Abstract
Inflammatory breast cancer (IBC) causes 7% of breast cancer deaths. The diagnosis is clinical (erythema, edema, peau d’orange involving at least one-third of the breast surface area). IBC is frequently diagnosed months after presentation. Imaging may not reveal a mass but thickening of the skin is frequently seen. Biopsy confirmation is mandatory, but dermal lymphatic invasion is seen only in approximately 60% of IBC cases. The onset is approximately 10 years earlier compared with non-IBC, and there are differences in the incidence and outcome across ethnicities. There is a higher prevalence of triple-negative and HER2+ subtypes; about 55% of IBC is hormone receptor positive (HR+) versus the predominantly HR+ non-IBCs. Areas of investigation to characterize IBC include assessment of stromal makeup, angio- and lymphangiogenesis, a variety of cytokines and chemokines, and stem cell and EMT compartments as well as specific pathways. Additional avenues being explored include immunotherapy. Trimodality therapy consisting of neoadjuvant chemotherapy, modified radical mastectomy, and local regional radiation treatment has improved outcome, but the median survival at 5 years for patients presenting with primary IBC is still only approximately 55%. Neoadjuvant therapy is tailored and sequentially upgraded in ongoing trials for phenotype-specific treatment efficacy (triple-negative vs. HER2+), with pathologic complete response as a surrogate being evaluated as a predictor of survival. For selected cases presenting with stage IV disease, there is a role for surgical intervention and radiation therapy to delay progression and to provide palliative therapy to the primary site. Improved systemic treatment options based on our evolving understanding of the biology (including learning more about metabolomics in the context of IBC), together with optimizing surgery and radiation treatment field and intensity, and optimization of adjuvant therapies will undoubtedly lead to better outcome.
Keywords
inflammatory breast cancer, epidemiology, neoadjuvant therapy, trimodality therapy, survival
Introduction and Historical Backdrop
Inflammatory breast cancer (IBC) was first described in 1814 by Sir Charles Bell as a “purple color on the skin over the tumor accompanied by shooting pains.” Thomas Bryant described the dermal lymphatic invasion as the cause for the inflammatory changes of the skin in 1887. IBC was referred to as mastitis carcinomatosa, carcinoma mastoids, and acute carcinoma of the breast before arriving at its current identifier inflammatory breast cancer coined by Lee and Tannenbaum in 1924. Although the clinical presentation is what remains the cornerstone of diagnosis, what truly defines this disease as a distinct clinicopathologic entity is much more diverse and includes the explosive clinical course, a still not well-defined molecular profile, its response to current therapies, and ultimately its prognosis. In this chapter, we explore the characteristics unique to IBC and ways to capitalize on what we know about this disease to optimize treatment.
Epidemiology
IBC comprises 2.5% of all breast cancers and 7% of all breast cancer–related deaths in the United States. It is estimated that 6231 people will be diagnosed with IBC this year alone and 2862 will die of the disease. The incidence of IBC has been reported to be higher in other countries, ranging from 5% in a series from Turkey to 17% of breast cancers diagnosed in a teaching hospital in Nigeria. With a stricter definition of clinical criteria (at least two of the three features of erythema, edema, and peau d’orange) the true prevalence in regions such as North Africa (in Tunisia in particular), are likely to be lower than previously reported, at 5% to 7% versus more than 50%. The mortality from IBC has decreased significantly over the past 2 decades with the 2-year disease free survival ranging from 62% for women diagnosed between 1990 and 1995, to 76% for women diagnosed between 2006 and 2010; but the median survival with IBC in that time period was only 2.9 years and remained inferior in comparison to 6.4 years for women presenting with locally advanced breast cancer. The improvements for women with IBC are largely due to advances in chemotherapy; developments in targeted therapy, primarily human epidermal growth factor (HER2); targeted therapy; and the general acceptance of trimodality (systemic therapy, surgery, and radiation) therapy. Rueth and coworkers showed 5-year survival to be 55% in nonmetastatic IBC patients treated with trimodality therapy compared with 42.9% in those treated with chemotherapy and surgery and 40.7% in those treated with surgery and radiotherapy only ( Fig. 64.1 ). At the time of initial diagnosis, however, more than 30% of patients have metastatic disease, most of whom will succumb to their disease. Despite all improvements, the median overall survival for newly diagnosed IBC is less than 4 years, and 5-year survival rate is approximately 30%, although in previous decades IBC was considered an overwhelmingly fatal disease.

Differences exist among races in the incidence and survival rates in IBC. It is more prevalent in African American, Hispanic American/Latina, and American Indian/Alaskan women than in Caucasian and Asian women. IBC has an earlier age of onset than noninflammatory breast cancer (non-IBC) with the majority of women being diagnosed between the ages of 40 and 59 years of age. The age of onset is earliest in Hispanic American/Latina women with a mean age at diagnosis of 50.5 years compared with 55.2 in African American women and 58.1 in Caucasian women. The median survival for Caucasian women is 47.6 months compared with 33.2 months in African American women and 43.1 months in Hispanic American women. Asian American women have the highest median survival of 49.7 months. The lowest median survival is found in American Indian/Alaskan women at 24.8 months.
Besides race, other predictors of poor prognosis exist, including being single, having grade 3 tumors, having a higher stage at diagnosis, not receiving radiation treatment, undergoing a partial mastectomy, and obesity. Higher education seems to have been associated with lower incidence of estrogen receptor (ER) and progesterone receptor (PR) positive IBC. Older age at pregnancy has not been consistently linked to IBC in hormone receptor (HR)-negative breast cancer; however, many series show a higher proportion of IBC among pregnant and lactating women. Additionally, premenopausal status has not been linked to IBC despite it being diagnosed more often in younger women than non-IBC.
Diagnosis
Clinical Presentation
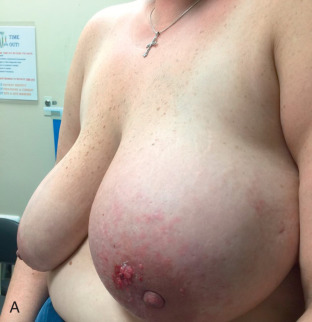
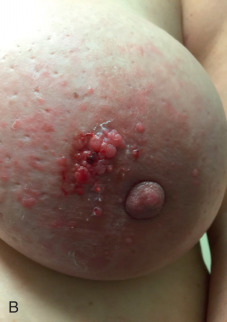
There are currently no known molecular or pathologic characteristics for a definitive diagnosis of IBC, and as such, IBC remains a clinical diagnosis. The current diagnostic definition as put forth by the American Joint Committee on Cancer is “a clinical-pathologic entity characterized by diffuse erythema and edema (peau d’orange) involving a third or more of the breast.” Physical examination may also reveal skin thickening and occasionally an underlying mass, although not consistently. IBC is categorized by the American Joint Committee on Cancer as a cT4d tumor and qualifies as at least a clinical stage III tumor. This definition is based primarily on the description put forth by Haagensen in 1971. Patients with IBC complain of an abrupt onset of symptoms including pain, swelling, and redness of the breast that typically develops in less than 3 months ( Fig. 64.2 ). The differential diagnosis includes mastitis with or without abscess, radiation change, locally advanced breast cancer, and primary breast lymphoma or other malignancies. Tissue diagnosis is necessary to document invasive carcinoma. History and physical examination are paramount in distinguishing this diagnosis from clinical mimickers. As a practical matter, because most IBC cases are first seen by health care providers not necessarily familiar with IBC, the absence of complete response to a trial of antibiotic therapy should heighten the clinician’s suspicion of IBC and prompt further investigation.
Imaging
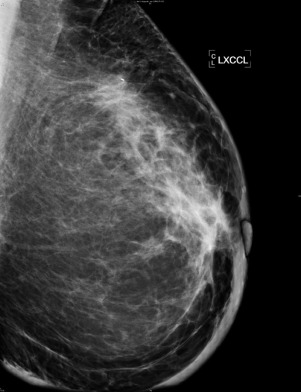
Diagnostic techniques used in IBC include mammography, ultrasonography, magnetic resonance imaging (MRI), and positron emission tomography with computed tomography (PET/CT). A retrospective review of 142 women diagnosed with IBC showed common findings seen on each modality. The most common signs on mammography include thickening of the skin (84%), trabecular thickening (81%), asymmetric focal density (61%), and microcalcifications (56%) ( Fig. 64.3 ). The increase in size of the skin thickness and trabecular thickening are often detected only compared with the contralateral side. Other findings were nipple retraction and axillary lymphadenopathy. An associated breast mass was identified only 16% of the time on mammography, and this is believed to be due to the increased breast density obscuring detection of a mass. Mammography is the least sensitive diagnostic tool available for IBC.
Ultrasound is more reliable than mammography in detecting IBC. It can demonstrate skin thickening more than 90% of the time. Detection of a mass was higher on ultrasonography (80%) than on mammogram, and it often appears as an irregular, hypoechoic mass with poorly defined margins. Even patients without a defined mass have extensive areas of parenchymal distortion seen on ultrasonography, which can be biopsied to make the diagnosis. Axillary, supraclavicular, and infraclavicular adenopathy is also detected on ultrasound in the majority of cases (93%, 50%, and 50% respectively).
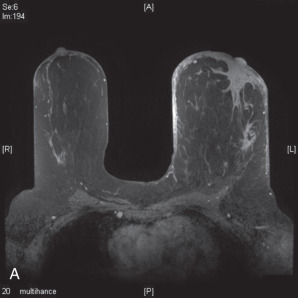
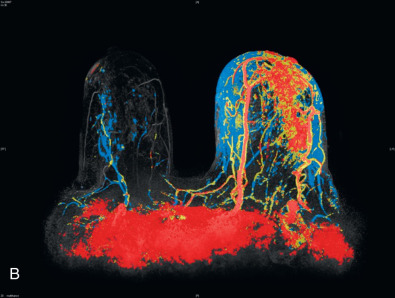
MRI can also show skin thickening and is more sensitive than mammography in detecting an underlying mass. Skin enhancement is also a common finding on MRI. In recent series, skin thickening and skin enhancement incidence ranged from 90% to100%. A series published by Yang and coworkers showed a primary breast lesion was shown in every MRI obtained as either nonmass enhancement or masslike enhancement ( Fig. 64.4 ). Axillary involvement was seen in the majority of cases as well (80%). For these reasons, MRI can be helpful in guiding core biopsies to establish the diagnosis. However, MRI does have limitations, including its decreased specificity. It cannot reliably distinguish between IBC and other inflammatory conditions of the breast, such as mastitis. Furthermore, the small size of the enclosure and duration of examination make it intolerable for some patients.
PET/CT is emerging as an imaging modality for IBC. Yang and coworkers reported that PET/CT documented multicentric disease in 63% of patients, nodal disease in 88% of patients and distant metastasis with its treatment-driving implications in 38% of patients. It can also detect dermal lymphatic invasion with prominent skin uptake. The major limitation of PET/CT is its cost compared with other imaging modalities.
Pathology
Although the diagnosis of IBC is clinical, biopsy to confirm presence of invasive disease is mandatory, and image-assisted biopsy of the primary mass is needed. Although not necessary for the diagnosis of IBC, tumor infiltration (rather than the presence of tumor emboli) of the dermal lymphatics supports the diagnosis, and skin punch biopsy may be useful. Biological markers, such as ER and PR receptors (HRs), and HER2 status need to be determined. Because of the extensive and explosive nature of IBC, currently available multigene panel assays are not considered specifically useful.
The majority of IBC are ductal in nature. They also often have a high histologic grade and are more likely HR negative. Only 44% of IBC were ER positive compared with locally advanced breast cancer (LABC), which had an ER-positive rate of 64%. PR positivity was 30% in IBC versus 51% in LABC. More recent reviews reveal similar findings with IBC having ER-positive 56% and PR positive 45% compared with 67% ER positive and PR positive 54% in LABC, with higher prevalence of lymphovascular space invasion noted as well. Among HR-positive IBCs, luminal A subtype occurs less frequently than in non-IBC.
Preclinical data do suggest alternative options for activating the estrogen signaling pathway in the absence of ER expression or alternative ER expressions such as the presence of ER α splice variants. One potential alternative pathway marker is GPR30, member of the G-protein-coupled receptor family implicated in bypassing the ER response and activating p-ERK 1/2. There are ongoing attempts to better target variants/mutants of the ER receptor, and its downstream pathways. As expected, ER positivity has been linked to better survival rates with a median survival of 4 years in those with ER-positive tumors compared with 2 years in ER-negative tumors.
HER2 positivity is found in 36% to 50% of IBC cases. Although HER2-positive tumors in general are associated with more aggressive disease, HER overexpression has not been linked to a difference in outcome compared with HER2-negative or non-IBC HER2-positive tumors. On the other hand, epidermal growth factor receptor (EGFR) is overexpressed in approximately 30% of patients with IBC and is associated with poor survival. Preclinical data suggest that the tumor microenvironment, specifically mesenchymal stromal cells may play a role increasing the expression of the EGFR receptors on the primary IBC cells and p-EGFR staining correlates between primary and stromal cells in patient samples. In xenograft experiments, and EGRF tyrosine kinase inhibitors do inhibit IBC metastasis.
There seems to be a higher prevalence in epithelial cadherin (E-cadherin) expression and cancer stem cell markers characterized by CD44+/CD24 low expression in IBC, which may explain some of the differentiating clinical features in comparison to non-IBC. Molecular drivers may also include the PI3K/AKT/mTOR and IL6/JAK2/STAT3 pathways, the latter of which is also involved in the proliferation of CD44+/CD24− breast cancer stem cells.
Although their specific role in the pathophysiology is unclear (i.e., cause-effect or bystander), an increased fraction of proliferating endothelial cells and lymph endothelial cells with overexpression of angiogenesis-related ( KDR, bFGF, NAG1, TEI1, and TIE2 ) and lymphangiogenesis-related (VEGF-C, VEGF-D, FLT-4) genes has been described. More recently, interleukin 8 and its receptors CXCR1 and 2 have been found to regulate breast cancer stem cell activity, and their role, as well as that of other cytokines and their interactions with stem cell regulators, may bear further investigation in IBC.
In one series, increased PDL1 expression was seen in 38% of 112 IBC samples out of the total of locally advanced 306 tumors tested. Comparatively, only 26% of non-IBC tested positive by messenger RNA assessment. PDL1 expression was a positive predictor for chemotherapy response in patients with IBC and was associated with a higher percentage of lymphocyte infiltration is such patients. There was greater degree of PDL1 expression seen in basal triple-negative and HER2-positive subtypes compared within ER-positive IBC.
Genomic markers including mutations in the p53 gene are also common in IBC and p53 mutations have been linked to worse clinical outcomes even compared with matched subtypes. Loss of WNT-inducible signaling protein 3 (WISP3) and the associated RHoC GTPase overexpression potentially acting as a transforming oncogene is also significantly more prevalent in IBC versus non-IBC.
Current studies are directed toward identifying further molecular biomarkers specific to IBC that will aid in targeting therapy. A 79-gene signature developed for IBC and used by the World IBC Consortium was tested in IBC versus non-IBC and was able to distinguish IBC, but when assessed against The Cancer Genome Atlas (TCGA) database the panel identified IBC-like signatures in 25% of the TCGA breast cancer set, hence the degree of specificity is unclear. Genomic profiling of 53 IBC specimens was carried out to identify responses to targeted therapies and demonstrated that 96% of the specimens had a genomic alteration with TP53 being the most common (62%). Targetable mutations ( FGFR1, BRCA2, and PTEN among others) have been noted in 51 of 53 IBC samples. Further understanding of the genomic profile of IBC may lead to better targeted therapies.
Medical Management and Trials
The main indication of neoadjuvant therapy is to downsize the primary tumor for breast preservation, but in the setting of IBC, the role of such therapy is to allow for the feasibility of modified radical mastectomy without leaving positive margins behind, and in parallel, to avoid development of systemic metastases in patients presenting with such aggressive disease. Locoregional management alone is technically insufficient because positive margins are the likely outcome, followed by local recurrence. Hence, trimodality therapy is the standard of care for primary IBC. Pathologic complete response (pCR) has become a US Food and Drug Administration–accepted surrogate marker allowing for incorporation of new agents into the neoadjuvant sequence. Increasing the rate of pCR as a potential surrogate of better relapse-free and overall survival has been the accepted paradigm in sequential series of neoadjuvant regimens.
Over the past several decades, anthracycline-based, and more recently taxane-containing neoadjuvant regimens have become standard choices for IBC, aligned along the evolution of neoadjuvant treatments for non-IBC cases. Prospective, randomized long-term data are not feasible in IBC alone, due to the rarity of the disease. However, in a large retrospective study, 15-year survival was the greatest (44%) in patients who accomplished pCR, versus 31% for those in partial response and 7% in those with less than partial response. Pathologic complete response in the axilla is a predictor of better 10-year survival of 44% versus 25.4% for patients with residual disease in the axilla after neoadjuvant therapy. The pCR rate after anthracycline-based chemotherapy is between 15% and 30%. More recent trials of dose-dense versus conventional administration of anthracycline- and taxane-containing regimens included IBC. In 100 IBC patients enrolled in a prospective neoadjuvant randomized trial post hoc analysis revealed that dose-dense neoadjuvant therapy did not provide significant advantage for pCR (12% vs. 10%, p = nonsignificant), or for disease-free or overall survival at a median follow-up of 69 months, in contrast to 567 patients with non-IBC, who benefited from dose-dense therapy. Disease-free survival was 45% and overall survival was 59% with dose-dense versus conventional neoadjuvant therapy at the median follow-up. In a trial comparing weekly versus conventional dosing of doxorubicin and cyclophosphamide followed by taxane-, pCR was trending in IBC in favor of the continuous arm, but 5-year projected disease-free survival was inferior for IBC at around 45%.
There is a higher reported percentage of HER2-overexpressing subtype among patients with IBC allowing for inclusion of HER2- targeting agents in the neoadjuvant regimen. In the HER2 setting, subset analysis of trastuzumab-containing neoadjuvant trials has shown a gradual increase in pCR rates in IBC as well as locally advanced breast cancer. Updated analysis of the randomized prospective NOAH trial testing the efficacy of trastuzumab in the neoadjuvant setting with a total of 10 cycles of chemotherapy with sequential anthracycline, taxane, and cyclophosphamide, methotrexate, and 5-fluorouracil, confirmed that pCR, particularly in HER2-positive locally advanced, and even more profoundly in IBC, is increased (36% pCR) versus chemotherapy alone. With trastuzumab then given in the adjuvant setting as well, the outcome at a median follow-up of 5.4 years was a 62% event-free survival in IBC (47 patients representing 20% of the patient population), even better than in 188 patients with locally advanced breast cancer.
More recent data revealed 40% pCR with dual antibody therapy of pertuzumab and trastuzumab, given with docetaxel; pCR predicted for longer 5-year disease free survival versus patients without pCR (85% vs. 76% (hazard ratio of 0.54 (95% CI 0.29–1.00), but of note, patients also received an adjuvant anthracycline-containing treatment. However, only 7% (29/417) of patients in this trial were enrolled with IBC. The rates of pCR vary depending on biological subtype. With longer duration of neoadjuvant therapy and with the inclusion of carboplatin with docetaxel, pertuzumab, and trastuzumab versus anthracycline-containing regimens, a high pCR of 62% was observed in the Tryphaena trial. However, only 13 of 225 patients (6%) were enrolled with IBC. An interesting study describing pCR with anthracycline-containing and trastuzumab and bevacizumab-inclusive neoadjuvant therapy resulted in 63.5% pathologic complete response in 52 patients with IBC. Patients also received adjuvant trastuzumab and bevacizumab. On follow-up, the 3-year disease-free survival was 68%, and an association with pCR (80% vs. 53%) and with lack of circulating tumor cells (disease-free survival of 95% in patients with pCR and without circulating tumor cells at baseline) was noted. Attempts to avoid anthracyclines and shorten the duration of treatment are ongoing with combinations of pertuzumab, trastuzumab, and a taxane in IBC and in trials allowing for enrollment of patients with IBC ( clinicaltrials.gov identifiers NCT01796197 and 01730833).
Data on IBC with triple-negative (ER-, PR-, and HER2-negative) characteristics are limited, because most neoadjuvant trials excluded such patients. Therefore the efficacy of double stranded, DNA-targeting agents, and poly (ADP-ribose) polymerase (PARP) inhibitors is unknown in IBC, although data suggest that such agents may induce higher pCR rates in triple negative, locally advanced breast cancers. Data on the utility of antiangiogenic agents are also sparse, but at least one phase II prospective randomized trial suggested that the addition of bevacizumab to anthracycline and taxane-based (in this case nab-paclitaxel) neoadjuvant therapy doubled the pCR rate to 30% (3 of 10 patients) versus 14.3% (2 of 14) among patients with IBC.
The current array of promising therapeutic agents is broad. In the neoadjuvant and possibly in the postsurgical adjuvant settings, based on the phenotype and genotype, targeting the EGFR receptor by erlotinib, dual targeting by afatinib, or by other novel bispecific (EGFR and HER2) tyrosine kinase inhibitors are under evaluation. Novel HER2-targeting agents such as neratinib, in addition to the currently available dual targeting with pertuzumab/trastuzumab or trastuzumab and HER2 tyrosine kinase inhibitor (TKI)-based strategy may contribute to better control of IBC with the potential to overcome HER2 mutations. Triple-negative, particularly DNA repair-deficient, IBC patients may benefit from platinum compounds and PARP inhibitors. An assessment of immunotherapeutic agents, including checkpoint inhibitors (PD1 or PDL1 inhibitors), particularly for PDL1 expressing patients, is needed. Targeted therapeutic agents based on molecular profiling of the tumor, and/or liquid biopsy samples (circulating tumor cells or cDNA) might provide better selection guidance for individual IBC patients for phase II trials of the appropriate agents.
Finally, there needs to be further exploration of stem cell and stroma-targeting strategies. Agents under consideration include the JAK2 inhibitor ruxolitinib and others. Chemokine and chemokine receptor targeting agents (against CXCR4 and CXCR7 as examples), antibodies aimed at E-cadherin, and stem cell–targeting agents as well as pathway-specific TKI are under evaluation as part of the neoadjuvant IBC regimens. Although not used as primary neoadjuvant strategy in IBC, for ER-positive disease, in the postsurgical setting, the potential for developing mutations in the ER may call for testing selective ER downregulators and CDK 4/6 inhibitors earlier in the treatment course. The focus of this chapter is primary IBC, but the preceding strategies may provide much-needed help for patients presenting or relapsing with IBC, whose overall survival is under 3 years.
Postneoadjuvant/Adjuvant Strategies
High-dose/dose-intense alkylator-containing therapies have yielded improved disease-free and overall survival, but the modality has not been tested sufficiently in a randomized prospective fashion, particularly in IBC, and is unlikely to be revisited. Current therapeutic trials are particularly focused on adjuvant treatment strategies in patients with residual disease after neoadjuvant therapies. Such strategies are likely to be equally applicable for IBC and non-IBC locally advanced breast cancer, although the biological differences need further investigations in order to select appropriate strategies. Some of the trials already in progress are designed for specific subsets. As selected examples, in patients with HER2-positive locally advanced disease, TDM-1 is being compared with trastuzumab in the adjuvant setting after evidence of residual disease postneoadjuvant therapy ( clinicaltrials.gov identifier NCT 01772472). Adjuvant cisplatin is being added with concurrent radiation for patients with residual triple-negative tumors (identifier NCT016748482). Also being tested is four cycles of adjuvant platinum therapy versus placebo (identifier NCT 02445391), the PARP inhibitor olaparib versus placebo ( clinicaltrials.gov identifier 02032823), and cisplatin with or without the PARP inhibitor rucaparib (identifier NCT01074970) in BRCA carriers. Adjuvant immunotherapy with pembrolizumab versus placebo and the others listed are all ongoing or about to launch, albeit not specifically in IBC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








