Innate Immunity
Introduction
Every living organism is confronted by continual intrusions from its environment. Our immune systems are equipped with a network of mechanisms to safeguard us from infectious microorganisms that would otherwise take advantage of our bodies for their own survival. In short, the immune system has evolved as a surveillance system poised to initiate and maintain protective responses against virtually any harmful foreign element we might encounter. These defenses range from physical and chemical barriers—elements of innate immunity—to highly sophisticated systems that constitute the adaptive immune system. Here, we describe the principle elements of innate immunity, which is a primordial immune defense system that is present from birth. The major role of this host defense system is to provide a rapid, first line of defense against pathogens. We will discuss the participating organs, cells, and molecular components of innate immunity and their physiological roles that, in many cases, include dynamic interactions with elements of the adaptive immune system. Thus, innate immune responses are important not only because they are an independent arm of the immune system but also because they profoundly influence the nature of adaptive immune responses.
Physical and Chemical Barriers of Innate Immunity
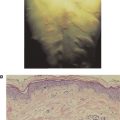
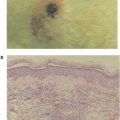
Most organisms and foreign substances cannot penetrate intact skin but can enter the body if the skin is damaged. Some microorganisms can enter through sebaceous glands and hair follicles. However, the acid pH of sweat and sebaceous secretions and the presence of various fatty acids and hydrolytic enzymes (e.g., lysozymes) all have some antimicrobial effects, therefore minimizing the importance of this route of infection. In addition, soluble proteins, including the interferons (see Chapter 12) and certain members of the complement system (see Chapter 14) found in the serum, contribute to nonspecific immunity. Interferons are a group of proteins made by cells in response to viral infection, which essentially induces a generalized antiviral state in surrounding cells. Activation of complement components in response to certain microorganisms results in a controlled enzymatic cascade, which targets the membrane of pathogenic organisms and leads to their destruction. An important innate immune mechanism involved in the protection of many areas of the body, including the respiratory and gastrointestinal tracts, involves the simple fact that surfaces in these areas are covered with mucous. In these areas, the mucous membrane barrier traps microorganisms, which are then swept away by ciliated epithelial cells toward the external openings. The hairs in the nostrils and the cough reflex are also helpful in preventing organisms from infecting the respiratory tract. The elimination of microorganisms from the respiratory tract is aided by pulmonary or alveolar macrophages, which, as we shall see later, are phagocytic cells able to engulf and destroy some microorganisms. Similarly, phagocytic cells called microglial cells provide innate immune defense within the central nervous system. Microorganisms that have penetrated the mucous membrane barrier can be phagocytized by macrophages or otherwise transported to lymph nodes, where many are destroyed. The environment of the gastrointestinal tract is made hostile to many microorganisms by other innate mechanisms, including the hydrolytic enzymes in saliva, the low pH of the stomach, and the proteolytic enzymes and bile in the small intestine. The low pH of the vagina serves a similar function.
Origin, Differentiation, and Characterization of Cells of the Innate Immune System
Once an invading microorganism has penetrated the various physical and chemical barriers, the next line of defense consists of various specialized cells whose purpose is to destroy the invader. These include the polymorphonuclear leukocytes (white blood cells containing a segmented lobular nucleus and include eosinophils, basophils, and neutrophils), monocytes, and macrophages, each of which is derived from hematopoietic precursor cells. The developmental pathways of each of the hematopoietic cells are shown in Figure 2.1. Cells of the innate immune system derive from myeloid precursors whereas cells associated with the adaptive immune system are derived from common lymphoid precursors.
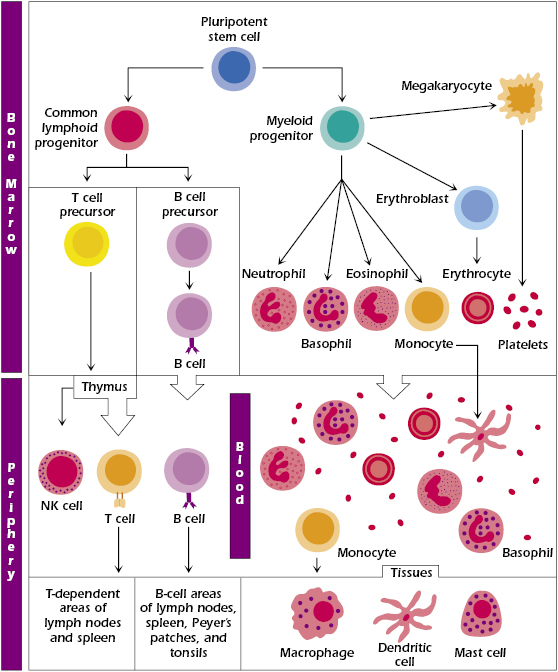
The immune system has evolved to exploit virtually each of the hematopoietic cell populations. As we have already pointed out, it is convenient to discuss the major arms of the immune system beginning with elements of the innate immune system followed by the adaptive immune system. But it is important to underscore the interrelationship of these two arms of our immune system. Clearly, they are interrelated developmentally due to their common hematopoietic precursor, the pluripotential stem cell. A classic example of their functional interrelationship is illustrated by the roles played by innate immune cells involved in antigen presentation. These so-called antigen-presenting cells (APCs) do just what their name implies: they present antigens (e.g., pieces of phagocytized bacteria) to T cells within the adaptive immune system. As will be discussed in great detail in subsequent chapters, T cells must interact with APCs that display antigens for which they are specific in order for the T cells to be activated to generate antigen-specific responses. Thus, while the title of this section implies that the cells described below are principally involved in innate immune responses, it is important to recognize their important role in adaptive immune responses (Chapter 3) at this early stage of study of the immune system.
Polymorphonuclear Leukocytes.
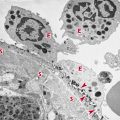
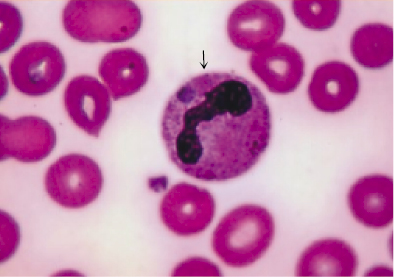
Polymorphonuclear (PMN) leukocytes are a population of cells also referred to as granulocytes. These include the basophils, eosinophils, and neutrophils. Granulocytes are short-lived phagocytic cells that contain the enzyme-rich lysosomes, which can facilitate destruction of infectious microorganisms (Figure 2.2). They also produce peroxide, superoxide radicals, and nitric oxide, which are toxic to many microorganisms. Some lysosomes also contain bactericidal proteins, such as lactoferrin. PMN leukocytes play a major role in protection against infection. Defects in PMN cell function are accompanied by chronic or recurrent infection.
Macrophages.
Macrophages are phagocytes derived from blood monocytes (Figure 2.3). The monocyte itself is a small, spherical cell with few projections, abundant cytoplasm, little endoplasmic reticulum, and many granules. Following migration of monocytes from the blood to various tissues, they undergo further differentiation into a variety of histologic forms, all of which play a role in phagocytosis, including the following:
- Kupffer cells, in the liver; large cells with many cytoplasmic projections
- Alveolar macrophages, in the lung
- Splenic macrophages, in the red pulp
- Peritoneal macrophages, free-floating in peritoneal fluid
- Microglial cells, in the central nervous tissue
Each of these macrophage populations constitutes part of the cellular members of the reticuloendothelial system (RES), which is widely distributed throughout the body. The major function of the RES is to phagocytize microorganisms and foreign substances that are in the bloodstream and in various tissues. The RES also functions in the destruction of aged and imperfect cells, such as erythrocytes.
Although associated with diverse names and locations, many of these cells share common features, such as the ability to bind and engulf particulate materials and antigens. Because of their location along capillaries, these cells are most likely to make first contact with invading pathogens and antigens and, as we shall see later, play a large part in the success of innate as well as adaptive immunity (also called acquired immunity).
In general, cells of the macrophage series have two major functions. One, as their name (“large eater”) implies, is to engulf and, with the aid of all the degradative enzymes in their lysosomal granules, break down trapped materials into simple amino acids, sugars, and other substances for excretion or reuse. Thus these cells play a key role in the removal of bacteria and parasites from the body. As noted above and discussed in detail in later chapters, the second major function of the macrophages is to take up antigens, process them by denaturation or partial digestion, and present them, on their surfaces, to antigen-specific T cells (i.e., the process of antigen presentation).
Dendritic Cells.
Dendritic cells (DCs) are critically important members of the innate immune system due to their highly efficient APC properties that enable them to trigger adaptive immune responses carried out by T cells (see Chapter 10). Like other innate immune cells, they recognize and phagocytize pathogens and other antigens but their ability to present antigens to T cells far exceeds that of other APCs. They are found as migrating dendritic cells in the blood, nonmigratory follicular dendritic cells (fDCs) in primary and secondary follicles of the B cell areas of lymph nodes and spleen (see Chapter 3), interdigitating cells of the thymus, and Langerhans cells in the skin. Another type of dendritic cell is the plasmacystoid DC (pDC). Unlike other DC subpopulations that are derived from myeloid precursor cells, pDCs are derived from lymphoid precursors. Like all DCs, pDCs display antigen-presenting function, but they are distinguished by their ability to produce large amounts of alpha/beta interferons (IFN-α/β) in response to viral and bacterial stimuli (see Chapter 12).
Natural Killer Cells.
Altered features of the membranes of abnormal cells, such as those found on virus-infected or cancer cells, are recognized by natural killer (NK) cells, which are cytotoxic. NK cells probably play a role in the early stages of viral infection or tumorogenesis, before the large numbers of activated cytotoxic T lymphocytes are generated. Histologically, NK cells are large granular lymphocytes. The intracellular granules contain preformed biologically potent molecules that are released when NK cells make contact with target cells. Some of these molecules cause the formation of pores in the membrane of the target cell, leading to its lysis. Other molecules enter the target cell and cause apoptosis (programmed cell death) of the target cell by enhanced fragmentation of its nuclear DNA. Hence, they are able to lyse certain virus-infected cells and tumor cells without prior stimulation.
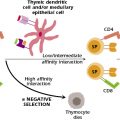
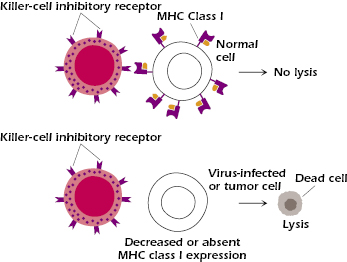
Unlike cytotoxic T lymphocytes, which recognize target cells in an antigen-specific fashion due to their expression of T cell receptors (TCRs), NK cells lack antigen-specific receptors. How, then, do they seek and destroy their targets? They do this by using a mechanism involving cell–cell contact, which allows them to determine whether a potential target cell has lost a particular self-antigen, namely, major histocompatibility complex (MHC) class I. MHC class I is expressed on virtually all nucleated cells. NK cells express receptors called killer-cell inhibitory receptors (KIR), which bind to MHC class I molecules expressed on normal cells. When ligated, KIRs inhibit downstream events that would otherwise cause the NK cell to be activated, causing degranulation and destruction of the target cells. Virus-infected or transformed (tumor) cells have significantly reduced numbers of MHC class I molecules on their surfaces. Thus, when such cells encounter NK cells, they fail to effectively engage KIRs and therefore become susceptible to NK cell-mediated cytotoxicity (Figure 2.4).
Natural Killer T Cells.
Recognized more than a decade ago, natural killer T (NKT) cells differentiate from thymic precursors through signals emanating from cortical thymocytes during TCR engagement. Like other T cells, these cells express TCRs, although with restricted variability. Their semi-invariant TCRs recognize a mammalian glycosphingolipid (isoglobotrihexosylceramide), as well as microbial α-glycuronylceramides found in the cell wall of Gram-negative, lipopolysaccharide (LPS)-negative bacteria. NKT cells are unique in terms of their functional status because they fall somewhere between the innate and the adaptive immune systems. Following activation, they secrete several regulatory cytokines, including interleukin-4 (IL-4) and interferon-γ and kill target cells via Fas–Fas ligand interactions that cause apoptosis (Chapter 12). NKT cells also regulate a range of immunopathological conditions, but the mechanisms and the ligands involved remain unknown.
Innate Lymphoid Cells.
Innate lymphoid cells (ILCs) constitute a heterogeneous family of innate immune cells that have been shown to aid in protective immunity at the acute phase of infections, tissue remodeling, anatomical containment of commensals microorganisms (e.g., microbiota that inhabit our gastrointestinal tract in a symbiotic relationship in which one species is benefited while the other is unaffected), wound healing, and in maintaining the epithelial integrity at mucosal sites. ILCs have also been associated with pathophysiological conditions, such as airway and gut inflammation. ILCs, which include a population called lymphoid tissue-inducer (LTi) cells, share a lymphoid morphology, are dependent on the interleukin-2 receptor γ (IL-2Rγ) chain (discussed in Chapter 12) for their development but lack the rearranged antigen receptors or markers that are expressed on cells of myeloid origin or cells of the adaptive immune system discussed in Chapter 3. Subsets of ILCs express cell-surface molecules that were previously thought to be restricted to NK cells. However, NK cells are distinct from the more recently discovered ILCs and can be divided into ILC subsets that are highly diverse with respect to their capacity of producing cytokines. This diversity matches that of T cells, and this has led to the hypotheses that ILCs act in early stages of the immune response against infectious microorganisms when the adaptive response is not yet operational.
From this brief outline, it can be seen that each of these cellular components of the innate immune system has diverse roles that serve the host’s initial attempt to eliminate foreign substances and pathogens to the generation of antigen-specific adaptive immune responses that ultimately give rise to long-term immunity. Finally, as producers of an array of cytokines, soluble mediators of immune responses (see Chapter 12), these cells influence the functional properties of many other cell types within the immune system. For example, they can enhance the phagocytic activity of macrophages to increase their killing of pathogens, as well as the cytotoxic effects of NK cells. Thus, innate immune cells are pivotal players in strategies employed by the immune system to ensure protection of the host against infectious microorganisms. They are also called into play whenever physical barriers of defense are compromised (e.g., skin wounds). In either case, mobilization of innate immune cells following injury or infection generates a physiologic response known as inflammation. This is discussed in more detail in the section that follows.
Pattern Recognition: The Hallmark of Innate Immune Responses
Now that we have outlined the origins and major characteristics of innate immune cells, we will discuss the sophisticated yet elegantly simple ways in which these cells initiate their host defense roles: pattern recognition. The underlying host defense mechanism associated with this arm of the immune system is the ability of innate cells and specific soluble mediators they produce to recognize and respond to evolutionarily conserved microbial structures termed pathogen-associated molecular patterns (PAMPs). Detection of PAMPs by innate immune cells occurs via soluble and cell-associated germline-encoded pattern recognition receptors (PRRs). It is important to note that this feature of innate immune recognition of pathogens differs markedly from recognition mechanisms associated with the adaptive immune system as illustrated in Figure 2.5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree