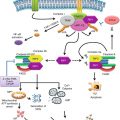
Fig. 2.1
Differentiation pathways of tumor-associated myeloid cells. Myeloid cells originate from hematopoietic stem cells (HSC) in the bone marrow. Here the networks that give rise to the various myeloid cell lineages in diverse compartments (bone marrow, blood/spleen, and tumor) and their precursors are illustrated. In the tumor tissue, macrophages and neutrophils display a gradient of differently polarized phenotypes whose extreme are M1–M2 for TAM and N1–N2 for neutrophils. CMP common myeloid progenitors, IMC immature myeloid cells, TEM Tie2-expressing monocytes, MDSC myeloid-derived suppressor cells, M–MDSC myeloid MDSC, G–MDSC granulocytic MDSC, TAM tumor-associated macrophages, TAN tumor-associated neutrophils, iDC immature dendritic cells, TADC tumor-associated dendritic cells
Results obtained so far clearly indicate that TAMC are major players in the connection between inflammation and cancer. Ongoing efforts, which led to a better understanding of their biological properties, indicated that myeloid cell-infiltrating growing tumor could have a prognostic value, thus representing an attractive target for novel biological therapies of tumors.
In this chapter, we will mainly focus on myeloid cells infiltrating tumors and mention soluble mediators involved in their recruitment or released by TAMC, which affect tumor progression and dissemination (cytokines, chemokines, and proteases). Furthermore, new therapeutic approaches based on targeting of tumor-infiltrating myeloid cells and/or soluble mediators will be discussed.
2.2 Heterogeneity of Myeloid Cells in the Tumor Microenvironment
2.2.1 Myeloid Subsets in the Tumor Microenvironment
Solid tumors are characterized by the presence of a leukocyte infiltrate including lymphocytes and myeloid cells from early stages. Growing evidence indicated that the leukocyte infiltrate has a prognostic value. For instance, it has been described that infiltrating T lymphocytes are associated with a favorable prognosis in colorectal cancer, melanoma, ovarian cancer, and breast cancer [12, 13]. In contrast, myeloid cells are most frequently associated with a poor prognosis [14]. TAMC (Fig. 2.1) comprise five distinct myeloid populations, namely, tumor-associated macrophages (TAM), monocytes expressing the angiopoietin-2 (Ang-2) receptor Tie2 (known as Tie2-expressing monocytes or TEM), myeloid-derived suppressor cells (MDSC), tumor-associated neutrophils (TAN), and tumor-associated dendritic cells (TADC).
Tumor-associated macrophages belong to the early infiltrating leukocyte populations within tumors, thus preceding lymphocytes, and are usually the most abundant immune population in the tumor microenvironment [6, 15]. They derive from blood monocytes actively recruited from the circulation into tumor tissues. Early studies demonstrated that appropriately stimulated macrophages are able to kill tumor cells in vitro; however, TAM, conditioned by the tumor microenvironment, loose the cytotoxic capability and rather exert several pro-tumoral functions, mediating cancer-related inflammation, angiogenesis, immunosuppression, tissue remodeling, and metastasis [16, 17, 6].
The heterogeneous behavior of TAM is a hallmark of myeloid cells and is oversimplified in a polarization concept with two extreme M1 and M2 phenotypes [18–20] with distinct and somehow opposite functions. M1 macrophages are classically activated by bacterial products and Th1 cytokines (e.g., LPS/interferon-γ). They are potent producers of inflammatory and immunostimulating cytokines, trigger adaptive responses, secrete reactive oxygen species (ROS) and nitrogen intermediates, and have cytotoxic effect towards transformed cells. On the other hand, M2 macrophages or alternatively activated macrophages differentiate in response to Th2 cytokines (e.g., interleukin (IL)-4, IL-13) [21]. In contrast to their M1 counterpart, M2 macrophages produce growth factors, leading to tissue repair and angiogenesis activation, have high scavenging activity, and inhibit adaptive immune responses [22, 14, 23, 11, 24]. Thus, macrophages are a very heterogeneous cell population, able to display different functions depending on the context. Macrophages can be either immunostimulatory at the beginning of the inflammatory response or immunosuppressive which dampen inflammation [25, 18, 14, 26, 27].
A similar dichotomy with polarization towards two extreme phenotypes (N1 and N2) has been also described for neutrophils [28]. Besides exerting antibacterial functions, neutrophils can infiltrate tumors playing a major role as key mediators in malignant transformation, tumor progression, and regulation of antitumor activity [29]. Tumor-associated neutrophils (TAN) have received interest only recently, mainly due to their short life span and the observation that tumor microenvironment can sustain and prolong the survival of polymorphonuclear leukocytes (PMN) [30, 31].
