entry. Herpesviruses have a lytic phase of replication that results in cell death and a latent phase of replication in which no or a very limited set of viral proteins are made.
Virus Replication
Herpesviruses use at least two principal methods to enter cells.5 Virus can enter cells by endocytosis and subsequent fusion of the virion envelope to the endocytic membrane, which allows entry of the nucleocapsid into the cytoplasm. Alternatively, the virion envelope can fuse to the cell membrane on the cell surface to deliver the nucleocapsid directly to the cytoplasm. Viral glycoproteins bind to receptors on the cell membrane, which allows entry into the cell. Herpesviruses often have more than one cell surface receptor (see Table 137-1). The viral nucleocapsid is transported from the cytoplasm to the nucleus, where the linear viral DNA circularizes, and thus DNA replication can begin. Replication proceeds in an orderly pattern of viral gene expression. The immediate-early genes are initially expressed that encode proteins that regulate viral gene expression. This is followed by the early viral proteins, many of which encode enzymes important for viral DNA replication or protein phosphorylation. Last, the late proteins are made, many of which encode structural proteins, including the viral glycoproteins and nucleocapsid proteins. Viral genes are transcribed in the nucleus, and the proteins are synthesized in the cytoplasm. Herpesvirus nucleocapsids are assembled in the nucleus and undergo envelopment at the inner nuclear membrane, de-envelopment at the outer nuclear membrane, and re-envelopment at the cytoplasmic membrane, and then exit the cell. Lytic replication of herpesviruses inhibits host cell RNA and protein synthesis.
Virus Latency and Reactivation
The mechanisms by which herpesviruses establish and maintain latency are not well understood. Some viruses, such as HSV, express one family of latency-associated transcripts that do not encode proteins but may be important to prevent apoptosis.6 Other viruses express proteins during latency. EBV expresses the EBV nuclear antigen 1 protein, which allows viral episomes to partition to dividing latently infected B cells.7 EBV and KSHV encode a number of latency proteins expressed in different types of virus-associated malignancies. Herpesvirus genomes exist in a circular, episomal form during latency in specific cell types. HHV-6 is an exception; up to 1% of healthy persons have HHV-6 integrated in their chromosomes.8
Several mechanisms have been proposed for maintaining latency in herpesviruses. These include expression of viral microRNAS during latency that inhibit expression of immediate-early viral genes9,10 and methylation of histone proteins associated with lytic genes, which results in compaction of chromatin and silencing of the lytic genes.11,12 In addition, certain viral and cellular proteins, which are normally in the nucleus of cells undergoing herpesvirus replication, may be sequestered to the cytoplasm during latency so that the viral proteins cannot activate gene expression.13
Latent viral genomes can reactivate to produce infectious virus. Reactivation can be stimulated in some virus-infected cells by radiation, trauma to nerves (in the case of the Alphaherpesvirinae), hyperthermia,14 or hypoxia.15 Reactivation is more common in immunosuppressed or immunocompromised hosts that have impaired T-cell immunity. For example, in the absence of antiviral prophylaxis 50% or more of seropositive transplant recipients reactivate HSV, EBV, HHV-6, and HHV-7. Stress can also lead to reactivation; CMV reactivation occurs in 33% of seropositive immunocompetent patients in intensive care units.16 Reactivation allows the virus to be transmitted to other individuals, thereby perpetuating virus infection over time to other generations.
Pathogenesis
Many herpesviruses, such as HSV, EBV, CMV, HHV-6, and HHV-7, are shed from the oral mucosa without symptoms despite the presence of neutralizing antibody and virus-specific T cells.17 It is during asymptomatic shedding, rather than during symptomatic disease, that most viruses are transmitted from person to person. In contrast, VZV is only transmitted when patients have varicella or zoster.
Symptomatic disease due to some herpesviruses is associated with lytic virus replication, resulting in skin lesions due to HSV or VZV or visceral lesions due to HSV, VZV, or CMV. Other diseases, such as erythema multiforme associated with HSV or hemolytic anemia associated with CMV or EBV, are due to the immune response to the virus. Most symptoms from infectious mononucleosis associated with EBV are due to the proliferation of T cells that respond to the infection rather than to lytic destruction of virus-infected B cells.
Most human encounters with herpesviruses are asymptomatic or induce very mild symptoms. Many persons infected with HSV-1 or HSV-2 are asymptomatic, and young children and infants infected with CMV and EBV are usually asymptomatic. In contrast, most persons infected with VZV present with chickenpox and most with HHV-6 have fever. Infections are rarely fatal except in highly immunocompromised persons. It is in the best interest of the virus for the host to survive so that the infection can be transmitted to others. Although some herpesviruses (e.g., HSV, VZV, CMV, HHV-6) infect a wide range of cells in the body, others (e.g., EBV, HHV-7, KSHV) have a more narrow host range.
Epidemiology
Nearly all adults are infected with HSV-1, VZV, EBV, HHV-6, and HHV-7 (Table 137-2). Fifteen to 50 percent of adults in the United States are infected with HSV-2, 40% to 70% are infected with CMV, and less than 5% are infected with KSHV. Rates of HSV-1, HSV-2, CMV, EBV, and KSHV are higher in developing countries than in the United States.
TABLE 137-2
Features of Herpesvirus Infections and Seroepidemiology
| VIRUS | PRIMARY INFECTION IN HEALTHY PERSONS | INFECTION IN IMMUNOCOMPROMISED PERSONS | SEROPREVALENCE (%) | ||
| Healthy Children | Healthy Adults | ||||
| United States | Developing World | ||||
| Herpes simplex virus 1 | Gingivostomatitis Keratoconjunctivitis Cutaneous herpes Genital herpes | Gingivostomatitis Keratoconjunctivitis Cutaneous herpes Visceral infections | 20-40 | 50-70 | 50-90 |
| Herpes simplex virus 2 | Genital herpes Cutaneous herpes Gingivostomatitis Aseptic meningitis Neonatal herpes | Genital herpes Cutaneous herpes Disseminated infection | 0-5 | 15-50 | 20-60 |
| Varicella-zoster virus | Varicella | Disseminated infection | 50-75 | 85-95 | 50-80 |
| Cytomegalovirus | Mononucleosis Hepatitis Congenital cytomegalic inclusion disease | Hepatitis Retinitis Other visceral infections | 10-30 | 40-70 | 40-80 |
| Epstein-Barr virus | Mononucleosis Hepatitis Encephalitis | Polyclonal and monoclonal lymphoproliferative syndromes Oral hairy leukoplakia | 10-30 | 80-95 | 90-100 |
| Human herpesvirus 6 | Exanthem subitum, infantile fever and seizures, encephalitis | Fever and rash Encephalitis Bone marrow suppression | 80-100 | 60-100 | 60-100 |
| Human herpesvirus 7 | Exanthem subitum, childhood fever and seizures, encephalitis | Encephalitis | 40-80 | 60-100 | 40-100 |
| Kaposi sarcoma–associated herpesvirus | Febrile exanthem Mononucleosis? | Kaposi sarcoma, Castleman disease, primary effusion lymphoma | <3 | <3 | 10-60 |
| Herpes B virus | Mucocutaneous lesions Encephalitis | Not reported | 0 | 0 | 0 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree