Intratumoral therapy with bacteria/bacterial products dates to at least the 1890s. Over the decades this has expanded beyond the use of microbes and microbial products to include chemicals, cancer chemotherapeutic agents, cytokines, recombinant organisms, and hybrid molecules. The appeal of this method of delivery is the ability to deliver high concentrations of the therapeutic agent directly to the tumor, often with minimal side effects. This article summarizes the use and efficacy of the various agents used in the past and present in the treatment of in-transit and satellite metastases in melanoma.
Key points
- •
Agents such as bacille Calmette-Guerin, interleukin-2, interferon, rose bengal, and granulocyte-macrophage colony-stimulating factor/viral recombinants are viable options for treatment of local-regional dermal and subcutaneous melanoma metastases through intralesional injections.
- •
Remission rates are significantly higher than when they are used systemically to treat metastatic disease, suggesting that the concentration of the agent within the tumor is a significant factor in determining response.
- •
The mechanism of the antitumor effect is postulated to be immunologic but a bystander effect caused by the inflammatory response is also possible.
Introduction
Intratumoral therapy with bacteria/bacterial products dates to at least the 1890s. It was in 1893 that William Coley published his case series of the deliberate infection of sarcomas with a mixture of Streptococcus pyogenes and Serratia marcescens . He concluded that bacterial infection could induce tumor regression. A scientific basis for this claim was provided by Zbar and Rapp who showed regression of subcutaneous implants of a diethyl-nitrosamine–induced hepatocellular carcinoma in inbred strain-2 male guinea pigs by intratumor injection of bacille Calmette-Guerin (BCG) with regression not only of the injected tumor but also regression of regional nodal metastases. The animals were resistant to rechallenge with the same tumor. This finding sparked renewed interest in intratumoral therapy as an approach not only to local tumor regression but also to inducing systemic antitumor immunity.
Over the ensuing decades, intratumoral therapy has expanded beyond the use of microbes and microbial products (eg, BCG, methanol extraction residue of BCG, purified protein derivative of Mycobacterium tuberculosis , vaccinia, Clostridium novyi NT [no toxin]) to chemicals (eg, dinitrochlorobenzene, rose bengal), cancer chemotherapeutic agents (eg, nitrogen mustard, 5-fluorouracil, imiquimod), cytokines (eg, interferon, interleukin-2 [IL-2], granulocyte-macrophage colony-stimulating factor [GM-CSF]), recombinant organisms (eg, vaccinia/GM-CSF, herpes simplex/GM-CSF, fowlpox/tumor antigen), and hybrid molecules (eg, mesothelin-diphtheria toxin gene). This list is incomplete and is being added to on a regular basis.
The obvious appeal of the intratumoral (intralesional/topical) approach to therapy is the ability to deliver a high concentration of the therapeutic agent directly to the tumor, usually with minimal systemic effects. The clinical experience in melanoma with the most extensively studied agents is reviewed in this article. Although regression of systemic disease has been more elusive, locoregional response rates approach 90%. We have found this approach invaluable in the treatment of dermal satellite and in-transit metastases, particularly those in cosmetically or functionally eloquent areas and also when the lesions encompass a large area defying surgical excision. This treatment can also be applicable in frail or medically compromised patients. To encourage more widespread application, this article provides detailed instructions on the use of some commercially available agents, such as BCG and the cytokines.
Introduction
Intratumoral therapy with bacteria/bacterial products dates to at least the 1890s. It was in 1893 that William Coley published his case series of the deliberate infection of sarcomas with a mixture of Streptococcus pyogenes and Serratia marcescens . He concluded that bacterial infection could induce tumor regression. A scientific basis for this claim was provided by Zbar and Rapp who showed regression of subcutaneous implants of a diethyl-nitrosamine–induced hepatocellular carcinoma in inbred strain-2 male guinea pigs by intratumor injection of bacille Calmette-Guerin (BCG) with regression not only of the injected tumor but also regression of regional nodal metastases. The animals were resistant to rechallenge with the same tumor. This finding sparked renewed interest in intratumoral therapy as an approach not only to local tumor regression but also to inducing systemic antitumor immunity.
Over the ensuing decades, intratumoral therapy has expanded beyond the use of microbes and microbial products (eg, BCG, methanol extraction residue of BCG, purified protein derivative of Mycobacterium tuberculosis , vaccinia, Clostridium novyi NT [no toxin]) to chemicals (eg, dinitrochlorobenzene, rose bengal), cancer chemotherapeutic agents (eg, nitrogen mustard, 5-fluorouracil, imiquimod), cytokines (eg, interferon, interleukin-2 [IL-2], granulocyte-macrophage colony-stimulating factor [GM-CSF]), recombinant organisms (eg, vaccinia/GM-CSF, herpes simplex/GM-CSF, fowlpox/tumor antigen), and hybrid molecules (eg, mesothelin-diphtheria toxin gene). This list is incomplete and is being added to on a regular basis.
The obvious appeal of the intratumoral (intralesional/topical) approach to therapy is the ability to deliver a high concentration of the therapeutic agent directly to the tumor, usually with minimal systemic effects. The clinical experience in melanoma with the most extensively studied agents is reviewed in this article. Although regression of systemic disease has been more elusive, locoregional response rates approach 90%. We have found this approach invaluable in the treatment of dermal satellite and in-transit metastases, particularly those in cosmetically or functionally eloquent areas and also when the lesions encompass a large area defying surgical excision. This treatment can also be applicable in frail or medically compromised patients. To encourage more widespread application, this article provides detailed instructions on the use of some commercially available agents, such as BCG and the cytokines.
Vaccinia
Vaccinia is a large poxvirus with a double-stranded DNA genome that closely resembles cowpox. It is the active constituent of the vaccine that eradicated smallpox. Vaccination has been discontinued and thus a large segment of the population is unprotected. Because of bioterrorism concerns, the vaccine is sequestered and no longer available for clinical use. Use in cancer treatment was prompted by its oncolytic nature and the ability of the virus to activate macrophages, professional antigen-presenting cells. This ability in turn could enhance systemic immunity. Vaccinia virus has been used for intralesional therapy for cutaneous melanoma.
In 1961, Belisario and Milton reported treating a 29-year-old woman with extensive in-transit deposits of melanoma with vaccinia virus intralesionally. All injected lesions resolved or decreased in size. Their work continued into the 1970s, studying both intralesional vaccinia and systemic immunization. Everall and colleagues reported a series of 48 patients with primary melanomas who were randomized to having the primary lesion injected with vaccinia 2 weeks before excision versus primary excision alone. Relapse-free survival was improved in the patients treated with vaccinia.
In anticipation of using vaccinia as a vector, Mastrangelo and colleagues treated 5 patients with metastatic melanoma accessible to injection with intralesional therapy with the wild-type vaccinia. All patients developed antivaccinia antibody titers. Despite this, infectivity could be maintained with repeat treatments. The injections were well tolerated. There were 1 complete (10+ years’ duration) and 3 partial remissions. However, this product is no longer available.
Bacille Calmette-Guerin
BCG is prepared from an attenuated strain of Mycobacterium bovis . It is similar enough to its wild ancestors to provide immunity against M tuberculosis (its original application was in the prevention of tuberculosis). BCG induces an intense inflammatory response at injection sites, triggering cytokine cascade and macrophage activation. Direct tumor cell killing is a bystander effect of the inflammation and systemic effects are mediated via its ability to activate macrophages, professional antigen-presenting cells. The ability of BCG to induce an antitumor immune response was shown by Zbar and Rapp. Intravesical BCG has been approved by the US Food and Drug Administration (FDA) for the treatment of superficial bladder cancer. One of its earliest anticancer uses was in the treatment of melanoma.
Dr Donald Morton was the first to explore this route of treatment. He reported his 7-year experience with 151 patients; 90% of injected lesions and 17% of uninjected lesions regressed. Responding lesions were intradermal, with subcutaneous lesions less likely to respond. Twenty-five percent of the responders remained disease free for between 1 and 6 years. Mastrangelo and colleagues reported a series of 15 patients treated every 2 weeks for a minimum of 5 treatments. There were 2 complete and 3 partial responders. Again, remissions were durable, lasting years to decades.
Storm and colleagues reported a series of patients with recurrent melanoma of the lower extremities treated initially with intralesional BCG. Twenty of 27 patients had complete or transient disease control. Nonresponders and those whose disease eventually progressed were treated with hyperthermic perfusion with l -phenylalanine mustard. Objective responses were seen in 7 of 9 patients. As with most therapies in oncology, a successful single agent is then combined with a second in an attempt to improve response rates. BCG was combined with topical imiquimod, an agent that signals the innate immune system through toll-like receptor-7. Kidner and colleagues treated 9 patients with broad areas of in-transit disease with intralesional BCG followed by topical imiquimod 5 to 7 days a week after an inflammatory response was seen with BCG. Patients were treated until complete remission or progression. Five of 9 patients achieved a complete response (CR) and 3 of the remaining 4 were rendered clinically disease free surgically. All patients were alive at 30 months of follow-up.
Overall, injected nodules disappear 90% of the time and in 20% of patients noninjected regional metastases regress as well. Regression of visceral disease remains elusive but has been reported. Toxicity consists primarily of injection site reactions; the sites most frequently ulcerate and require 6 to 8 weeks to heal. About 2 hours after the injection the patient experiences flulike symptoms that are easily controlled with antipyretics. A BCG bacteremia can occur, resulting in granulomatous changes in the liver and lungs. BCG infections are readily eradicated with isoniazid. The systemic toxicity of BCG is likely related to a transient BCG bacteremia resulting from the intratumoral injection under pressure. This toxicity can be largely avoided by using the scarification approach (discussed later). The systemic toxicity of BCG has been most systematically reported for intravesical use in bladder cancer.
So why is BCG so uncommonly used in clinical practice? A therapy with a 90% response rate is unheard of in most tumor types. We believe it is secondary to a lack of training and exposure to the technique in fellowship. Although intralesional therapy is not unique to melanoma it is most commonly used in this disease, and therefore only trainees at tertiary care institutions with large populations of patients with melanoma would have exposure to this technique. It is not difficult and can be used in any medical oncologist’s or oncologic surgeon’s office.
Mechanics of injecting bacille Calmette-Guerin
To inject BCG, the first step is the proper selection of target lesions. They should be dermal or cutaneous. As noted earlier, subcutaneous lesions do not respond well because they do not erupt at the surface. Second, the most distal lesions should be injected first, because up to 20% of uninjected lesions in the draining basin respond. Third, they should be in a nonirradiated field because areas of prior irradiated skin do not respond well.
Once the target lesions have been identified, the patient is premedicated with acetaminophen or ibuprofen. The vial of BCG (Merck Sharpe & Dohme, NDC 0005206020) containing 1 × 10 8 to 8 × 10 8 organisms (as colony forming units [CFU]) is reconstituted with 1 mL of sterile water. The contents of the vial are then diluted to a total volume of 10 mL of sterile water for a 1:10 dilution (1–8 × 10 7 CFU/mL). One milliliter is then drawn up in a 1-mL Luer Lock syringe with a 25-gauge needle. The selected lesions are cleaned with alcohol and then the needle is inserted from the base of the lesion toward the center. The BCG is then injected slowly. Each lesion should accommodate between 0.05 (0.5–4 × 10 6 CFU) and 0.10 mL (1–8 × 10 6 CFU). Because the solution is under pressure, wait 30 to 60 seconds before withdrawing the needle so that the BCG is not allowed to run out of the lesion.
We typically inject 0.4 to 0.8 mL of the 1:10 dilution of BCG in the first session depending on the number of lesions. Instructions are given to the patient to redose the acetaminophen or ibuprofen every 6 to 8 hours for the first 24 hours. If fever of more than 38.0°C (100.4°F) persists for more than 48 hours we typically consider treatment with isoniazid.
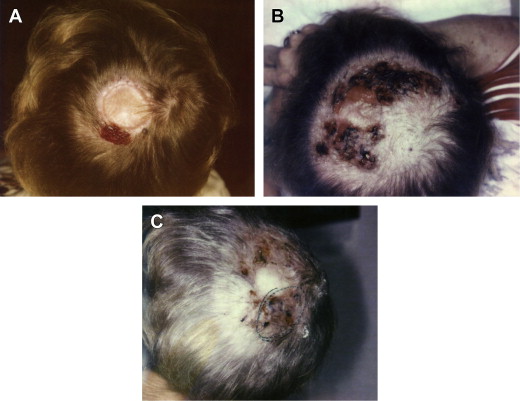
The patient returns for the second treatment in 2 to 3 weeks’ time. If there is minimal induration or erythema we continue retreatment at a 1:10 dilution. If they have an intensely suppurative reaction we retreat at a 1:20 dilution and typically inject previously uninjected lesions, again working distal to proximal. This process creates an abscess. The drainage and erythema become intense, because this is what is intended: a large immune reaction ( Fig. 1 ).