A particular small subset of TAMC is represented by Tie2-expressing monocytes (TEM): they express several monocyte/macrophage markers, along with the angiopoetin-2 receptor, Tie2, and are endowed with proangiogenic properties [32–35]. Tie2-expressing monocytes can be distinguished from the majority of TAM by their surface marker profile (Tie2+, CD11b+) and their preferential localization to areas of angiogenesis [33], while they are largely missing in nonneoplastic area adjacent to tumors [35]. Indeed, Tie2 is constitutively expressed at low levels by a substantial fraction (20 %) of circulating monocytes and is overexpressed upon monocyte homing into growing tumors or regenerating tissues [33, 36]. Following Ang-2 stimulation, Tie2+ monocytes acquire an M2-like phenotype, with increased expression of IL-10, CCL17, arginase 1 (Arg-1), and scavenger and mannose receptors and low expression of proinflammatory molecules such as IL-12 and TNF-α [37, 38].
Myeloid-derived suppressor cells (MDSC) are a heterogeneous population of immature myeloid cells, having the ability to suppress T-cell functions [39, 40]. They are derived from myeloid progenitors in bone marrows which do not differentiate into mature granulocytes, macrophages, or dendritic cells. MDSC have been isolated from blood, spleen, and bone marrow of tumor-bearing mice and infiltrate the tumor tissue, where local tumor-associated factors promote their activation [41]. In tumor-bearing mice, two main subsets of MDSC were identified: monocytic MDSC (M-MDSC), characterized by CD11b+, Ly6G−, and Ly6Chigh, and granulocytic MDSC (G-MDSC), characterized by CD11b+, Ly6Ghigh, and Ly6C− [42]. M-MDSC were shown to govern the ability of differentiating into monocytes (macrophages) and (DC), whereas G-MDSC do not possess this potential [43]. These subsets are functionally different: M-MDSC-mediated immunosuppression is based on upregulation of inducible nitric oxide synthase (iNOS), expression of Arg-1, and production of suppressive cytokines, whereas G-MDSC-mediated immunosuppression is characterized by antigen-specific responses (including ROS release requiring prolonged MDSC and T-cell contacts) [44]. Tumor-associated MDSC generally exhibit an M2-like phenotype, while M1 and M2 phenotypes could coexist in some mouse tumor models [45, 46].
Human MDSC are still poorly defined [47], even if they have been isolated from blood of patients with glioblastoma, colon cancer, breast cancer, lung cancer, or kidney cancer [48–52]. Recent studies have proposed that human MDSC have a characteristic CD34+, CD33+, CD11b+, and HLA-DR− profile [42]. Similarly to the murine counterpart, human MDSC are divided into two main subsets: monocytic MDSC (M-MDSC), characterized by the expression of CD14, and granulocytic MDSC (G-MDSC), identified by positivity for CD15.
A small number of dendritic (DC) are found in most human and murine neoplasms. Similarly to macrophages and neutrophils, plasticity is a main feature of these cells. DC are differentially localized in tumors; for example, in breast cancer immature langerin+ DC are interspersed within the tumor mass, whereas more mature CD83+, DC-LAMP+ DC are confined to the peritumoral area [53]. In contrast to TAM, tumor-associated dendritic cells (TADC) were found in the invasive front of papillary thyroid carcinoma [54]. Growing evidences demonstrate that the majority of TADC found within the tumor microenvironment have an immature phenotype (iDC) [55–57]. The immature stage of TADC is responsible for the tolerogenic response of adaptive immunity against tumors and strongly contributes to tumor immune evasion [58].
2.2.2 Recruitment of Myeloid Cells in Tumors
TAMC derive from monocytes and granulocytes, extravasated from the circulation and infiltrating the tumor mass. Recruitment of blood cells into tumors is mediated by chemoattractants released by tumor and stromal cells. CC chemokine 2 (CCL2), originally known as monocyte chemotactic protein 1 (MCP1), was the first relevant tumor-derived chemotactic factor described [59, 60]. Several other chemokines attracting myeloid cells have been identified, including CCL5, CCL7, CCL8, and CXC chemokine 1 (CXCL1) and CXCL12 [61–63]. Furthermore, urokinase plasminogen activator (uPA); growth factors such as colony-stimulating factor (CSF)-1, transforming growth factor β (TGF-β), basic fibroblast growth factor (bFGF, also known as FGF-2), and vascular endothelial growth factor (VEGF); and antimicrobial peptides (e.g., human beta-defensin-3) were shown to be involved in myeloid recruitment into neoplastic tissues [64, 9, 65–67].
The prototypic chemoattractant for neutrophils, CXCL8, is mainly responsible for the recruitment of TAN; other related chemokines of the CXC subfamily are also involved, including CXCL1, CXCL2, and CXCL6 [68, 69]. Moreover, tumor-derived TGF-β can promote neutrophil migration [70].
CC chemokine receptor 2 (CCR2), CCL2 receptor, CXCL12, CXCL5, and stem cell factor (SCF, also known as KIT ligand) play a pivotal role in the recruitment of MDSC into tumors [71–73]; in addition, Bv8, also known as prokineticine 2 (PROK2), might be essential for MDSC recruitment [74, 75]. Finally, the proinflammatory proteins S-100A9 and S-100A8, produced by MDSC, are implicated in an autocrine loop promoting accumulation of suppressor cells into tumors [76, 77].
TEM do not express CCR2 and are therefore recruited towards tumors by different mechanisms [35, 78, 79]. Other CC chemokines, such as CCL3, CCL5, and CCL8, are produced by tumor cells and could play a role in TEM recruitment [80]. Ang-2, overexpressed by tumor cells and inflamed tissues, has been shown to exert a chemotactic effect on Tie2-expressing blood monocytes in vitro, suggesting that the Ang-2/Tie2 axis might be involved in recruiting TEM into tumors [81, 32, 35, 34, 82]. In addition, recent data suggest the involvement of the CXCL12-CXCR4 homing axis for TEM infiltration [82].
In recent years, it has been shown that tumor-derived factors such as VEGF, CXCL12, CXCL8, β-defensins, and hepatocyte growth factor (HGF) are secreted into the bloodstream and are believed to attract iDC into the tumor bed [83–86]. Moreover, CCL20, CCL7, as well as the receptors CCR5 and CCR6 were demonstrated to be important for TADC recruitment towards the tumor [87].
Proliferation can also contribute to sustaining TAMC levels in solid tumors. A paracrine loop has been evidenced for TAM, with production of colony-stimulating factor 1 (CSF-1) by murine fibrosarcoma cells acting on TAM-expressing CSF-1 receptor (CSF-1R) [88]. A finding confirmed more recently by Condeelis and Pollard [89] showed the effect of epidermal growth factor (EGF) produced by TAM and tumor-derived CSF-1 on recruitment and survival of macrophages during tumor growth. Indeed, macrophage proliferation has been demonstrated to occur during type II inflammation [90].
2.2.3 Tumor-Derived Factors Affecting Myeloid Differentiation and Polarized Functions
Upon arrival in the tumor, monocytes differentiate to macrophages primarily in response to CSF-1 produced by tumor cells. Although coexistence of diverse TAM subpopulations with distinct functions depending on tumor stage and geographical localization within the same tumor has been proposed, they mostly have an M2-like phenotype [91]. Many different studies demonstrated that M2 (pro-tumoral) TAM polarization is driven by cytokines and other signals released in the tumor microenvironment [92]. Among these IL-10, IL-6, CCL2, CSF-1, and prostaglandin E2 (PGE2) were reported to promote M2-like polarization [93, 94]. TGF-β is overexpressed by tumor cells and plays a crucial role in promoting an immunosuppressive phenotype, in addition to driving N2 polarization of TAN [31].
Many tumor-derived factors were implicated in MDSC expansion such as GM-CSF, M-CSF, IL-6, IL-1β, VEGF, and PGE2 [44, 95]. In addition, Bronte and coworkers recently found that cytokine-mediated induction of MDSC was completely dependent on the transcription factor CCAT/enhancer-binding protein b (C/EBPb), shown to function as a master regulator in this process [96]. Further it was proposed that a combination of at least two signals is necessary for MDSC functionality and expansion, for example, GM-CSF, inhibiting maturation of myeloid cells, and a proinflammatory molecule such as interferon-γ (INF-γ) [41].
Soluble factors released by tumor cells (i.e., IL-10, VEGF, TGF-β, etc.) contribute to keep DC in an immature pro-tumorigenic phenotype. Furthermore, in preclinical studies of breast cancer, it was shown that tumor-derived factors altered DC maturation by secretion of thymic stromal lymphopoietin (TSLP), which in turn induces the expression and secretion of the OX40 ligand, a molecule that contributes to sustain the M2-like phenotype of TAM.
2.3 Pro-tumoral Functions of Tumor-Associated Myeloid Cells
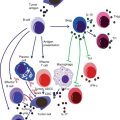
Myeloid cells exposed to the tumor microenvironment most frequently promote tumor progression. They can secrete soluble factors which support proliferation and invasion of tumor cells, activate angiogenesis, and promote resistance to therapies (Fig. 2.2). High TAM or TAN infiltration generally correlates with poor patient outcome [97, 6, 16, 11, 98–101], but few exceptions to this finding are also reported. For instance, in colorectal cancer (CRC) contrasting results reported that TAM density is associated with positive or negative patient outcome [102, 103–105]. On the same line, TAN infiltrate is associated with a favorable prognosis in patients with gastric carcinomas [106], but also with more aggressive pancreatic tumors [107]. Macrophage subsets might have distinct roles, as observed in lung adenocarcinoma were the number of CD204+ TAM showed a strong association with poor patient outcome, while the CD68+ TAM population did not [108]. The concept that not only the number and the presence of specific cell subsets but also the localization of infiltrating cells might have specific functions and predictive values is increasingly emerging. Accordingly, peritumoral TAM density with high expression of co-stimulatory molecules (CD80 and CD86) was associated with better patient survival in CRC, whereas the same cell population within the tumors did not have any predictive value [109, 110]. Thus, TAMC exert complex roles on growing tumors affecting different aspects of tumor progression, i.e. tumor cell proliferation and survival, angiogenesis, tumor dissemination, and resistance to therapies.
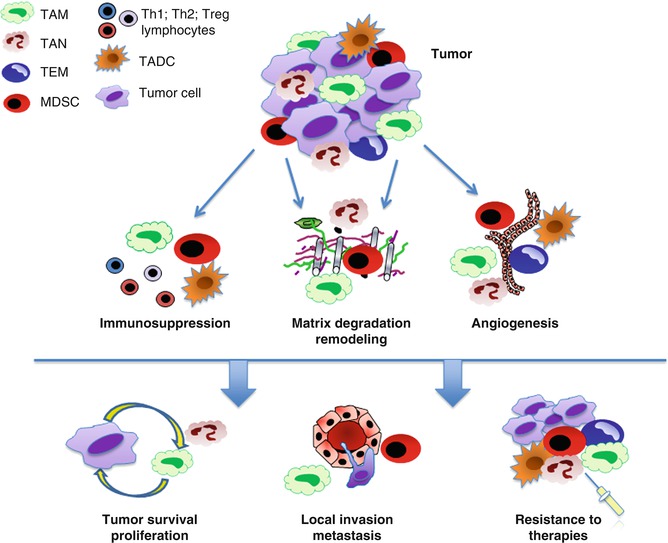

Fig. 2.2
Pro-tumoral functions of tumor-associated myeloid cells. TAMC exposed to the tumor microenvironment exert several pro-tumoral functions, including promotion of angiogenesis, matrix degradation, and suppression of adaptive immunity. These effects are mediated through the release of soluble factors (i.e., cytokines, growth and proangiogenic factors, proteolytic enzymes, etc.) and result in higher tumor survival and proliferation, local invasion and dissemination, resistance to therapies
2.3.1 Tumor Proliferation and Survival
TAM were shown to have the ability to promote tumor growth directly through the production of trophic and activating factors for stromal and cancer cells (EGF, bFGF, VEGF, platelet-derived growth factor β [PDGF], TGF-β) [111, 112, 6, 113] in response to stimuli from the tumor microenvironment. For example, IL-13 and IL-4 produced by CD4+ T-cell-infiltrating tumors, such as breast cancer, led to the production and secretion of EGF by TAM [114]. Moreover, production of proinflammatory cytokines, including TNF-α and IL-6, by TAM and other cells of the tumor microenvironment (e.g., epithelial cells), sustains tumor growth and inhibits apoptosis [115–119].
Several lines of evidence suggest that TAN are required for the rapid growth of tumor cells and their depletion inhibits tumor development [120, 28]. Proteins stored within neutrophil granules (e.g., elastase) may have a role in tumor initiation [121]. In addition, neutrophil-derived ROS have been associated with DNA damage [122]. TAN were shown to be able to produce soluble factors (cytokines and chemokines, HGF, oncostatin M), driving processes like angiogenesis, wound healing, and hematopoiesis and thus exerting a role in tumor promotion and growth [123–125, 121, 101]. For instance, HGF released by neutrophils enhances the invasiveness of human cholangiocellular and hepatocellular carcinoma cells in vitro, and HGF levels in bronchoalveolar lavage fluids were found to correlate with neutrophil number in patients with bronchoalveolar carcinomas, which further correlates with poor patient prognosis [101].
2.3.2 Angiogenesis
To sustain the increased metabolic demand of growing tumors, the development of a tumor vasculature is required. VEGF is the primary, but not the only, angiogenic factor released by tumor cells and is involved in the “angiogenic switch” that can occur at various stages of tumor progression, depending on the tumor type and the microenvironment. Other factors are involved, including PDGF-β, bFGF, angiopoietins, and CXCL12 (SDF-1) [126]. Tumor-associated myeloid cells were shown to contribute to tumor angiogenesis by production of growth factors, cytokines, and proteases [80] such as VEGFA, Bv8, and metalloproteases (MMP) [10, 127, 65, 128].
The prototypic myeloid cell with angiogenic properties is the Tie2 monocyte [32, 35]. TEM can be found in close proximity to nascent blood vessels within solid tumors. In addition, TEM depletion completely prevented neovascularization in preclinical models (spontaneous pancreatic adenocarcinoma, human glioma grown orthotopically in the mouse) [33]. Interestingly, TEM ablation did not affect the number of infiltrating TAM or TAN, suggesting that TEM are an entity on their own and not just precursors of TAM [35]. How TEM stimulate angiogenesis has not been clarified yet, but preliminary indications in murine tumor models point to the fact that perivascular TEM secrete bFGF. It is believed that release of such factors in close proximity to vessels could directly stimulate angiogenesis or MMP9 secretion, which in turn would release growth factors entrapped within the extracellular matrix (ECM).
TAM have also a profound influence on the regulation of tumor angiogenesis [129]. It was demonstrated in several preclinical studies that TAM positively correlated with microvascular density (MVD) [130–133]. Lin and coworkers were the first to describe the direct role of TAM in driving the “angiogenic switch” in a spontaneous mammary carcinoma mouse model [134]. Likewise, depletion of monocytes by clodronate treatment in a preclinical model with Lewis lung carcinoma led to lower TAM infiltration and angiogenesis, further underlining the importance and the involvement of macrophages in tumor angiogenesis [135].
TAM express various molecules modulating angiogenesis, such as VEGF, bFGF, TNF-α, IL-1β, CXCL8, cyclooxygenase 2 (COX2, also known as PTGS2), plasminogen activator, uPA, PDGF-β, MMP7, MMP9, and MMP12 [136]. Hypoxia exerts a crucial role in the upregulation of gene transcription in TAM, promoting VEGF expression [137–141]. Other recent studies showed a direct involvement of TAM in tumor angiogenesis and neovascularization via transdifferentiation into endothelial cells when stimulated by angiogenic factors [142, 143].
More recent studies have shown that MDSC can contribute to tumor angiogenesis. In a preclinical model for colon cancer, MDSC positively correlated with tumor growth rate and blood vessel density [144]. Moreover, tumor angiogenesis was significantly lowered by blocking Bv8 with a neutralizing antibody, a treatment that significantly reduced the number of MDSC [74]. Metalloproteases, particularly MMP9, MMP2, MMP13, and MMP14, produced by MDSC, were shown to enhance VEGF bioavailability by mobilization from the ECM [144, 145]. Increased recruitment of MDSC has also been demonstrated in the presence of hypoxia, possibly stimulating tumor angiogenesis [126, 74]. Parallel to TEM, MDSC were also observed to be localized in the vicinity of blood vessels. Under certain conditions, some MDSC acquire endothelial cell shape, start to express endothelial markers including CD31 and VEGFR2, and are eventually incorporated into the tumor endothelium [144].
TAN were shown to rapidly release VEGF from internal storage compartments, leading to endothelial proliferation and tubule formation [146, 147]. In addition, TNF-α and GM-CSF secreted by tumor cells were shown to trigger the release of proangiogenic chemokines by TAN. The number of TAN in myxofibrosarcoma positively correlated with tumor MVD [148]. Furthermore, in a xenograft mouse model of human melanoma where cancer cells were engineered to constitutively produce CXCL6, it was found that the number of TAN as well as angiogenesis was markedly increased [149]. Studies in the RIP1-TAG2 mouse model for pancreatic carcinogenesis revealed formation of dysplastic, neutrophil-bearing, angiogenic islets upon malignant transformation. In the abovementioned model, neutrophil depletion of the islets led to dramatically lowered angiogenesis [150].
In recent years, it has become more and more apparent that iDC make a profound contribution to tumor angiogenesis [85]. TNF-α and CXCL8 produced by iDC from ovarian cancer ascites triggered the release of various growth factors from EC [85, 151]. Moreover, iDC were shown to release osteopontin which promotes monocyte secretion of the proangiogenic IL-1β [152]. Finally, it was recently observed that iDC produced high levels of VEGF and CXCL8 under hypoxic conditions, which, in turn, might inhibit DC maturation and further promote angiogenesis via this autocrine loop [153, 151].
2.3.3 Cancer Cell Dissemination
The major cause of death in cancer results from therapy-resistant metastases. Stephen Paget’s conclusion in the late nineteenth century that the metastatic process depends on cross talk between selected cancer cells (the “seeds”) and a specific organ microenvironment (“the soil”) is still valid and is experimentally confirmed [154, 155]. Tumor metastasis is a complex multistep process, during which malignant cells spread from the primary tumor site to secondary distant organs. The different steps of cancer cell dissemination can be subdivided into local invasion, entry into the bloodstream (intravasation), survival in the bloodstream, extravasation, and colonization [156]. Mesenchymal, endothelial, and immune cells are required to form an appropriate microenvironment for tumor progression [157]. Immune cells, particularly macrophages, neutrophils, T lymphocytes, and natural killer (NK) cells, are major sources of proteases that degrade the host tissue, allowing cancer cells to disseminate.
The set of proteolytic enzymes found in tumor microenvironment comprises matrix metalloproteases, serine proteases, and cysteine proteases (i.e., cathepsin) [158–162]. Matrix proteases exert essential functions in physiological conditions as active regulators of postnatal tissue development and remodeling. In addition, they are important for tissue repair in response to injury and regulate cancer progression modulating the tumor microenvironment, particularly the leukocyte infiltrate [163]. MMP were shown to activate TGF-β, which is an important regulator of T-cell and TAN functions [164]. Proteases also produce specific cleavage fragments of target chemokines with independent biological activity, ranging from anergic products (CXCL7, CXCL4, CXCL1), antagonists (CCL7), or more potent chemoattractants (CXCL8), thereby modulating the leukocyte composition within a tumor [165–167].
Besides their influence on the tumor infiltrate, proteases were shown to promote cancer cell invasion and intravasation. The cleavage of cell-adhesion molecules like E-cadherin induces the disruption of cell-cell junctions leading to loosening of cell-cell contacts which, together with ECM protein turnover, facilitated cancer cell migration and invasion into the surrounding tissue and vasculature. Tight regulation of the single proteases within the tumor microenvironment allows the control of tumor cell invasion [168].
After invasion to the surrounding tissues, cancer cells enter the blood circulatory system directly or indirectly via the lymphatic system. Since the majority of circulating tumor cells (CTC) are eliminated by NK cells [169], only about 0.01 % of CTC survive in the bloodstream [157]. Platelets play a key role in hematogenous metastasis and contribute to the survival of CTC in the bloodstream by both thrombin-dependent and thrombin-independent mechanisms [170]. After a passage into the bloodstream, CTC adhere to vessel walls for extravasation when they are in the vicinity of secondary metastatic organs. Circulating tumor cells take advantage of the capability of neutrophils and platelets to produce and secrete adhesion molecules, such as integrins and selectins which all aid the nearby CTC to adhere and ultimately extravasate [170, 171].
The arrest of cancer cells to specific organs seems to be primarily “mechanical” [172]. However, chemokines and chemokine receptors are also involved in organ-specific colonization, which finally drive cells along tissue-specific chemokine gradients. Furthermore, a non-chemokine pathway also exists, in which immune cells support organ-specific cancer cell dissemination. One example is represented by the two inflammatory mediators S100-A8 and S100-A9, which were shown to promote metastasis through serum amyloid A 3 (SAA-3) [173].
The subsequent growth of arrested tumor cells will depend on the molecular interactions between cancer cells and the microenvironment of the new organ. Although cancer cells are sometimes said to “home” to specific organs (e.g., breast tumors metastasizing to bone), it is more likely that this organ specificity is due to efficient organ-specific growth rather than preferential “homing” of cells to a particular organ.
It has been suggested that tumor cells can influence the microenvironment of secondary organs promoting the formation of a pre-metastatic niche [174, 175]. Tumor-derived factors and HSC are crucial components of the pre-metastatic niche. VEGF derived from tumor cells promote recruitment to the secondary organs of VEGFR1-expressing HSC that induce fibronectin and MMP9 expression by resident fibroblasts, creating favorable conditions for settlement of future metastases [176]. Other soluble factors released by tumor cells can promote the formation of pre-metastatic niche. In a murine model of breast cancer, tumor cells were found to induce production of CCL17 and CCL22 in the lung; both attracting CCR4+ tumor and immune cells which establish a microenvironment for metastases settlement at secondary organs [177]. Moreover, it was demonstrated that the prototypic hypoxia-induced protein lysyl oxidase (LOX), often found in tumors, leads to cross-linking of collagen IV in basement membranes, in addition to recruitment of CD11b+ myeloid cells which adhere to the abovementioned collagen meshwork. The captured CD11b+ myeloid cells were shown to secrete MMP2, which facilitated invasion and recruitment of metastasizing tumor cells [178].
TAMC, TAM and MDSC in particular, are important players of tumor progression and metastatic colonization through the cross talk with tumor cells. For instance, macrophages play a crucial role in conferring an invasive phenotype to epidermal keratinocytes from Snail transgenic mice [179]. TAM contribute to cancer cell dissemination by releasing enzymes involved in degradation of the ECM (i.e., MMP and cathepsin) [168, 161, 180, 76], or motility factors. Recently we found that tumor-derived soluble factors, particularly CSF-1, activate a transcription program in macrophages resulting in upregulation of a series of genes, especially migration-stimulating factor (MSF). MSF is a truncated isoform of human fibronectin 1, physiologically expressed during fetal life and upregulated in M2-like macrophages [181, 182]. MSF exerts a chemotactic effect on tumor cells, indicating that macrophage products released in the tumor microenvironment can support the pro-invasive phenotype of tumor cells [181]. An example of the cross talk between TAM and tumor cells involved in metastatic colonization is shown in breast cancer, where EGF secreted by TAM increases migration and invasion of neighboring breast cancer cells which express high levels of EGF receptor (EGFR). On the other hand, cancer cells secrete high levels of CSF-1, a main chemoattractant for TAM which expresses the cognate receptor CSF-R1. Therapies aiming at inhibiting this cross talk by blocking CSF-R1 and/or EGFR were shown to be successful [183, 184]. Macrophages and their reciprocal cross talk with tumor cells are mandatory for tumor cell migration, regardless of the factor inducing cell invasion (i.e., SDF-1).
A myeloid cell population involved in tumor progression, including invasion, is represented by MDSC. A direct role for MDSC in tumor metastasis has not been demonstrated; however, a connection was suggested by the study on mice deficient for the TGF-β receptor type 2 (TGF-β-R2), in which MDSC were concentrated on the invasive margin. In addition, it is possible to reduce lung metastases by antagonizing CXCR2 and CXCR4, two receptors involved in homing of MDSC [145]. As previously mentioned, PGE2 and the proinflammatory molecule S100A9 have been identified as main effectors of MDSC accumulation and function. Accordingly, S100A9 deficient mice rejected implantation of colorectal cancer, while administration of wild-type MDSC reverted the phenotype and colorectal cancer cells could successfully engraft [76]. In addition, TGF-β was demonstrated to be instrumental in MDSC homing, mediated via CXCL12-CXCR4 and CXCL5-CXCR2 axis in a preclinical mammary cancer model [145].
2.3.4 Suppression of Adaptive Immunity
Besides the effect on tumor growth and dissemination, TAMC have also the potential to suppress the adaptive immune response, leading to cancer immune evasion [185].
M2-like polarized tumor-infiltrating macrophages are characterized by an immunosuppressive phenotype, with production of high levels of the immunosuppressive cytokines IL-10 and TGF-β and reduced expressions of IL-12 [19, 186, 92, 187, 188]. In addition, they have reduced tumoricidal activity and are poor in antigen presentation [189]. Furthermore, TAM secret chemokines, such as CCL17 or CCL22, that preferentially attract Th1, Th2, and T regulatory (Treg) lymphocytes with defective cytotoxic functions, or such as CCL18, that recruit naïve T cells which become anergic in contact with M2 macrophages and iDC [8, 190–192].
MDSC play a prominent role in the inhibition of tumor-specific immune responses. MDSC localized within the tumor microenvironment has an M2-like phenotype and mediate immunosuppression through multiple pathways, that is, production of Arg-1 [193], iNOS [194, 195], ROI, and suppressive cytokines including IL-10 and TGF-β [196], or via the activation and recruitment of Treg [196, 197]. MDSC inhibit homing to lymph nodes of CD4+ and CD8+ T cells and suppress their activation [198, 199]. It was found that cysteine uptake by MDSC limited its availability for uptake by T cells, which in turn disables their activation and renders them nonfunctional. Furthermore, it was shown that posttranslational T-cell receptor modifications mediated via generation of peroxynitrite species led to anergy of effector CD8+ T cells [196]. MDSC can also impair innate immunity through cross talk with macrophages which led to decreased production of IL-12 by macrophages and increased production of IL-10 by MDSC, thus driving a polarization towards an M2-like phenotype [200].
In addition to the above described mechanisms in TAM and MDSC, TADC were found to be involved in suppression of adaptive immunity. One mechanism leading to the induction of tumor-specific T-cell tolerance was via upregulation of inhibitory molecules such as B7-H1 [201] or by inducing the expression of Arg-1 [202]. Moreover, it was shown that the induction of oxygen-dependent pathways led to the downregulation of CD3 epsilon and T-cell apoptosis [203]. Furthermore, Muller and coworkers demonstrated that upregulation of indoleamine 2,3-dioxygenase (IDO) in TADC contributed to immunosuppression [204].
2.4 Selected Aspects of Therapeutic Targeting of TAMC
The above summarized data describing the pro-tumoral role of the myeloid infiltrate of tumors make clear that TAMC are reasonable targets for novel therapeutic approaches. As illustrated above, TAMC can directly promote tumor cell growth releasing growth factors and proangiogenic molecules, in addition to suppression of tumor-specific immune responses. Strategies explored in the last years are focused on the stoppage of the mechanisms leading to suppression of lymphocyte activity and, on the other side, on the reduction of recruitment of myeloid cells and repolarization of M2-like pro-tumoral cells to proinflammatory M1 macrophages. There is a wide range of preclinical and clinical research aimed at eliminating or reprogramming TAMC [39]: here we only mention some examples of the results obtained so far in this growing field of anticancer research.
Many studies have shown that targeting TAM might be a successful strategy to limit tumor growth and metastasization and to achieve better therapeutic responses [32, 44, 59, 82, 189, 205, 206, 207]. One example is represented by bisphosphonates [208] traditionally used in the clinic to treat osteoporosis, which were shown to be very effective in depleting TAM and inhibiting angiogenesis as well as metastatic spread in preclinical animal models for breast cancer [209, 210]. Furthermore, Germano and coworkers recently showed that specific targeting of macrophages with the marine antitumor agent trabectedin was very successful in four different preclinical tumor animal models [211].
An alternative strategy is to target circulating monocytes known as precursors of TAM. Two candidate molecules are the M-CSF receptor (solely expressed by monocyte-macrophages) and the chemokine CCL2, involved in monocyte recruitment within tumors. Since preclinical studies on prostate and colon cancer [212–215] identified CCR2+Ly6C+ cells as targets involved in cancer progression and metastasis, CCL2 antibodies are currently investigated for therapeutic applications in human cancer treatment. Another approach to affect TAM specifically is to try to reeducate them to become tumoricidal or, in terms of polarization, to try to repolarize them towards an M1 phenotype. Several successful trials using CpG-oligodeoxynucleotide (TLR9 agonists) were performed in combination with anti-IL-10 receptor or anti-CD40 antibodies, which reverted pro-tumoral M2-like TAM to M1 macrophages displaying antitumor activity [216–218]. Rolny et al. recently demonstrated that skewing of M2 TAM towards M1 leads to effective antitumoral activity of host histidine-rich glycoprotein (HRG), which in consequence leads to inhibition of angiogenesis and promoted antitumor immune responses [219]. Gazzaniga and coworkers reported promising results using the molecule legumain, which targets M2 polarized TAM specifically, and was able to induce a robust CD8+ T-cell answer leading to reduced tumor growth and inhibition of tumor angiogenesis [220]. Furthermore, it was shown that zoledronic acid was able to revert M2 towards M1 TAM and inhibit breast carcinogenesis by targeting the mevalonate pathway [221]. Moreover, it was demonstrated that direct reeducation of TAM using the prototypical M1 polarizing cytokine INF-γ [222] is successful in promoting antitumor activity in minimal residual disease [8]. In line with the abovementioned results are the findings that inhibition of M2 polarization led to restoration of M1 proinflammatory phenotype and inhibition of tumor growth in several preclinical animal models [92, 223, 224].
To counteract the pro-tumoral activities of MDSC, two general strategies can be envisaged; the first consists of transforming these immature cells into mature cells devoid of suppressive activity, and the second is focused on blocking MDSC suppressive functions. Depletion of MDSC producing high levels of TGF-β (in an IL-13-dependent manner) led to the restoration of T-cell-mediated immunosurveillance in a preclinical mouse model for fibrosarcoma [225]. Several studies have shown that metabolites of all-trans-retinoic acid are able to differentiate MDSC into DC and macrophages, reducing MDSC accumulation [226, 227]. This effect was demonstrated to be beneficial for patients suffering from metastasizing renal cancer, since in these patient less circulating MDSC were detected in the bloodstream [228]. Furthermore, one of the beneficial effects of the anticancer drug gemcitabine is its potential to eliminate MDSC without affecting T, B, NK cells, or macrophages [229].
The second possibility to counteract MDSC function is to block their inhibitory function, for example, by using COX2 inhibitors, phosphodiesterase (PDE5), and nonsteroidal anti-inflammatory drugs releasing NO [44]. Blocking of IL-1β inhibits cancer progression and metastasis [230] and decreases MDSC accumulation and suppressive activity [42]. Moreover, the proangiogenic chemokine Bv8 was shown to be important for mobilization and homing of MDSC to tumor sites and therefore qualifies as an interesting therapeutic target [74].
Complete neutrophil depletion in already immunocompromised patients is not desirable; therefore, the strategy of choice concerning TAN might be to disturb their tumor homing ability, in other words to interfere with their ability to migrate. To this purpose, preclinical experiments using anti-CXCR2 antibodies were performed and were shown to be successful [231]. Furthermore, considering the well-documented key role of TGF-β in skewing TAN towards a N2 phenotype, this cytokine keeps promising potential for treatment [70, 31].
Some studies indicate that blocking IL-10 together with the administration of CpG oligonucleotides are able to unblock the functionally paralyzed TADCs and to reactivate antitumor responses [232]. Another strategy enhancing immunotherapy might be targeting of soluble factors like VEGF, IL-10, TGF-β, gangliosides, and others, which are all tumor secreted factors leading to abnormal differentiation of DC, often leaving them in an immature state [233]. Other and more recent strategies make use of siRNA nano-complexes which lead to reprogramming of TADC from an immunosuppressive to an activated anticancer phenotype [234]. Furthermore, it was shown that in situ stimulated CD40 and toll-like receptor 3 (TLR3) TADC were successfully transformed from immunosuppressive to immunostimulatory cells [235]. More recently it was demonstrated that delivery of regulatory miRNA, particularly miRNA 155 in a nanoparticle formulation, leads to reprogramming of immunosuppressive TADC to highly active antitumoral TADC which provoked regression of established ovarian tumors [236].
In light of the recent results, tumor therapy with drugs targeting the inflammatory tumor microenvironment in combination with treatment aimed at defeating TAM, TAN, and other myeloid cells holds promise for the future.
2.5 Concluding Remarks
In recent years, it has become clear that inflammation has an essential role in tumor promotion [1–6]. The inflammatory tumor microenvironment, mainly consisting of soluble factors and host cells, has a predominant role in all aspects of the disease (progression, angiogenesis, immune surveillance). In particular, a heterogeneous group of myeloid cells is the most consistent host cell component of solid tumor [8, 9]. TAM, TEM, MDSC, TAN, and TADC display distinct specialized functions, as well as overlapping activities (e.g. angiogenesis). Tumor and stromal cells release different chemoattractants involved in the recruitment of myeloid cells from the blood into the growing tumor. Cytokines and other soluble factors released in the tumor microenvironment can contribute to induce a protumoral phenotype, promoting M2 polarization of TAM [92], N2 polarization of TAN [31], MDSC expansion [41], or preventing maturation of DCs. Thus the different TAMC populations potentially represent a target for new therapeutic approaches aimed at breaking the protumoral networks established by cancer-associated myeloid cells.
Acknowledgments
The authors would like to gratefully acknowledge the financial supports of the European Research Council (ERC project HIIS), the European Commission (FP7-HEALTH-2011-ADITEC-280873), the Italian Association for Cancer Research, the Italian Ministry of Health and University, Fondazione CARIPLO (project 2009–2582), and Regione Lombardia (project Metadistretti – SEPSIS).
References
1.
Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339(6117):286–91.PubMedCentralPubMed
2.
Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22(1):33–40.PubMed
3.
DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012;246(1):379–400.PubMed
4.
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–81.PubMed
6.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44.PubMed
7.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99.PubMedCentralPubMed
8.
Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231–7.PubMed
9.
Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117(5):1155–66.PubMedCentralPubMed
10.
Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–96.PubMed
11.
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51.PubMed
12.
Laghi L, Bianchi P, Miranda E, Balladore E, Pacetti V, Grizzi F, et al. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10(9):877–84.PubMed
13.
Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–66.PubMed
14.
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–95.PubMedCentralPubMed
15.
Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67(19):9518–27.PubMed
16.
Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–8.PubMed
17.
Dinapoli MR, Calderon CL, Lopez DM. The altered tumoricidal capacity of macrophages isolated from tumor-bearing mice is related to reduce expression of the inducible nitric oxide synthase gene. J Exp Med. 1996;183(4):1323–9.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree