INTRODUCTION
Inflammatory breast carcinoma (IBC) is a very aggressive disease and accounts for less than 6% of all breast cancers in the United States. It is characterized by a rapidly progressive clinical course and diagnosed on the basis of distinct skin manifestations that are often confused with inflammation, hence its name. These manifestations include erythema, edema, skin nodules, and nipple retraction. A characteristic infiltration of the dermal lymphatics with tumor emboli is often seen by pathology but is not required to establish a diagnosis.
Although clinically more than 50% of patients do not have a detectable breast mass at the time of diagnosis, up to 85% have already metastasized disease that has spread to the regional lymph nodes, while more than 30% present with gross distant metastasis (1). Not surprisingly, owing to its high metastatic potential, IBC is associated with a 5-year overall survival (OS) rate of no more than 55% (2,3). Multimodality treatment in the form of neoadjuvant chemotherapy, surgery, and radiation aims to prevent metastatic failure as well as achieve local control.
EPIDEMIOLOGIC CHARACTERISTICS
The epidemiology of IBC has been a challenging area to study due to a number of factors. The rarity of the disease combined with its rapid progression and short OS mean that there are few single centers able to recruit an adequate sample of patients for investigation. In addition, confusion about its clinical definition has led to difficulties in comparing the results of different studies. As a result, the primary source of epidemiologic data comes from large national registries, which are limited by nonuniform data collection, inability to capture comprehensive details on risk factors, and in the case of IBC, varying case definitions.
To address these issues, the Morgan Welch Inflammatory Breast Cancer Research Program and Clinic at the University of Texas, MD Anderson Cancer Center (MDACC) spearheaded the development of an international IBC Registry. The IBC Registry prospectively collects tissue, serum, plasma, whole blood, imaging, clinical, and epidemiological data from patients with IBC, allowing a more comprehensive examination of the underlying mechanisms associated with the development of this lethal disease.
The incidence of IBC is reported to range from 1% to 6% of all breast cancers diagnosed in the United States (4). Higher proportions have been reported in North Africa, specifically in Tunisia and Egypt, where IBC accounts for 6% and 10% of all breast cancers, respectively (5,6,7). Data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program have demonstrated a rise in the incidence of IBC over time, although this trend seems to be slowing. Between 1973 and 2002, the incidence of IBC is reported to have increased at an annual rate that ranged from 1.23% to 4.35% per year, depending on the specific database analyzed and the case definition used (4,8,9). In comparison, during the same time period, breast cancer as a whole saw a slower rise of 0.42% per year (95% CI, 0.14% to 0.71%) (9).
Inflammatory breast carcinoma is commonly lethal and associated with a high risk of early recurrence (10). Women diagnosed with IBC have a statistically significant poorer survival than women with noninflammatory locally advanced breast cancer (LABC) (P < .001), with a median OS of 2.9 years and a 5-year survival rate of 30% to 40% (4,11). This was also true for patients presenting with stage IV disease. Patients with stage IV IBC had a shorter median OS than stage IV non-IBC (2.3 vs 3.4 years, P = .004) (12). Also, although IBC accounted for only 2.5% of all breast cancer cases in the period 1988 to 2000, it led to 7% of all breast cancer–specific deaths (4).
Higher mortality rates were reported for African American women, according to data from SEER, with a median survival of 2.0 versus 2.9 years when compared with Caucasians (P < .001) (4,13). The early age at diagnosis and poor survival outcomes observed among African American patients with IBC suggests epidemiological differences between different ethnic groups that could involve an interplay of genetic, environmental, and lifestyle risk factors.
Earlier reports suggested a trend for a modest improvement in mortality rates for IBC (4). This trend was compared to a similar improvement seen in non-IBC and attributed to improvements in multidisciplinary treatment, the effect of new therapies, and an increasing awareness of IBC. However, more recently, this trend toward improved survival was not supported by retrospective data at our institution (11). The authors looked at the survival of 398 women with IBC treated over a period of 30 years (between January 1974 and April 2005). After adjusting for patient and disease characteristics, Cox proportional hazards models did not show an association between year of diagnosis and the risk of recurrence or death.
RISK FACTORS
While clearly established risk factors have been associated with the development of non-IBC, this is not the case with IBC, which is much rarer and not consistently defined. For example, while family history, menopausal status, and age at menarche are important risk factors for the development of non-IBC, they have not been confirmed as risk factors for IBC. Despite these limitations, several important epidemiologic factors appear to be consistently associated with IBC (Table 29-1).
| Risk Factor | Degree of Association |
|---|---|
• Younger age at diagnosis • African American ethnicity • Increased body mass index (≥30) • North Africa • Ever pregnant • Younger age at live first birth • Duration of breast feeding | +++ +++ +++ ++ + + + |
According to an analysis of breast cancer cases diagnosed in the SEER 9 Registries between 1988 and 2000 (n = 180,224), IBC developed at a younger age (median 57 years) when compared with non-IBC (61.9 years) (9). Young age at diagnosis was also seen in earlier population-based studies (8,14,15). In addition, these studies found that age of onset varied according to ethnicity, with African American women presenting at a younger age than Caucasians (15). Age at diagnosis also varied between genders, and men usually were diagnosed 10 years later than women (median 66.5 vs 57 years, P < .001) (9).
African Americans have at least a 50% higher incidence of IBC than whites in the United States (3.1 vs 2.2 per 100,000 woman-years, p < .001) (4). The higher incidence of IBC in African Americans has been confirmed in four different population-based studies (4,8,14,15). Three of these studies used the SEER dataset to cover different time periods; the fourth used the North American Association of Central Cancer Registries (NAACCR) database.
Moreover, African Americans were diagnosed at a younger age (median 55.2 vs 58.1 years) and had a poorer prognosis when compared with Caucasians. Of note, Hispanic women have the youngest mean age of onset of the disease (median 50.5 years) (15).
A recent SEER study that examined patients with breast cancer (IBC and non-IBC) diagnosed between 1990 and 2000 suggested that the inferior outcome seen in African American women was independent of stage or inflammatory status (13).
One of the most consistent risk factors associated with IBC is a high body mass index (BMI). In contrast to non-IBC, for which elevated BMI is a risk factor in postmenopausal patients only, high BMI is associated with IBC in both pre- and postmenopausal patients. In a case-comparison study conducted at MDACC, patients with IBC weighed more (median weight 77.6 kg) than those with non-IBC (70.0 kg) or those with history of other cancers (68.0 kg) (8). After adjusting for other factors, women with the highest BMI (>26 kg/m2) had an increased risk for development of IBC compared to those with non-IBC (odds ratio 2.54). This was irrespective of menopausal status, age at menarche, or family history of breast cancer.
In the largest case-control study to evaluate the association between risk factors for breast cancer and the development of IBC, Schairer et al compared 617 patients with IBC to three reference groups: LABC (n = 7,600), noninflammatory breast cancer not involving the chest wall or skin (n = 1,151), and healthy controls (n = 93,654). They found that high BMI at diagnosis was associated with an increased risk of IBC irrespective of menopausal status or hormone receptor expression (16).
The impact of obesity and menopausal status on IBC survival was also evaluated in a cohort of 177 women patients with IBC seen at MDACC between 1974 and1993. After adjusting for axillary lymph node involvement and chemotherapy protocol, premenopausal obese women had significantly worse survival compared to postmenopausal obese women (hazard ratio [HR] 1.51) (17). After stratifying by menopausal status, postmenopausal obese women had significantly worse survival than those who were not obese (HR 1.86). These findings suggest that factors associated with a higher BMI at diagnosis may contribute to shorter IBC survival among postmenopausal women but not premenopausal women, who were found to have poorer survival regardless of body size.
Finally, the prognostic value of BMI at diagnosis was evaluated in a retrospective study of 602 patients with LABC, which included a subset of patients with IBC (18%). Obese patients were more commonly associated with a diagnosis of IBC compared with overweight and normal or underweight groups (p = .01) (18). Patients with LABC who were obese or overweight had a significantly worse OS and relapse-free survival (RFS) and a higher incidence of visceral recurrence compared with normal/underweight patients.
Overall, these results are of great importance for IBC because obesity is a modifiable risk factor, and preventive strategies aimed at reducing obesity may yield significant rewards.
The incidence of IBC has been observed to vary according to geographic location. Using the SEER program registries from 1992 to 2002, rates ranged from 2.064 per 100,000 woman-years in San Jose-Monterey (California) up to 3.042 cases per 100,000 in Los Angeles (9). Similarly, reports have suggested that North Africa is associated with the highest rates in the world, with countries such as Tunisia and Egypt reporting proportions of 6% and 10% of all breast cancers, respectively (5,6,7). These variations are currently under study and may suggest underlying differences in environmental and lifestyle or genetic risk factors. Alternatively, differences in case definitions and the lack of specificity associated with clinical identification of IBC have somewhat limited the value of these comparisons.
Data suggest that socioeconomic position (SEP) can affect both the incidence and the outcome of IBC. Whereas the incidence of breast cancer overall has been associated with higher SEP, several studies have suggested a higher incidence rate of IBC among patients with lower SEP (13,16). Likewise, in a large, nested case-control study of patients identified from the Breast Cancer Surveillance Consortium database (1994-2009), the risk of developing IBC gradually decreased with increasing level of education (16).
On the other hand, differences in SEP are associated with different exposure patterns to risk factors and with differences in disease awareness and access to health care, leading to diagnostic delays, mismanagement, and potentially worse OS. This may explain why the association between lower SEP and poorer survival was not significant for IBC after adjustment for other prognostic factors (16).
Although IBC is diagnosed at a younger age than non-IBC, there does not appear to be a consistent association between IBC and premenopausal status. Of the patients evaluated at MDACC, 49% of patients with IBC were premenopausal compared to 39% of non-IBC patients (8). Similarly, while patients with IBC presented with earlier age at menarche than patients with non-IBC (12.2 vs 12.7 years), this difference was not statistically significant. A study conducted in Pakistan did not show a significant difference in menopausal status among the various comparison groups, including IBC (19). Larger studies are required to establish relationships between IBC and menstrual history.
Early age at first birth has also been associated with the development of IBC. Women with aggressive breast cancer, including IBC, are more likely to have their first child before the age of 20 when compared to patients with nonaggressive breast cancer (9). A later age at first birth was associated with a reduced risk of developing IBC that was estrogen receptor (ER) negative compared to locally advanced and early breast cancer (16).
An association with pregnancy or lactation has been suggested in earlier reports (20). In a study by Bonnier et al, IBC accounted for approximately 21% to 26% of breast cancer tumors in patients who developed breast cancer during or after pregnancy (21). Later studies reported that the risk of pregnancy seen with IBC was not different from the risk seen in non-IBC (22). Results from our group suggest that lack of breast-feeding history maybe associated with the development of specific IBC subtypes (ER+, triple negative). No association between oral contraceptive use and development of IBC has been found (8).
Approximately 15% of women with breast cancer have a positive family history of breast cancer in a first-degree relative, while 5% to 10% of breast cancers are directly attributed to heredity. In a study comparing 68 patients with IBC and 143 patients with non-IBC seen at MDACC, 13% of patients with IBC reported a positive family history of breast cancer, compared with 8% of patients with non-IBC; this was not statistically significant (23). However, in a study conducted in Pakistan, 20% of patients with IBC reported a positive family history of breast cancer, compared to 5% in the non-IBC group, which was statistically significant (19).
Using data from the Breast Cancer Surveillance Consortium database (1994-2009), Schairer et al also found that the presence of a first-degree family history of breast cancer was associated with increased risk of developing IBC (HR 1.6; 95% CI, 1.1 to 2.2) (16).
A host of other risk factors have been explored in IBC, including blood type and area of residence. A higher proportion of Tunisian patients with IBC have blood type A. In addition, a larger proportion of patients lived in rural locations (20). Smoking and alcohol use were not associated with a risk of developing IBC (23). Other studies have suggested a link between IBC and a variant of mouse mammary tumor virus (24). This relationship remains under investigation.
MOLECULAR PATHOGENESIS
Attempts to distinguish between IBC and non-IBC at the molecular level have so far not been successful. Inflammatory breast carcinoma shares some of the molecular characteristics associated with breast cancer in general, as well as some important differences.
Gene expression profiles: There has been no shortage of gene expression studies aimed at distinguishing IBC from non-IBC at the genomic level (25,26,27). Although several gene expression studies of IBC tumors have revealed useful and promising results, none has yielded an IBC-specific signature.
