Abstract
Traditionally breast cancer has been classified according to morphologic features. Despite emerging era of personalized medicine and availability of molecular testing, the histologic classification remains valuable. Different patterns of breast cancer are known to have different clinical presentations, and some of the patterns are associated with a specific prognosis. Grading of breast cancer also provides additional prognostic information. Routine analysis for estrogen and progesterone receptor and HER2/neu has shown that breast cancer is not biologically a single disease. Another group of breast cancers has also emerged. This group lacks all three markers and is classified as “triple-negative” carcinoma.
Keywords
invasive breast carcinoma, molecular and histologic classification, personalized medicine
Molecular classifications, the availability of targeted therapies, and the emerging observations provided by pharmacogenomics and molecular studies underscore that breast cancer could not, and should not, be treated as one disease.
Since the 1990s, tremendous advances in cancer treatment have been achieved. To achieve a higher cure rate, a comprehensive database on all breast cancers is required. Once all data are integrated into a final report, the results can be more easily retrieved by local and national tumor registries. Currently the national registry for Surveillance, Epidemiology, and End Results (SEER) gathers its information from local tumor registries. Because the original data are incomplete, available data through the SEER registry do not provide a complete summary regarding the outcome of many cancers. Personalized medicine cannot be achieved without a thorough understanding and careful follow-up of a large number of histologically and biochemically well-defined and similar breast cancer subsets. The purpose of this chapter is to highlight the histologic subtype and link morphologic findings to those of biochemical results and potentially to the molecular subtypes of breast cancer.
Traditionally breast cancer has been classified according to the morphologic features. Despite the emerging era of personalized medicine and the availability of molecular testing, the traditional pathologic classification of infiltrating breast carcinoma remains valuable. Pathologists were one of the first groups to recognize that breast cancer was a morphologically heterogeneous group of diseases. Different patterns of breast cancer were noted to have different clinical presentations, and some of the patterns were associated with specific prognoses. In addition, pathologists were able to describe a grading system that seemed to be an independent prognostic indicator. Once tumors were routinely analyzed for the presence of estrogen and progesterone receptors (ER/PR), others in the scientific community realized that breast cancers are quite heterogeneous. During the late 1980s and early 1990s, it was recognized that some breast cancers show HER2/neu protein overexpression or ERBB2 gene amplification. This group represented a subset of cancers that had a worse prognosis, independent of their clinical or pathologic stage. After all tumors were routinely examined for ER/PR and HER2/neu amplification, another group of cancers emerged. This group of breast cancers did not express hormonal receptors and showed no evidence of HER2 amplification. These tumors were classified as “triple-negative” carcinomas (TNBCs). Recent studies have shown that even TNBCs represent a heterogeneous group of cancers.
Molecular classifications, the availability of targeted therapies, and the emerging observations provided by pharmacogenomics and molecular studies underscore that breast cancer could not, and should not, be treated as one disease.
Traditional histopathological classification of breast cancer, including the World Health Organization (WHO) classification remains important; however, other information must be considered before any treatment decision. In this chapter, an attempt is made to delineate how all available information including traditional histopathological classification, the commonly used grading system, and the result of ancillary tests can be incorporated. This approach allows us to better understand the biology of cancer and might allow us to link traditional information to molecular subtyping.
Tremendous advances in cancer treatment have been achieved during the past two decades. To achieve a higher cure rate, more comprehensive data on all breast cancers is required. Once all data are integrated into a final report, the results can be easier to retrieve by the local and national tumor registries. Currently the national registry for Surveillance, Epidemiology, and End Results (SEER) gathers its information from local tumor registries. Because the original data are not complete, available data through the SEER registry do not provide a complete summary regarding the outcome of many cancers. Personalized medicine cannot be achieved without a thorough understanding and careful follow- up of a large number of histologically and biochemically well-defined and similar breast cancer subsets.
Molecular Classification
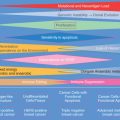
Molecular classifications have contributed to the paradigm shift that human breast cancer is not one disease. Breast cancer is considered to show at least five major molecular subtypes, each characterized by distinct gene expression profiles. This is of paramount importance because the patients with varying subtypes have different clinical outcomes, and each responds differently to treatment ( Fig. 10.1 ).
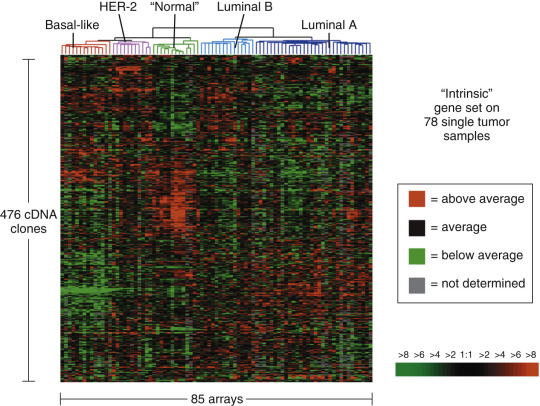
By definition, luminal type cancers express hormonal receptors (ER/PR). According to this classification, luminal A cancers express both the ER and PR, but HER2 amplification is absent. They are also marked by low levels of proliferation markers. The second group is luminal B which also express ER and PR and may, or may not, be HER2. Luminal B tumors have a higher level of expression of proliferation markers compared with luminal A. The great majority of invasive breast cancers belong to one of these two groups. As it has been known for many decades that ER status is a predictive biomarker in the treatment of breast cancer. Luminal A and B subtypes are phenotypically characterized by the expression of ER; the overall survival of patients with luminal A cancers is significantly greater than patients with luminal B cancers. The level of ki67, a proliferation marker, plays an important role in luminal B cancers. High levels of proliferation have been shown to be an independent prognostic indicator in lymph node–negative cancers. The HER2 overexpressing subtype occurs in approximately 10% to 20% of breast cancers. These patients respond to HER2-directed therapy such as trastuzumab, a humanized monoclonal antibody directed to the external domain of HER2 (or ERBB2 ) transmembrane tyrosine kinase, and lapatinib, a small molecule inhibitor of HER1 (epidermal growth factor receptor) and HER2. HER2 overexpressing cancers and basal-like subtypes have higher initial responses to anthracycline-based chemotherapy than the luminal subtypes and have worse prognosis and shorter disease-free survival. As evident from the Table 10.1 TNBC represent 20% to 25% of all invasive cancers. This group does not represent a biologically uniform cancer; in fact it is very heterogeneous. Table 10.1 suggests that a great majority of basal-like tumors express markers such as CK5/6 and P63; other studies show a more diverse immunoprofile. The subtypes defined by gene expression arrays can also be approximated by standard immunohistochemical stains (see Table 10.1 ). Another study by Cheang and colleagues has confirmed that using additional immunostains, such as CK5/6 and EGFR, could identify a group of TNBCs with a worse prognosis.
| Subtype | Percent | DDS | Outcome , a OS | Standard IHC |
|---|---|---|---|---|
| Luminal A | 51–61 | 75 | 90 | ER+, PRþ, HER2- |
| Luminal B | 14–16 | 47 | 40 | ER+ and/or PR+, HER2+ |
| HER2 | 7–9 | 34 | 31 | ER and PR-, HER2+ |
| Basal | 11–20 | 18 | 0 | ER, PR, and HER2–, Cytokeratin 5/6 and EGFR+ |
| Unclassified | 2–6 | NA | NA | Negative for all markers |
a Percent of patients without distant metastases or alive with systemic therapy at 5 years.
The heterogeneity of TNBCs has been known for many years. Recent studies have identified that TNBCs have various types of long noncoding RNAs (ncRNAs), defined as RNA molecules longer than 200 nucleotides in length that do not belong to known categories of small RNAs are involved in a spectrum of biological processes, such as development and maintenance of pluripotency. On the basis of this system, Lui and colleagues were able to classify TNBCs into four distinct clusters, including an immunomodulatory subtype (IM), a luminal androgen receptor subtype (LAR), a mesenchymal-like subtype (MES), and a basal-like and immune suppressed (BLIS) subtype.
Increasingly, clinical trials are being designed according to the different molecular subtypes of breast cancer. In contrast, earlier clinical trials required that patients have only histologic confirmation of breast cancer. Nevertheless the initial histologic classification remains important in establishing the initial diagnosis.
Histopathologic Classification
Understanding the histopathologic features of breast cancer remains a necessary element for the appropriate management of breast cancer. This histopathological classification system has been well established for many years. The latest WHO classification of breast cancer recognizes 20 subtypes of breast cancer. There have been two general approaches to prognostication via histopathologic analysis. The first categorizes breast carcinomas based on specific features, recognizing the so-called special type of carcinomas. The second parameter evaluates the grade of the cancer. Two grading systems, Bloom-Richardson and Nottingham, are routinely applied to all invasive cancers. Both of these systems consider similar aspects of the tumor including nuclear pleomorphism, extent of gland formation, and mitotic index. Using this approach has allowed the pathologists and clinical teams to categorize patients with a very good prognosis to a very poor prognosis. Classifying tumors based on their morphology and assigning a grade are a standard component of pathology reports.
Histologic Types of Invasive Carcinoma
Invasive Mammary Carcinoma, Not Otherwise Specified
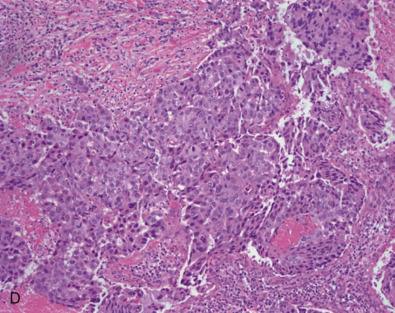
Early classifications of breast carcinomas used the term lobular for tumors commonly associated with lobular carcinoma in situ. Because ducts were the other source of epithelium within the breast, lesions that did not have a lobular pattern were referred to as ductal . As highlighted in the WHO classification system of invasive breast cancer, 40% to 75% of breast cancers are currently classified as invasive mammary carcinoma, not otherwise specified (IDC NOS). Breast cancers are known to originate from terminal duct-lobular structures; the term ductal is a misnomer. These tumors are best classified as IDC NOS. Nevertheless the term ductal is used by both clinicians and pathologists (hence the acronym IDC ). These tumors are poorly characterized and represent a heterogeneous group of cancers based on gross appearance, morphologic features, grade, and the prognostic biomarkers. In addition, some familial breast cancers with known BRCA1 and BRCA2 mutations are classified as mammary carcinoma, NOS (invasive ductal carcinoma). The majority of carcinomas with familial BRCA1 and BRCA2 mutation are higher grade. Many familial BRCA cancers are TNBC and show basal subtype differentiation. Despite numerous publications, immunostains for basal subtype markers are not routinely applied to separate basal subtype cancers from the other invasive mammary carcinoma, NOS. Fig. 10.2 shows a composite image of four separate cancers with various morphologic features and grades. All four cancers are classified as mammary carcinoma, NOS. There is clearly a need to further divide this group.
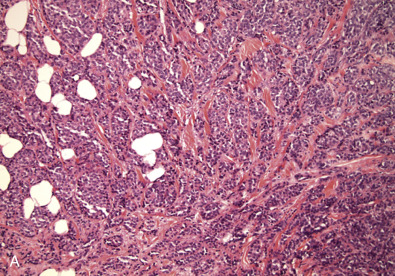
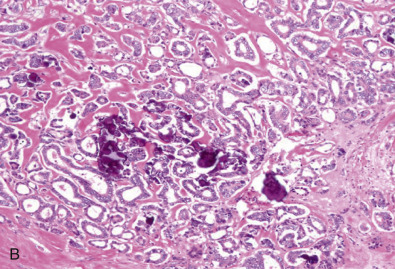
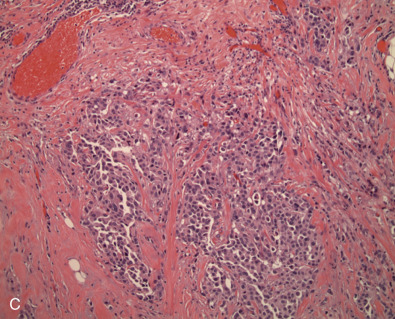
Invasive Lobular Carcinoma
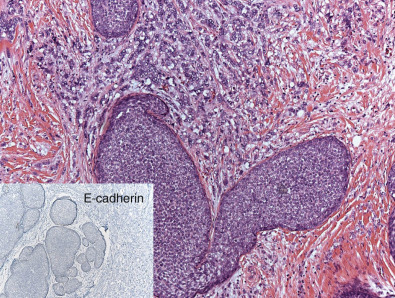
Approximately 5% to 15% of invasive carcinomas are classified as invasive lobular (ILC). More than 90% of the tumor should show lobular features to be classified as such. If 50% to 90% of the tumor shows lobular features, these cancers are currently classified as ductal-lobular carcinoma. The incidence of ILC has been increasing since the 1980s. The increase in incidence might be related to hormone replacement therapy. An alternative explanation for the increased incidence may be the significant improvement in detection methodology. Many of these cancers are known to grow along the fibrous septae and do not form a distinct mass. On occasion, patients have developed metastatic disease, and the primary site was only subsequently recognized using magnetic resonance imaging (MRI). Morphologically, ILCs show single discohesive cells that infiltrate the fibrous connective tissue. Tumor cells often form a concentric pattern around normal ductal structures, showing the characteristic targetoid pattern of ILC. These tumors are known to be more often multicentric. The incidence of contralateral tumors, particularly synchronous tumors is reported to be as high as 5% to 19%. This rate is higher than those reported for IDC NOS. Several morphologic patterns are recognized. The most common type is the “classic type” composed of a uniform, discohesive cell population. The pleomorphic variant may either grow as single file pleomorphic cells or form globular aggregates. The tubulolobular variant shows both evidence of tubule formation and the characteristic lobular feature of single file tumor cells. The last type represents a mixture of ductal carcinoma NOS, admixed with a single file of tumor cells. More than 95% of these tumors are luminal type and strongly express ER.
Loss of heterozygosity of the 16q chromosomal regions and the absence of epithelial cadherin (E-cadherin) expression are common findings in invasive lobular carcinomas. ILC and LCIS show a decrease or absence of E-cadherin expression while showing aberrant expression of p120 catenin. Recent studies suggest lack of functionality of E-cadherin with aberrant expression of p120 is more useful in detecting these cancers than absence of E-cadherin alone. The majority of ILC cases do not express basal cell markers such as CK5/6, CK14, or CK17. Fig. 10.3 shows a composite image of invasive lobular carcinoma. Unfortunately pathologists have not uniformly applied criteria for this diagnosis; historically ILC was thought to have a better prognosis than other subtypes ; however, recent data do not completely support this assumption. The majority of investigators agree that these tumors are responsive to endocrine therapy and respond relatively poorly to chemotherapy. It remains important to clearly separate ER-positive and HER2-negative ILC from rare ILCs that lack ER and/or overexpress HER2.
Tubular Carcinoma
Tubular carcinomas are grade I invasive carcinomas that express ER and PR but not HER2. This pattern strongly correlates with the luminal A molecular subtype. Distant metastatic potential is highly unlikely when this tumor is present in pure form. The diagnosis is made when characteristic angulated tubules, composed of a single layer of relatively uniform cells with no significant pleomorphism, comprise at least 90% of the carcinoma ( Fig. 10.4 ). These neoplastic tubules are haphazardly arranged and are often found infiltrating between existing benign structures. Low-grade ductal carcinoma in situ and atypical ductal hyperplasia are common associated findings.
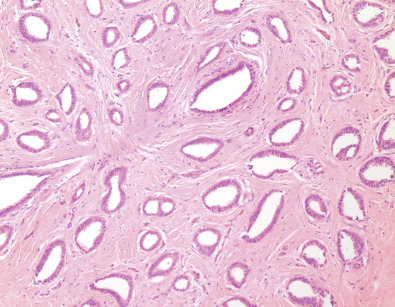
The prognosis for patients with tubular carcinoma depends on the purity of the histologic pattern. In the classic series of 54 cases reported by Cooper, Patchefsky, and Krall, all 12 patients whose carcinoma was composed purely of the characteristic low-grade, angulated tubules survived 15 years, regardless of tumor size.
In screening programs, tubular carcinoma represents 9% of detected carcinomas, whereas in mammographic series, this special type of carcinoma is responsible for as many as 27% of detected carcinomas. Mammographic features include a spiculated mass, with or without associated microcalcifications, or, less commonly, asymmetric density and architectural distortion with associated calcifications.
Tubular carcinoma represents only approximately 3% to 5% of all invasive carcinomas; thus the significance may be lost when cases are grouped and analyzed only by stage. The importance of tubular carcinoma lies with therapeutic decisions for individual patients. It is more likely to occur in older patients. The survival of patients with tubular carcinoma is generally similar to that of the general population, and systemic adjuvant therapy may be avoided in these patients. A review of surgical therapy states that for cases of pure tubular carcinoma with an adequate negative margin, mastectomy, radiation, or even axillary lymph node dissection may be unnecessary.
Invasive Cribriform Carcinoma
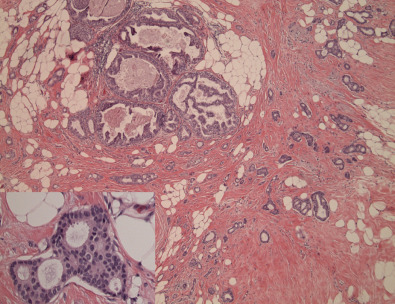
Closely related, histologically and biologically, to tubular carcinoma is invasive cribriform carcinoma (ICC). Histopathologically, these carcinomas infiltrate the stroma as islands of cells that have the same appearance as cribriform-type ductal carcinoma in situ ( Fig. 10.5 ). Differentiating cribriform in situ from ICC may be difficult because of distortion and scarring. Irregular clustering of cellular islands signifies an invasive process. Another helpful feature is that the invasive islands in ICC are usually evenly spaced and often of uniform size. One-fourth of cases have intermixed areas of tubular carcinoma. Because both tubular carcinoma and ICC have equally excellent prognoses, this feature has no bearing on an otherwise excellent prognosis. In studies of pure tubular carcinoma and ICC, the presence of one or two positive low axillary lymph nodes did not adversely affect survival. More than 90% of tumors should show this pattern because the presence of carcinoma that does not conform to special-type criteria increases the likelihood not only of nodal involvement but also of shorter survival. Similar to tubular carcinomas, these tumors are strongly ER/PR-positive, HER2-negative, and ki67 low. They also belong to the luminal A molecular subtype of breast cancer.
Mucinous Carcinoma
Mucinous (colloid) carcinoma, when present in its pure form, is also associated with an excellent prognosis. Its defining histologic characteristic is extracellular pools of mucin in which low-grade tumor aggregates that appear to be suspended ( Fig. 10.6 ). As with tubular carcinoma, the importance of pure patterns is essential to ensure an excellent prognosis (90% 10-year survival) in the absence of adjuvant chemotherapy. Other studies confirm that the excellent prognosis of mucinous carcinoma is confined to pure examples of this special type of breast carcinoma.
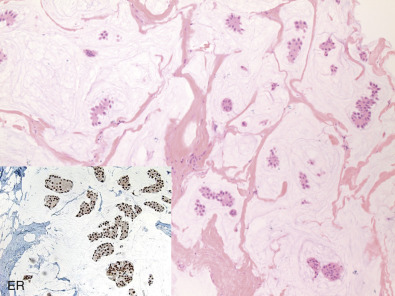
Pure and mixed mucinous carcinomas also have different mammographic appearances. On mammograms, pure mucinous carcinomas have a circumscribed, lobular contour (corresponding histologically to pools of extracellular mucin), whereas mixed carcinomas have an ill-defined, irregular contour. This lack of circumscription corresponds histologically to the interface between invasive carcinoma and the often-fibrotic stroma. Mucinous carcinomas are usually grade I, ER/PR-positive, and HER2-negative. This pattern strongly correlates with the luminal A molecular subtype.
Medullary Carcinoma
Medullary carcinomas (MC) have characteristic mammographic, clinical, and pathologic correlates. Medullary carcinoma is a common phenotype of hereditary breast cancer and is found in women who are at risk for cancer because of mutations in the tumor suppressor gene BRCA1 . This observation is less common in patients with BRCA2 and those with no known germline mutation. This pattern is observed in 7.8% to 13% of BRCA1 patients compared with 2% in the general public. This genetic characteristic is in large part attributable to the young age of the patients. Medullary features have been reported in 35% to 60% of tumors arising in BRCA1 patients. Multifactorial analysis has shown that high mitotic count, pushing tumor margins, and a lymphocytic infiltrate are independently associated with a BRCA1 mutation. Medullary carcinoma of the breast is rarely associated with microsatellite instability.
The distinctive smooth, pushing border of medullary carcinoma is reflected mammographically as a sharply circumscribed mass. Grossly, medullary carcinoma has a uniform, soft consistency. The essential histologic features include islands of tumor cells having irregular borders, without sharp edges, that are often connected ( Fig. 10.7 ). These islands do not invade the adjacent breast tissue, but they appear to push against it instead, resulting in a smooth interface with the adjacent normal breast tissue. Unlike the special-type carcinomas discussed earlier, medullary carcinoma is characterized by nuclei that have pronounced anaplastic features. The nuclei are large and pleomorphic, with clumped chromatin, frequent nucleoli, and readily identifiable mitotic figures. The other required histologic feature is a prominent infiltrate of lymphocytes and plasma cells in the loose connective tissue between the cellular islands.
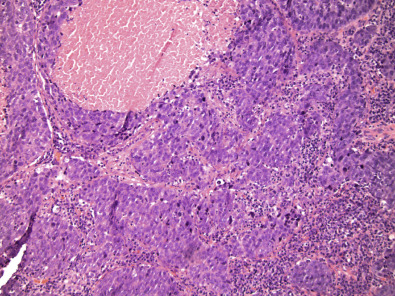
The preceding overview highlights the challenge of correctly diagnosing breast cancer. First, these tumors are known to be triple negative; they do not express ER/PR or HER2 and have very high ki67. A study conducted on medullary found tumors with high level of ki67 even within this morphology that are more likely to be lymph node positive. This study also showed that many of these tumors show strong staining diffusely for p53. A recent study done by Park and colleagues confirms that the prognosis of these patients does not significantly differ from the other high-grade invasive ductal carcinoma; therefore the same treatment protocol should be considered for these tumors as those with TNBC.
Micropapillary Carcinoma
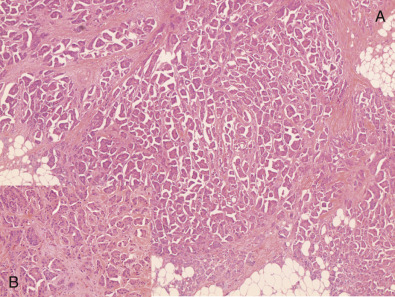
Micropapillary carcinoma is characterized by small clusters of tumor cells lying within clear stromal spaces, resembling dilated vascular channels ( Fig. 10.8 ). Many of these spaces are not lined by endothelial cells. Micropapillary carcinomas may manifest florid but early lymphovascular invasion. These cancers show relatively high proliferation with moderate to severe cytologic atypia. The majority of these tumors are ER positive, and more than 50% are HER2 positive.
Secretory Carcinoma
This tumor was originally recognized in young patients and represents 0.15% of all invasive cancers; rarely, it can also affect older women. The characteristic histologic feature is the presence of abundant intracellular and extracellular clear areas that contain secretions. Most examples are associated with discontinuous fibrous tissue that is often prominent within the lesion. The secretory material stains with periodic acid–Schiff stain and other mucosubstance stains. Features that correlate with an excellent prognosis include young age, tumor diameter less than 2 cm, and well-demarcated borders with no stromal invasion at the periphery of the lesion. A literature review suggests that some of these cancers express ER and PR and are usually HER2 negative. A 2012 report described this tumor in an 8-year-old female. The majority of investigators suggest no additional systemic treatment for this cancer.
Salivary Gland–Type Breast Carcinoma
These patterns are rare. They display the same morphology as in the salivary gland. The most important salivary gland type of tumor in the breast is adenoid cystic carcinoma.
Adenoid Cystic Carcinoma
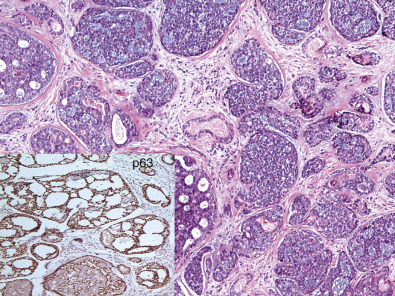
Adenoid cystic carcinoma of the breast is associated with an excellent prognosis. These tumors represent less than 0.1% of all invasive breast cancers, and despite showing basal-like features, lack of HER2 gene amplification, and lack of ER/PR expression, they usually remain local and have an excellent prognosis. Fig. 10.9 presents an image of adenoid cystic carcinoma.
Mucoepidermoid Carcinoma
This tumor shows the same histopathologic features as its salivary gland counterpart. The salient features mucin production and squamous differentiation can, however, be a nonspecific feature of mammary carcinoma no specific type (NST). The identification of mucoepidermoid carcinoma is probably important only when it is a low-grade tumor. Case reports of high-grade mucoepidermoid carcinoma have demonstrated an aggressive course.
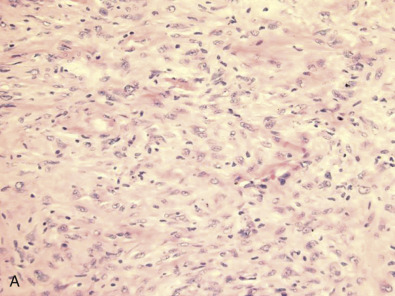
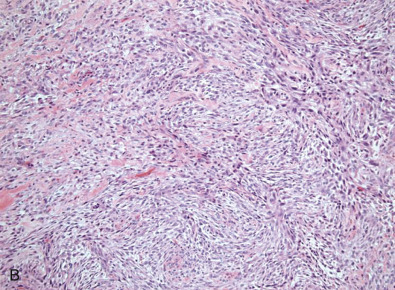
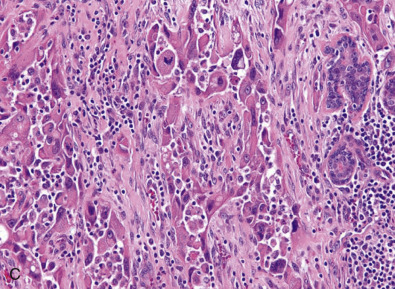
Metaplastic Carcinoma
Metaplastic breast cancer (MBC) encompasses almost 25% of all breast cancers and includes many morphologic patterns, including squamous cell carcinoma, spindle cell carcinoma, adenosquamous cell carcinoma, matrix producing carcinomas, and carcinomas with true malignant mesenchymal component. Essentially 100% of these cancers diffusely or focally express basal-like markers and usually lack ER/PR and HER2. Fig. 10.10 shows a composite image of several metaplastic carcinomas. The incidence of TNBC is remarkably higher in MBC than in IDC NOS. The presence of certain metaplastic elements has been associated with varying prognoses. The presence of high-grade spindled or pleomorphic components, for example, has been associated with aggressive behavior such as metastases, whereas the low-grade, fibromatosis-like metaplastic carcinomas with bland spindled cells have a high risk of local recurrence but minimal risk of metastatic spread. Molecular and genomic analyses have suggested that MBCs are enriched in the epithelial-to-mesenchymal transition and cancer stem cell characteristics. Despite aggressive local and systemic management strategies, patients with MBC have suboptimal breast cancer outcomes. A review of a large database has shown that many of these tumors are larger at the time of presentation than IDC NOS. This review also shows that the majority of patients are treated very aggressively; however, the prognosis remains poor, especially compared with other breast cancers. A subset of these tumors show prominent squamous differentiation. Primary squamous cell carcinoma of the breast in a pure pattern is distinctly unusual and often cystic; it may also assume a solid pattern with keratinization. The importance in its recognition lies with better understanding in the event of later metastases. Fig. 10.11 shows gross and microscopic images of an invasive squamous cell carcinoma.