Key points
- •
Image guided techniques are the most common invasive therapy for unresectable hepatocellular carcinoma (HCC).
- •
Level 1 evidence supports survival benefit chemoembolization.
- •
New embolic devices may offer benefits not currenlty realized.
Background
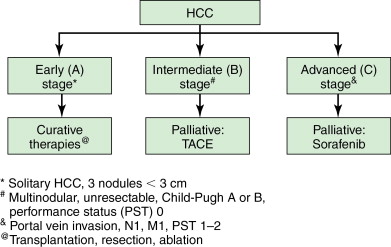
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third most common cause of cancer-related death. Although the highest prevalence is still in sub-Saharan Africa and Asia, the incidence in the United States is rising along with the rise in hepatitis B and C and the longer survival of cirrhotic patients. As novel therapeutic options have emerged and been refined, the manner in which HCC is treated has changed dramatically compared with just two decades ago. The approach to a patient with HCC has been reasonably standardized as a result of several international guidelines. Now, there is a reproducible algorithm that determines whether a patient undergoes transplantation, surgical resection, ablation, transarterial treatment, or systemic chemotherapy ( Figure 8-1 ). It should be noted that a minorty of HCC patients can be treated with resection or transplantation, and percutaneous ablation and transarterial techniques have emerged as essential therapies in the treatment algorithm for HCC.
This chapter focuses on these ablative and transarterial techniques. Percutaneous ethanol injection (PEI) was introduced in the 1980s as the first effective ablative technique. Since then, the thermal systems radiofrequency (RF), microwave (MW), and cryoablation have gained wide acceptance, with the most data having been gathered for RF ablation (RFA). The newest method, though largely experimental in the treatment of HCC, is irreversible electroporation (IRE).
Transarterial therapy for HCC was first reported in the late 1970s. In the 1980s, several reports discussed the feasibility of combining embolic and chemotherapeutic agents. Two seminal randomized controlled trials in 2002 were the first to demonstrate a survival benefit in patients with unresectable HCC treated with transarterial chemoembolization. , Phase 1 and 2 clinical trials on doxorubicin eluting embolic beads in the treatment of unresectable HCC were conducted in the mid 2000s in an attempt to standardize the procedure and improve the pharmacokinetic profile of chemoembolization. During the past 10 years, nonocclusive alternatives to transarterial embolization (TAE)/transarterial chemoembolization (TACE) have received growing interest. The following pages discuss the technical details of these approaches, their indications, complications, and their relative value in the treatment of unresectable HCC.
Percutaneous ablation
The technical details of the various percutaneous ablative techniques named above can be found in Chapter 2 . The discussion here will focus on workup and preoperative imaging, data-based patient selection, complications, and postprocedural care and imaging.
Patient preparation and preoperative imaging
Either magnetic resonance imaging (MRI) or computed tomography (CT) with arterial and portal contrast phases should be obtained to identify the location, number, and size of tumors to be treated. The imaging is essential to determine whether the lesions are amenable to ablation. The case should be explicitly discussed with the referring physician or tumor board, and the patient’s disease stage should be characterized by the combination of imaging findings, laboratory results, and functional status. Blood product replacement should be performed if the international normalized ratio (INR) is >1.5 and the platelets are <50,000/mm . Aspirin and/or clopidogrel should be held at least 5 days prior to the procedure if possible. Imaging will also guide whether ultrasonography, CT, or a combination will be used to ablate the lesion(s).
Ablative techniques
Radiofrequency ablation
As stated above, most of the data on percutaneous techniques has been generated for radiofrequency ablation (RFA), so the current recommendations primarily apply to this ablative modality ( Figure 8-2 ). RFA relies on thermal heat created by an electrical current passing through the electrode embedded in the tumor. Patients who should be considered for RFA are those with very-early-stage and early-stage HCC. Very early stage is defined as having a performance status of 0, being Child-Pugh Class A, and having a single tumor <2 cm in size. The treatment options for these patients are surgical resection and ablation, and the decision is made based largely on anatomic location. Lesions that should be resected include those in a subcapsular or perivascular distribution, as ablation is occasionally unsafe and less effective in these respective locations. Presuming the patient is a good surgical candidate (normal liver function, absence of portal hypertension), the 5-year survival rate following anatomic resection is >75%. , Livraghi et al. conducted a retrospective analysis of 218 very-early-stage HCC patients treated with RFA and found an overall 55% 5-year survival rate, which climbed to 68% in patients whose initial tumor was deemed resectable, indicating a favorable comparison to surgery. Cho et al. simulated a randomized trial between resection and RFA based on previous data and concluded that RFA followed by resection (if necessary, in the event of local failure) had a nearly identical survival to primary resection. In conclusion, the current recommendations favor surgical resection in patients with very-early-stage disease; however, for nonresectable tumors, RFA offers excellent 5-year survival and spares significantly more hepatic parenchyma than anatomic resection.
Early-stage HCC patients, defined as having intact liver function (Child-Pugh Class A or B) and a solitary tumor 2–5 cm or 3 tumors <3 cm, can undergo resection, transplantation, or ablation. The decision as to which to pursue is highly patient and tumor specific. Head-to-head comparisons between RFA and resection have been conflicting, with one randomized trial demonstrating essentially no difference, and the other favoring resection. Other factors to consider are the size of the tumor; lesions greater than 3 cm are not as effectively treated at the margins by RFA. As stated above, the heat-sink effect caused by vessels adjacent to the lesion limit the effectiveness of RFA. Other technologies, including MW ablation, may overcome some of these limitations of RFA, but further studies are required for confirmation.
Contraindications include diffuse or infiltrative tumors, those with vascular invasion, and distant disease. Relative contraindications include proximity to the gallbladder and gastrointestinal viscera, and a central location. Iatrogenic cholecystitis is usually temporary. The colon is most susceptible to thermal damage, possibly because of its fixed position and thin wall. A central tumor position has a higher risk for biliary tract violation.
The estimated mortality rate is 0.1%–0.5%, with major complication rates ranging from 2% to 3%. Complications include thermal burns, intraperitoneal hemorrhage, bile duct injury, and visceral injury. Special note should be made of tumor tract seeding, estimated to have a 0.5% incidence, for which many practitioners ablate the tract itself.
Percutaneous chemical injection
PEI is the oldest of the percutaneous ablative techniques. It relies on the dispersion of ethanol within the lesion, which results in coagulation necrosis. When compared to RFA for early-stage HCC, PEI shows a less favorable survival profile. It also has been shown to have higher recurrence rates, postulated to be secondary to inhomogeneous infiltration of a lesion and the inability to create an ablation margin that would encompass satellite lesions. However, in patients with very-early-stage HCC, PEI can be effective when tumor location makes the lesion unresectable or suboptimal for RFA. It is also much less costly than the thermal ablative technologies.
Percutaneous acetic acid injection works on a similar principle to PEI, with the theoretical benefit compared to PEI of being able to dissolve intratumoral septations and achieve higher homogeneity. It compares favorably with PEI, but has not been widely adopted.
Microwave ablation
MW technology causes local excitation of water molecules to generate tissue heat via friction, and is capable of creating much higher intratumoral temperatures than RFA ( Figure 8-3 ). Moreover, MW ablation is capable of creating a larger ablation zone and is not affected by adjacent vasculature. That being said, the one randomized trial that compared MW ablation with RFA failed to demonstrate a survival benefit. However, MW technology has improved markedly since this trial, and those results may be obsolete.
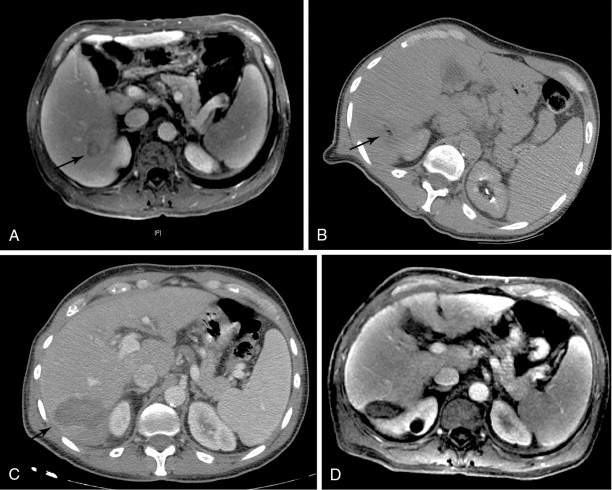
Cryoablation
Cryoablation relies on freezing temperatures to induce tumor cell death. It has the potential advantage of being able to see the developing “ice ball” as a hypodense oval during the ablation. However, it suffers from having very limited data to support its use in the setting of HCC. A 2011 study demonstrated a 60% 3-year survival in 40 patients with a 70% local control rate; however, the major complication rate was 12%. It should be noted that Child-Pugh class and tumor size were not controlled for, so the relevance of this study to early-stage HCC is questionable.
Irreversible electroporation
IRE is predicated on membrane disruption and subsequent cell death in the treated area. Its theoretical advantages are precision and nonthermal tumor kill, allowing for treatment of lesions adjacent to vasculature without injury to these vessels. No significant data are yet available to evaluate this upcoming technology in the setting of HCC.
Postprocedural management and imaging
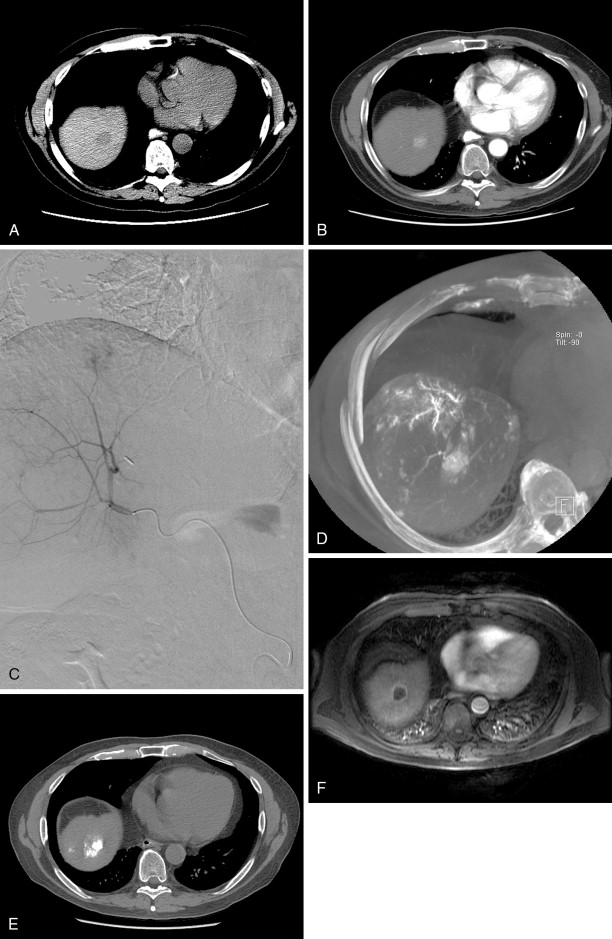
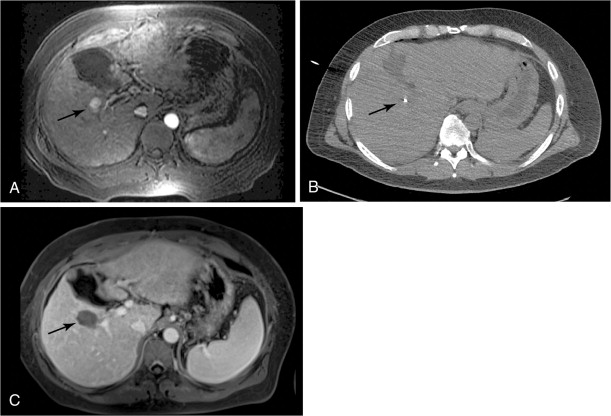
Patients should be kept in the hospital overnight for pain control and monitoring. Most patients can safely be discharged the day following the procedure. Some practices will obtain contrast-enhanced cross-sectional imaging several days after the procedure. It should be noted that a benign hyperenhancing smooth rim is often seen at this time, representing reactive hyperemia ( Figure 8-3 , C ). This appearance should not be confused with residual tumor. A 1-month postablation scan is the standard, at which time nodular or irregular enhancement can be signs of residual tumor. Repeat ablation or alternative modalities can then be considered.
Transarterial embolization and chemoembolization
Clinical indications
Table 8-1 lists the circumstances under which TACE is employed. The most common indication is for the treatment of unresectable HCC. Figure 8-1 demonstrates the Barcelona Clinic Liver Cancer (BCLC) schematic and highlights the patients who are most appropriate for transarterial therapies. As shown in the algorithm, those patients with Child-Pugh Class A or B, with unresectable intermediate-stage tumors, are the most appropriate candidates according to several society guidelines.
| Current |
|---|
| Unresectable, intermediate stage HCC, Child-Pugh class A or B * |
| Combined with percutaneous ablation |
| Bridge to transplantation |
| Downstaging for transplantation |
| Combined with surgical resection |
| FUTURE/ONGOING |
| Combined with systemic therapy |
The BCLC recommendation comes primarily from the two randomized clinical trials mentioned above that demonstrated a survival advantage for patients with unresectable HCC treated with conventional transarterial chemoembolization (cTACE) compared with best supportive care. , It should be noted that three other randomized controlled trials failed to show a survival benefit in patients treated with cTACE. Several meta-analyses that were subsequently conducted supported the role of cTACE in treating unresectable HCC. , When understanding these data, it is important to note that patient selection likely accounted for the differences between the positive and negative trials. In the positive studies, patients were less than 75 years old, did not have portal vein thrombosis, had no hepatic decompensation (ascites, encephalopathy, variceal bleeding), and had preserved liver function. Of note, the largest case series comes from Japan, in which data collected for more than 8000 patients demonstrated a mean survival of 34 months.
Several trials have examined bland embolization in the setting of unresectable HCC. Bruix et al. demonstrated no survival advantage when 80 patients were randomized to receive bland embolization; however, these patients received embolic treatment that is not considered standard of care in most institutions, including proximal deployment of steel coils. In contrast, other studies using bland embolization have shown a meaningful survival benefit. Covey et al. demonstrated 86%, 74%, and 47% survival at 1, 2, and 3 years, respectively, in patients with mostly Okuda stage I disease with recurrent HCC after resection. The largest study was conducted by Maluccio et al., who reported a median survival of 21 months and 1-, 2-, and 3-year survival rates of, respectively, 66%, 46%, and 33% overall, and 84%, 66%, and 51% when patients with portal vein invasion or extrahepatic disease were excluded.
Drug-eluting bead (DEB)-TACE has also shown efficacy in the treatment of unresectable HCC. Poon et al. conducted phase 1 and 2 clinical trials examining safety and efficacy in patients with unresectable HCC and showed that a dose of 150 mg of doxorubicin was tolerable with a response rate (partial plus complete) of 70%. Varela et al. demonstrated a 1- and 2-year survival of 92.5% and 88.9% at 1 and 2 years in patients with unresectable HCC treated with DEB-TACE. Malagari et al. reported on 71 patients with unresectable HCC and Child-Pugh Class A or B disease treated with DEB-TACE and showed an overall response rate of more than 80% and a 30-month survival of 88%. The first phase 2 trial in the United States demonstrated 1- and 2-year survival rates of 65% and 55% in 20 patients.
Transarterial therapies have also been used to improve the treatment efficacy of RFA. Limitations of RFA include higher recurrence in tumors greater than 3 cm and poor efficacy for tumors close to hepatic vessels secondary to a heat sink phenomenon. Transarterial techniques have been used in combination with RFA in both of these circumstances. Maluccio et al. compared surgical resection with combination RFA and transarterial embolization and found a trend toward improved survival in the combination group, although this finding was not statistically significant. Morimoto et al. found slower tumor progression in patients with solitary HCCs between 3 and 5 cm treated with both RFA and TACE than in those treated with RFA alone. Combination therapy may also be of value in patients with recurrent HCC after surgical resection, with lower intrahepatic recurrence compared with RFA or TACE alone.
The transplant population represents a special category of unresectable HCC patients. For patients within the Milan criteria for transplantation, transarterial methods have been used to maintain their tumors within the size and number required for transplantation. Although the survival benefit has not been firmly established, some studies have noted that a better response to transarterial therapy is associated with higher posttransplant survival. In select patients, transarterial therapy has successfully downstaged tumors so that these patients could be listed for transplant. Several studies have shown similar posttransplant survival in patients who were successfully downstaged and transplanted to those who were always within the Milan criteria before transplant, with one study demonstrating a 70% success rate in downstaging tumors and a 4-year posttransplant intention to treat survival of 69%.
Combining transarterial techniques with surgical resection has also been explored. In a prospective study randomizing patients with tumors larger than 5 cm to upfront cTACE or surgical resection, no difference in survival was detected. However, those patients who responded to cTACE and ultimately underwent surgical resection had a longer survival than those who did not respond, suggesting that transarterial therapy response may be predictive of a good outcome after resection.
Comparison of techniques
Currently, there is no consensus on which of the techniques are most efficacious in the treatment of HCC for the indications listed above. The strongest evidence supports cTACE in the treatment of intermediate unresectable HCC, as stated previously. However, this evidence is far from conclusive. The following paragraphs compare the three major techniques: cTACE versus TAE, cTACE versus DEB-TACE, and TAE versus DEB-TACE.
cTACE versus TAE
The two aforementioned randomized trials in 2002 , demonstrating a significant survival benefit of cTACE over supportive care for unresectable HCC legitimized cTACE as the embolization method of choice for this disease. However, no direct large-scale comparison between bland embolization and cTACE has been performed. The Barcelona study, which treated patients with both cTACE and bland embolization, was halted early and was not powered to detect a difference between the two embolic therapies. A meta-analysis looking at pooled data of bland and cTACE treated patients demonstrated similar 2-year survival between the groups. A retrospective analysis published in 2006 demonstrated similar survival rates at 6 months, 1 year, and 2 years to those seen with cTACE.
The underwhelming clinical evidence for cTACE over bland embolization has made some suggest that it is the embolic effect rather than the chemotherapeutic effect that predominates in transarterial tumor kill. Several lines of evidence favor this theory. First, lipiodol has embolic properties of its own. Moreover, pharmacokinetic studies have shown that lipiodol poorly “retains” chemotherapeutic drugs, and that the systemic doses from cTACE are similar to those seen with systemic infusion. Proponents of bland embolization also argue that it results in less pruning of the hepatic arterial circulation compared with cTACE because of the lack of chemotherapeutic toxicity on the endothelium. This argument has yet to be validated prospectively, however. They also cite the lack of systemic toxicity that bland embolization offers.
Overall, there is no strong evidence to recommend one embolic strategy over the other when comparing bland embolization to cTACE.
cTACE versus DEB-TACE
Drug eluting beads operate on the principle that both tumor ischemia and cytotoxicity are necessary for maximal tumor kill. They also arose out of a desire to create uniformity during embolization that is not seen with cTACE. Moreover, DEB-TACE aims to reduce some of the systemic toxicity and side effects seen with cTACE.
Several comparisons between DEB-TACE and cTACE have been made. The PRECISION V trial demonstrated a reduction in drug-related adverse events and liver toxicity in the DEB-TACE arm, as well as a trend toward higher rates of complete response, objective response, and disease control in the DEB-TACE arm, and a statistically significant improvement in tumor response in patients with more advanced disease. However, no survival advantage was seen between the 2 groups.
Larger multicenter randomized controlled trials are needed to convincingly support the use of one technique over the other.
DEB versus bland embolization
The crux of this comparison is whether the addition of drug elution from similar-sized beads results in better tumor response and overall survival. There are relatively few trials, however, that have looked at this question. Malagari et al. compared 84 patients with HCC who received either bland embolization or DEB-TACE, and found a longer time to progression in the DEB-TACE arm and higher recurrence rate in the bland embolization arm. Limited head-to-head survival data have been collected.
The question as to which of these three techniques is superior has yet to be answered. Randomized trials comparing standard methodologies for each are underway and will potentially offer some clarity.
Patient preparation and preoperative imaging
Relevant cross-sectional imaging, either magnetic resonance or computed tomography with arterial and portal contrast phases, should be reviewed to delineate the location of tumors and the extent of tumor burden. Moreover, the cross-sectional imaging will determine patency of the portal vein, the presence of extrahepatic disease, and arterial anatomy. The indication for embolization, whether it is to palliate or bridge to transplantation, should be explicitly discussed with the referring physician or tumor board. Important laboratory values to obtain or review include liver function tests (esp. serum bilirubin), serum creatinine, a complete blood count (including hemoglobin and platelet counts), and coagulation parameters (i.e., INR). Although practices vary, if the INR is >1.5 and the platelets are <50,000/mm , blood products should be administered before the procedure. If the patient has a history of biliary tree manipulation or ductal dilation, there is a higher risk of postembolization liver abscess formation, and prophylactic antibiotics should be administered preprocedurally. A history of contrast allergy should prompt appropriate premedication to prevent a contrast reaction during the procedure.
The patient’s medication history should also be carefully reviewed. If possible, the patient should be off any antiplatelet agent (aspirin, clopidogrel) for 5 days before the procedure to avoid complications of arterial puncture.
Contraindications to transarterial therapy are listed in Table 8-2 . A relative contraindication is portal vein thrombosis. However, Georgiades et al. demonstrated that cTACE could safely be performed in patients with portal vein thrombosis. Other contraindications are related to poor hepatic function and decompensation, including Child-Pugh Class C disease, elevated bilirubin, and depressed serum albumin. No definite cutoff can be cited, although many practitioners become wary above a bilirubin of 2.0 mg/dL. Tumor biology also clearly affects the outcome of patients treated with transarterial therapies, with tumors >5 cm, tumor involving >50% of the liver, bilobar disease, more than three nodules, and elevated α-fetoprotein (AFP) conferring worse outcomes. ,
| ADVANCED LIVER DISEASE |
|---|
| Child Pugh Class C |
| Moderate-severe ascites |
| Encephalopathy |
| Active gastrointestinal bleeding |
| Bilirubin >2 * |
| Hypoalbuminemia (<2.5 mg/dL) * |
| Active transaminitis |
| Performance status >2 |
| SYSTEMIC PARAMETERS |
| Neutropenia † |
| Cardiomyopathy † |
| Renal insufficiency |
* Relative contraindication; different practices have different thresholds
† Potentially exacerbated by doxorubicin administration, n/a for TAE
Procedural technique
After access is gained most commonly via the common femoral artery, abdominal aortography is performed to determine the arterial anatomy. This step can be omitted at the discretion of the interventionalist if the procedure is a repeat embolization; however, given that tumors parasitize the vasculature, if a significant time has elapsed between treatments, repeat aortography should be considered. Aortography will uncover the origins of the celiac artery and superior mesenteric artery (SMA), determine whether hepatic arterial anatomy is conventional or aberrant, and identify other potentially significant branches such as the left gastric artery, intercostal arteries, or the inferior phrenic arteries. Moreover, stenoses can be either visualized or inferred based on direction of flow (e.g., reversal of flow in the gastroduodenal artery implies a celiac stenosis). Third, the angle of the origin and the course of the celiac artery or the SMA can be identified to guide catheter choice.
The celiac artery can be engaged with a number of 4- or 5-French catheters. The cobra, Sos, or Simmons catheters are used most commonly. Assuming conventional anatomy, the catheter is advanced into the common hepatic artery, where digital subtraction angiography (DSA) in multiple planes can be performed to delineate relevant anatomy. Contrast injections of approximately 3–5 mL/s, for a total volume of 15–20 mL, can determine the course of the hepatic arterial branches and the supply to hypervascular hepatomas. Alternatively, if available, contrast-enhanced cone-beam computed tomography (CBCT) could be employed to derive similar information, with three-dimensional postprocessing of the acquired images adding powerful anatomical insights. A recent publication demonstrated that CBCT did not significantly add to the radiation exposure during a case and could adequately substitute for multiple DSA runs. During these contrast injections, careful attention should be paid to arteriovenous shunting, given that particles may pass through these shunts and embolize to the lung. Additionally, extrahepatic branches to bowel should be recognized so that they may be either bypassed or prophylactically coil embolized.
Once the number and distribution of the tumors are identified, an embolization strategy can take shape. An important factor to consider is the patient’s liver function. If the serum bilirubin is >2, a superselective approach is most prudent to minimize further damage to uninvolved hepatocytes. This superselective approach also has the advantage of concentrating the dose of the agent to maximize tumoricidal effects. However, if multiple tumors are present, several territories feed a single tumor, and/or if the patient’s liver function is good, a less selective approach may be attempted.
The combination of a microcatheter and microwire is then used to select the branches to be embolized. If conventional TACE is chosen ( Figure 8-4 ), then a mixture of the chemotherapeutic agent and lipiodol, typically in a 1:1 or 2:1 ratio, is given until one of several endpoints, including 2–5 beat stasis or portal venous staining, is observed. It should be noted that there is marked worldwide variation in the cytotoxic agents used, ranging from single-agent doxorubicin to combination infusion of doxorubicin, mitomycin C, and cisplatin. The practitioner can elect to give Gelfoam or embolization particles after the infusion of the lipiodol/chemotherapeutic drug, although the embolic effects of lipiodol may be sufficient to make this second step unnecessary. If DEB-TACE is selected ( Figure 8-5 ), the doxorubicin-laden beads are mixed with a saline-to-contrast ratio of 1:1 and infused until the same endpoints are observed. Administration should be reasonably slow, as slow as 1 mL/min. There is no definitive data supporting the use of a particular bead size currently, although studies are ongoing. A trend toward smaller beads (100–300 μm) can be attributed to the ability to create a more distal embolization. Care should be taken to avoid reflux to prevent nontarget embolization. The amount of drug used is variable, but ranges from 50 to 150 mg of doxorubicin. If bland embolization ( Figure 8-6 ) is selected, 40–500-μm particles can be used with static endpoints at the discretion of the practitioner.