In breast cancer, the success associated with the identification of several gene expression subtypes of prognostic significance has led to their incorporation in the treatment strategy for women with nonmetastatic breast cancer (28). These molecular subtypes are classified into luminal (ER-related genes), HER2 (HER2-related genes), and basal subtypes. Despite differences in their relative frequency, the molecular subtypes of IBC are similar to those expressed in non-IBC (26,29). Likewise, there are no unique differences between triple-negative IBC and non-IBC at the messenger RNA gene expression level (30).
RhoC GTPase overexpression/loss of WNT1 Inducible Signaling Pathway Protein 3 (WISP3): The transforming oncogene RhoC GTPase (guanosine triphosphatase) is one of the most upregulated genes in IBC and is found in 91% of IBC tumors compared to 38% in non-IBC tumors in some studies (31). RhoC GTPase overexpression is thought to be involved in tumor invasion and increased expression of cyclin D1, vascular endothelin growth factor (VEGF), fibronectin, and caveolin-2 (31). The overexpression of RhoC GTPase, combined with the loss of the tumor suppressor gene WISP3/WINT-1, was found to characterize the IBC cell line SUM149 and is believed to be partially responsible for the aggressive phenotype of IBC tumors (32,33).
E-cadherin overexpression: E-cadherin is a cell adhesion molecule that is lost in malignant progression and is thought to promote tumor cell metastasis. Loss of E-cadherin is considered to be a fundamental event in epithelial-mesenchymal transition (EMT) that is associated with tumor metastasis and stem cell–like phenotypes. Paradoxically, E-cadherin is overexpressed in IBC and appears to be necessary for tumor emboli formation by enhancing tumor cell–cell contact (34).
Increased expression of E-cadherin and nonsialylated mucin-1 (MUC1) has been identified in up to 100% of IBC tumors compared to 68% of non-IBC tumors in one study (35). In another study using a xenograft model, a 10- to 20-fold increased expression of E-cadherin and MUC1 was noted and thought to contribute to the passive dissemination of tumor emboli in IBC (36). Dual overexpression of these two proteins in IBC is thought to play a role in the aggressive, invasive nature of IBC (37).
p53 mutation: In breast cancer, studies have linked p53 mutation to worse prognosis (38). In a study performed at MDACC, p53 overexpression was noted in up to 58% of IBC tumors and was independent of histologic grade (39). Patients with p53 overexpression were younger (median age 45.2 vs 52.2 years; P = .02) and had lower 5-year progression-free survival rates (35% vs 55%; P = .03) and 5-year OS rates (44% vs 54%; P = .4). Patients with tumors overexpressing p53 had an 8.6-fold higher risk of death in multivariate analysis. These results were in line with previous studies (40).
Angiogenesis: Inflammatory breast carcinoma is a vascular tumor with overexpression of lymphangiogenic factors such as VEGF and Flt-4 (34,41). A significantly higher intratumoral microvessel density has been reported in IBC tumors compared to non-IBC tumors (42). Evidence also suggests that IBC tumors exhibit the phenomenon of vasculogenic mimicry, by which tumors form vessel-like structures in the absence of endothelial cells. These structures act as a supplementary blood supply by which tumor tissue is able to nourish itself. Both the WIBC-9 and the Mary-X xenograft models have demonstrated a role for vasculogenic mimicry in IBC (43).
Inflammation: The degree to which inflammation plays a role in inflammatory breast cancer remains largely unknown. Recent evidence comes from the constitutive activation of major inflammatory signaling pathways (nuclear factor kappa B [NF-κB], cyclooxygenase 2 [COX-2], and JAK/STAT [Janus kinase/signal transducer and activator of transcription]) as well as in vitro, in vivo, and patient studies (44). Similarly, inflammatory cytokines and chemokines such as interleukin 6, transforming growth factor beta, tumor necrosis factor alpha (TNF-α), and gamma interferon are involved in all steps of tumorigenesis in IBC. These findings have prompted the testing of several compounds for their anti-inflammatory activity, with the aim of developing therapeutic or prevention strategies for IBC (44).
Cancer stem cells: Some evidence suggests that IBC cells have characteristics similar to cancer stem cells (45,46). The IBC tumors as well as the SUM149 IBC cell line and the Mary-X preclinical model of IBC have all been shown to express stem cell surface markers (CD44+/CD24–/low and aldehyde dehydrogenase 1 [ALDH-1] enzyme production) as assessed by the ALDEFLUOR assay (46,47). Expression of ALDH-1 in samples from patients diagnosed with IBC was associated with poor prognosis (47). Using microarray analysis, IBC was found to express genes known to be associated with breast cancer stem cells (48). A study using a xenograft model of IBC has demonstrated the potential to reverse the EMT process by which epithelial cells are thought to acquire stem cell–like properties (49).
Other molecular mechanisms: Overexpression of epidermal growth factor receptor (EGFR) is observed in more than 30% of patients with IBC. Patients with EGFR-positive tumors have an increased risk of recurrence and worse 5-year OS rates (50). Elevated levels of extracellular signal-regulated kinase (ERK) have been reported in both SUM149 and KPL-4 IBC cell lines (49). Dual blockade of EGFR and ERK1/2 phosphorylation in SUM19 cells was associated with decreased mitogen-activated protein kinase (MAPK) signaling and induced apoptosis (51).
On the other hand, WISP3 was able to modulate insulinlike growth factor (IGF) signaling in SUM149 cells, which may be an effective therapeutic target for IBC (32,52).
The expanded knowledge gained from preclinical studies has resulted in several promising molecular targets for directed therapy (Table 29-2).
| Category | Molecular Marker | Pharmacologic Class |
|---|---|---|
| Oncogenes | Her-2/neu | mAbs, TKIs |
| RhoC GTPase | FTIs | |
| Tumor suppressor genes | p53 | Gene therapy, p53-stabilizing agents |
| PTEN | Proteasome inhibitors, PI3K inhibitors | |
| Angiogenesis modulators | Tie-2 | Tie-2 kinase inhibitor |
| VEGF | TKIs, mAbs | |
| Flt-1/Flk-1 | TKIs, mAbs | |
| E-cadherin | E-cadherin inhibitors | |
| MUC1 | MUC1 inhibitors, PIAS | |
| RhoC GTPase | FTIs |
CLINICAL PRESENTATION
The clinical identification of IBC is frequently confused with more common benign diseases and requires a high index of suspicion on the part of the diagnosing physician. On suspicion, the physician must obtain pathologic evidence of breast carcinoma to establish the presence of malignancy. Once evidence of breast carcinoma is obtained, the presence of dermal lymphatic invasion helps to confirm the diagnosis but is neither required nor sufficient to make the diagnosis (53).
The affected breast typically presents with a rapid onset of erythema, tenderness, edema, and swelling, accompanied by an enlargement of the draining lymph nodes, frequently in the absence of a breast mass. This clinical picture closely mimics common inflammatory conditions of the breast, such as simple bacterial mastitis (54). As a result, many patients with IBC unnecessarily undergo repeated courses of antibiotics before a diagnosis is made.
On the other hand, in the presence of a breast mass, these symptoms are often confused with LABC with secondary erythematous skin involvement. The lack of specific pathologic or molecular characteristics for IBC means that the distinction between IBC and non-IBC is based mainly on clinical grounds.
The confusing clinical presentation of IBC often leads to unnecessary delays in diagnosis and treatment. Haagensen reported that the median duration of symptoms before diagnosis was 2.5 months compared to 5 months for non-IBC (55). However, a recent study examining the impact of delayed diagnosis, defined as more than 60 days from the time of first contact with a physician, found no effect on patient outcome (56).
Inflammatory breast carcinoma commonly presents unilaterally, although bilateral disease can occur (57). Haagensen’s original description of IBC outlined a list of characteristic symptoms and their corresponding frequencies (55). The author’s criteria included breast mass (57%), redness of the skin (57%), breast enlargement (48%), pain in breast or nipple (29%), breast tenderness (16%), generalized breast hardness (16%), nipple retraction (13%), edema of the skin (13%), axillary mass (9%), and warmness of the skin (8%).
One of the defining characteristics of IBC is the rapid onset of skin changes. Patients with primary IBC exhibit symptoms characterized by rapid onset erythema occurring less than 6 months from the diagnosis of breast cancer (58). In contrast, those presenting with delayed erythema occurring more than 6 months after breast cancer diagnosis are considered to have neglected breast cancer with secondary erythematous changes.
Skin changes, in the form of erythema and edema, are a distinguishing feature of IBC and should involve at least one-third of the skin overlying the breast to establish the diagnosis. Patients who present with skin changes involving less than one-third of the skin should not be classified as having IBC.
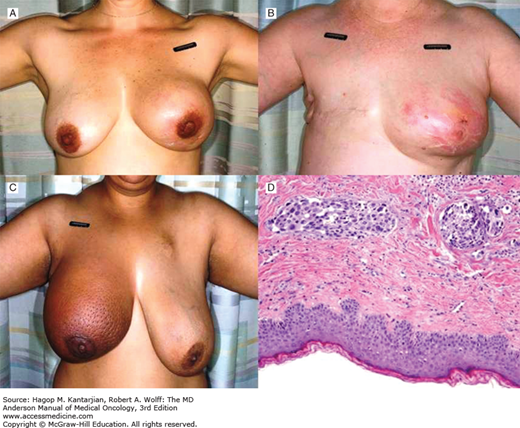
Erythema tends to be one of the earliest manifestations and typically presents with a mottled pink discoloration of the skin in the affected breast, which may be associated with a sensation of heat. Comparison with the contralateral, unaffected breast helps to identify erythema (Figs. 29-1A, 29-1B). During the course of its evolution, the degree of erythema may change from a flush of pink to red to bronze; it even may briefly fade and give the misleading picture of disease regression (54). In more severe cases, skin discoloration involves the entire breast and may change to dark red or purple. These manifestations can vary in African American women, for whom skin erythema is not the predominant symptom, leading to more difficulties in diagnosis. In these cases, peau d’orange changes may be easier to identify (Fig. 29-1C).
FIGURE 29-1
Clinical appearance of inflammatory breast cancer. A. Erythema and enlargement noted when compared to normal breast. B. Diffuse erythema of the left breast in a woman with prior history of right breast cancer. C. Peau d’orange appearance of the skin of the right breast. D. Photomicrograph of breast biopsy from a woman with inflammatory breast cancer showing normal-appearing epidermis (bottom of figure) with tumor cells infiltrating the lymphatic channels of the dermis.
On the other hand, edema of the skin results from blockage of the draining lymphatics. This also leads to swelling of the breast and exaggeration of pits around hair follicles, giving the characteristic peau d’orange (orange peel) appearance. This can cause generalized breast induration as well as wheals and ridging (59).
Rapid swelling of the breast results from breast edema and in some cases can increase in size two- to threefold in a period of a few weeks.
At the time of diagnosis, almost all patients present with lymph node metastasis, and up to 30% also have distant metastasis at presentation (1). The ipsilateral axillary lymph nodes are the most common areas of spread, followed by ipsilateral supraclavicular lymph nodes. Contralateral and distant lymph node spread are also common, especially in more advanced and neglected cases (1). On physical examination, lymph nodes are usually enlarged, palpable, and fixed and in extreme cases may be accompanied with arm lymphedema.
Other clinical features not considered diagnostic of IBC include nipple involvement, itching, and axillary pain. The nipple may be flattened and retracted and appear blistered or associated with an area of crusting (1). Ulceration is not a common feature of IBC and generally suggests neglected LABC.
A distinction must be made between primary IBC and secondary IBC, which is the development of inflammatory changes in a breast or chest wall after a history of non-IBC in either the same or opposite breast. The outcomes from these disease entities are likely very different. In a recent review of eight patients with secondary IBC identified from the Inflammatory Breast Cancer Registry database, patients commonly presented with redness at the surgical scar site, peau d’orange, a diffuse rash, or chest wall nodules, which, unlike primary IBC, can become ulcerated (59,60). An early study at MDACC identified 96 patients with secondary IBC diagnosed between 1954 and 1981 and suggested there were no major differences in clinical course or outcome between primary and secondary IBC (61). This conclusion should be interpreted cautiously. In view of the recent progress achieved in the treatment of non-IBC, secondary IBC is likely to have a different outcome compared to primary IBC, and the two entities should be clearly distinguished.
Inflammatory breast carcinoma is often misdiagnosed as an infection of the breast, such as cellulitis, bacterial mastitis, or breast abscess, which are treated with repeated courses of anti-inflammatory or antibiotic treatment (54). A high degree of suspicion and the absence of fever, pain, leukocytosis, or other symptoms and signs associated with inflammation help distinguish IBC from breast infection. Acute bacterial mastitis commonly occurs during lactation and resolves in several days. Other infectious entities that can mimic IBC include erysipelas, which is usually caused by group A streptococcus.
Inflammatory breast cancer is also commonly confused with locally advanced neglected breast cancer with secondary erythematous changes. This condition presents with delayed erythema occurring more than 6 months after the initial diagnosis of breast cancer (58).
Paget disease of the nipple can also mimic IBC but generally develops more slowly and is usually associated with destruction of the nipple (54). Radiation dermatitis, in its acute phase, may also appear to be IBC; however, desquamation of the skin usually occurs with resolution of skin changes in 2 to 3 weeks.
Other less-common conditions that may be confused with IBC include rare breast malignancies such as sarcoma, inflammatory metastatic melanoma, or breast lymphoma; distant breast metastasis from another primary cancer may also produce a similar clinical picture (Table 29-3).
| Infectious conditions: |
|
| Benign (noninfectious) conditions: |
|
| Malignant conditions: |
|
Inflammatory breast carcinoma is a rapidly progressive disease and is characterized by high rates of locoregional and distant recurrence. In a pooled analysis of 10 clinical trials conducted at MDACC, patterns of recurrence in 240 patients with IBC were compared to 831 patients with stage-matched non-IBC (10). All patients were reported to have received similar multidisciplinary treatment. Patients with IBC had a higher cumulative incidence of recurrence at 5 years compared to individuals with non-IBC (64.8% vs 43.4%; P < .0001) and a lower 5-year OS rate (40.5% vs 63.2%; P < .0001). Inflammatory breast carcinoma was associated with significantly higher rates of soft tissue recurrence (skin and lymph nodes) both locoregionally and at distant sites. There was no difference between IBC and non-IBC in terms of distant recurrence to the bone or viscera.
Using data from the SEER registries, Schairer et al compared the risk of contralateral breast cancer in 5,631 patients with IBC versus and 174,634 patients with comparably staged non-IBC among women diagnosed with first breast cancer between 1973 and 2006 (62). Contralateral breast cancer was further divided into recurrent/metastatic (occurring between 2 and 23 months of diagnosis) and independent second primary breast cancer (occurring in the opposite breast 2 or more years after the first diagnosis). Absolute risk of contralateral breast cancer was higher in IBC (4.9% vs 1.1% at 2 years), irrespective of age or hormonal status. The majority of IBC events occurred within 2 to 23 months from the first diagnosis, reflecting a higher risk of recurrence compared to non-IBC. The risk of contralateral breast cancer continued to be higher for those with IBC, up to 15 years from the time of first diagnosis.
PATHOLOGY
Obtaining a core biopsy with evidence of malignancy is considered the cornerstone of diagnosing IBC. Two additional skin punch biopsies are strongly recommended to detect dermal lymphatic tumor emboli and confirm the diagnosis.
It should be emphasized that IBC is not a true inflammatory process and does not demonstrate any of the pathologic hallmarks of inflammation, such as the presence of inflammatory cells or pus formation.
One of the most striking gross features of IBC is that it commonly presents in the absence of a dominant mass. The cancer usually presents as clumps of tumor cells within the lymphovascular spaces of the skin. These microscopic lesions are known as lymphovascular tumor emboli and represent the pathologic hallmark of the disease as well as explain most of its clinical manifestations. As a result, IBC often presents with multicentric disease, in some cases bilaterally, and has a high propensity to spread to the regional lymph nodes and the distant organs.
Otherwise, the gross pathology of IBC tends to correspond with its clinical characteristics and includes erythema, thickening of the skin, and generalized enlargement of the breast due to edema.
In conjunction with the clinical features associated with IBC, several histopathological features have also been identified. Histologically, IBC tumors are characterized as being of higher tumor grade, with small areas of invasive carcinoma and extensive vascular tumor emboli associated with large tumor-free skip areas (Fig. 29-1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree