Anthony W. Chow
Infections of the Oral Cavity, Neck, and Head
Infections of the oral cavity are most commonly odontogenic in origin. Odontogenic orofacial infections include dental caries, pulpitis, periapical abscess, gingivitis, periodontal disease, and infections in the deep fascial spaces. Complications such as intracranial, retropharyngeal, or pleuropulmonary extension and hematogenous dissemination to heart valves, prosthetic devices, and other metastatic foci, although rare, clearly indicate the potentially serious nature of these infections. Nonodontogenic infections of the oral cavity include ulcerative mucositis, which complicates radiation and chemotherapy; noma (gangrenous stomatitis); and infection of the major salivary glands. Suppurative orofacial infections can also arise from the oronasopharynx, middle ear and mastoids, and paranasal sinuses; these are discussed in Chapters 59, 62, and 63, respectively.
Infections of the neck and head in the adult most commonly result from human or animal bites, trauma, irradiation, and surgical procedures. In children, cervical adenitis and thyroiditis caused by bacteria or viruses are more common. Rarely do embryologic cysts in the neck region become secondarily infected. These are considered separately from oral infections because they frequently involve different microflora and necessitate alternative approaches to diagnosis and therapy.
Microbiologic Considerations
The microbiota associated with odontogenic infections are complex and generally reflect the indigenous oral flora. Such infections are typically polymicrobial, and invasiveness is often influenced by synergistic interactions of multiple microbial species. Moreover, certain species or combinations may be more invasive or more resistant to therapy than others.1 Despite this complexity, there is strong evidence of a causative role of specific microorganisms in different forms of odontogenic infections.2 Because the microflora associated with these infections are typically polymicrobial, the components of these complex flora do not necessarily have equal pathogenic potential, and the numerically predominant cultivatable microflora may not be the most pathogenic. Furthermore, it may not be necessary to eradicate the complete microflora for effective therapy. In addition, results of surveys with molecular tools indicate a level of diversity in the human subgingival microflora that cannot be recognized by conventional culture techniques.3 More than 700 bacterial species from the oral cavity have been identified.4 In most instances, the cultivatable microflora probably represent less than 1% of the total extant population, as estimated by microscopy or other means.5 Nevertheless, an appreciation of the indigenous oral flora and the host factors that may modify its composition, as well as knowledge of the most common microorganisms implicated in different odontogenic and nonodontogenic infections, should provide a more rational approach to the management of such infections arising from the oral cavity.
Unique Niches of the Indigenous Oral Flora
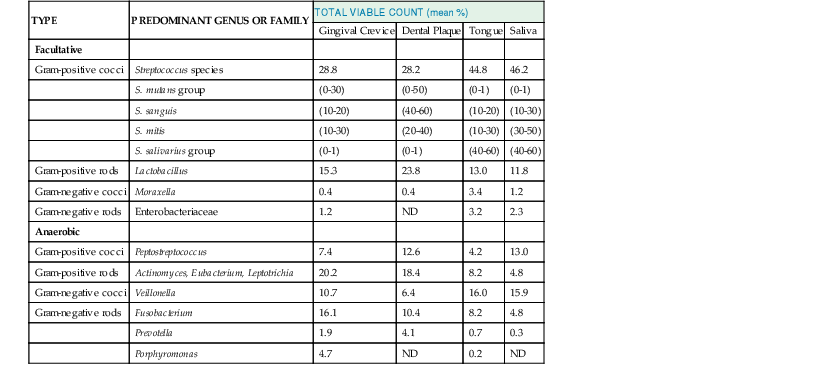
The oral cavity cannot be regarded as a single, uniform environment. Although representative species of microorganisms can be isolated from most areas of the mouth, certain sites such as the tongue, tooth surface, gingival crevice, and saliva are favored for colonization by specific organisms (Table 65-1).6
Quantitative studies indicate that obligate anaerobes constitute a large and important part of the residential oral flora. In the gingival crevice of healthy adults, for example, the total microscopic counts averaged 2.7 × 1011 microorganisms per gram of wet weight. The total cultivatable anaerobic bacteria averaged 1.8 × 1011 microorganisms per gram, whereas facultative bacteria averaged 2.2 × 1010 microorganisms per gram, which is an eightfold difference. Overall, Streptococcus, Finegoldia, Peptostreptococcus, Veillonella, Lactobacillus, Corynebacterium, and Actinomyces account for more than 80% of the total cultivatable oral flora. Facultative gram-negative rods are uncommon in healthy adults but may be more prominent in seriously ill, hospitalized, and elderly patients. Unique ecologic niches have been observed.6 For example, Streptococcus sanguis, Streptococcus mutans, and Streptococcus mitis, as well as Actinomyces viscosus, preferentially colonize the tooth surface. In contrast, Streptococcus salivarius and Veillonella spp. have a predilection for the tongue and buccal mucosa. Fusobacterium, Porphyromonas, Prevotella, and anaerobic spirochetes appear concentrated in the gingival crevice.7 The cultivable microbiota in the saliva most closely resemble those on the dorsum of the tongue. Factors that appear to govern these localization patterns include selective adherence characteristics of certain bacteria for various types of cells, local environmental conditions such as oxygen tension, oxidation-reduction potential (Eh) and pH, interbacterial coaggregation, and microbial inhibition.6 Apart from anatomic considerations, numerous factors such as age, pregnancy, diet and nutrition, eruption of deciduous dentition, oral hygiene, smoking habits, the presence of dental caries or periodontal disease, antimicrobial therapy, hospitalization, and genetic or racial factors may influence the composition of the oral flora.8
Microbial Specificity in Odontogenic Infections
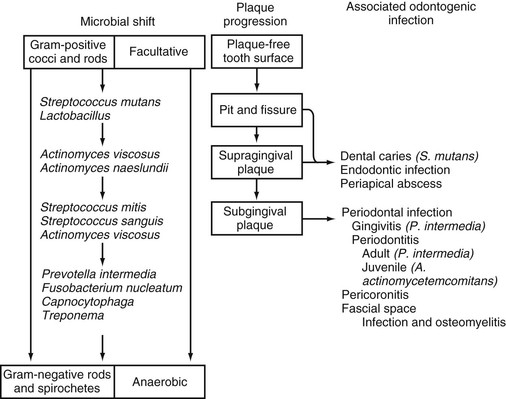
Of importance is that the normal commensal microflora are closely adapted to their unique ecologic niches in the oral cavity within well-established structures known as biofilms. These highly organized microorganisms are encased in an extracellular matrix composed mainly of polysaccharides and exist in a relatively protected environment. Under normal “healthy” conditions, these commensal bacteria maintain an effective and nondestructive inflammatory barrier against potential pathogens.9 Under pathologic conditions, however, this microbial homeostasis is disrupted, and the commensal microbiota shifts to a pathogenic form, which results in inflammation and tissue destruction. Only certain microorganisms residing within dental plaques are cariogenic or periodontopathic (i.e., the “specific” plaque hypothesis of dental caries and periodontal disease).10 This microbial specificity demonstrated for different odontogenic infections probably reflects the acquisition of unique microflora during the development of a supragingival dental plaque and its progression to a subgingival dental plaque. Plaques that accumulate above the gingival margin are composed mainly of gram-positive facultative and microaerophilic cocci and rods; plaques that accumulate below the gingival margin are composed mainly of gram-negative anaerobic rods and motile forms, including spirochetes (Fig. 65-1).11 Microorganisms residing within the supragingival plaque are characterized by their ability to adhere to the tooth surface and by their saccharolytic activity. Microorganisms in the subgingival plaque are frequently asaccharolytic but proteolytic, and they need not be adherent.
Important differences in bacterial compositions have been noted for dental caries, gingivitis, and different forms of periodontitis in comparison with cultures from healthy tissues.10 An etiologic association of S. mutans in dental caries has been firmly established.12 S. mutans is the only organism consistently isolated from all decayed dental fissures and is the only organism consistently found in greater numbers in carious teeth than in noncarious teeth. The infectious and transmissible nature of this organism in dental caries has been demonstrated in both experimental animals and in longitudinal studies in humans.12
Similarly, in gingivitis and periodontitis, a unique and specific bacterial composition of the subgingival plaque has been identified.12,13 In the healthy periodontium, the microflora is sparse and consists mainly of gram-positive organisms such as Streptococcus oralis, S. sanguis, and Actinomyces spp. In the presence of gingivitis, the predominant subgingival flora shift to a greater proportion of anaerobic gram-negative rods, and Prevotella intermedia (formerly Bacteroides intermedius), Capnocytophaga spp., Finegoldia magna, and Peptostreptococcus spp. are most commonly isolated. In adults with “established” periodontitis, the flora further increases in complexity, with a preponderance of anaerobic gram-negative and motile organisms and spirochetes. Porphyromonas gingivalis (formerly Bacteroides gingivalis), P. intermedia, Aggregatibacter actinomycetemcomitans, Tannerella forsythensis (formerly Bacteroides forsythus), and Treponema denticola are most commonly isolated. In juvenile or “early-onset” periodontitis, a clinical variant seen primarily in adolescents, the subgingival plaque consists mainly of saccharolytic organisms, with A. actinomycetemcomitans and Capnocytophaga spp. as the most common identifiable species. P. gingivalis is rarely found in this condition.
In suppurative odontogenic infections such as periapical abscesses or deep fascial space infections, polymicrobial flora are usually present; the predominant isolates are Fusobacterium nucleatum, pigmented Bacteroides spp., Peptostreptococcus spp., Actinomyces spp., and Streptococcus spp.14 Except in patients with serious underlying illnesses, facultative gram-negative bacilli and Staphylococcus aureus are uncommonly isolated.
Pathogenetic Mechanisms
The mechanisms by which pathogenic microorganisms in the oral cavity can cause disease are varied. To some extent, these microbes must be able to adhere to mucosal or tooth surfaces, resist elimination by mechanical means such as flushing by oral fluids, compete for space and nutrients with other resident flora, evade host defenses, and penetrate host tissues. The ability to attach to mucosal and tooth surfaces appears important for both commensal and pathogenic microbes.15
For example, a 36-kDa fimbrial protein identified in S. sanguis and Streptococcus parasanguis allows these organisms to bind to hydroxyapatite on the tooth surface and is apparently an important virulence factor for infective endocarditis.16 In the case of P. gingivalis, various proteases, as well as collagenase and hyaluronidase, may enhance binding to fibroblasts and matrix proteins by degrading host proteins, thus exposing cryptic receptors for the microorganisms.17
Microorganisms that cause dental caries, such as S. mutans and Streptococcus sobrinus, reside within the supragingival plaque and are both acidogenic (able to produce acid) and aciduric (able to grow at low pH). They readily colonize the tooth surface shortly after tooth eruption but do not become cariogenic until they are exposed to dietary sucrose.18 Fermentation of dietary sucrose by acidogenic plaque bacteria lowers the pH on the tooth surface, promoting demineralization and eventually tooth decay. S. mutans can also use sucrose to produce extracellular adhesive polymers, known as glucans, which enable S. mutans to stick avidly to the tooth surface, facilitating cariogenesis in the underlying structures.19 In the healthy host, at least three mechanisms protect the tooth from carious decay: (1) the cleaning action of the tongue and buccal membranes, which removes any food particles from the proximity of the tooth; (2) the buffering effect of saliva, which has a neutral pH that washes away bacterial acids, and provides essential substrates for remineralization and repair of damaged tooth surfaces; and (3) the protective effect of an acellular bacteria-free coating of salivary origin on the tooth surface, known as the acquired pellicle, which acts as a surface barrier to most dietary and bacterial acids and other proteolytic substances. In the absence of tooth brushing and flossing, the acquired pellicle becomes rapidly colonized and is replaced by the bacterial plaque. It is not surprising, therefore, that carious lesions occur most often in areas inaccessible to the self-cleaning mechanisms of the mouth and on the occlusal surfaces and sites that are protected from the reaches of the toothbrush.
Periodontal disease is caused mainly by selective periodontopathic microorganisms within the subgingival dental plaque, which penetrate the gingival epithelium, elicit an inflammatory host response, and ultimately cause destruction of the periodontium.10 This tissue destruction results in apical migration of gingival tissues (gingival recession), loss of periodontal attachment, and an increase in the depth of the gingival crevice (periodontal pockets). Specific virulence factors such as lipopolysaccharide and proteolytic enzymes play a role in this destruction. For example, several oral microorganisms associated with periodontitis, including A. actinomycetemcomitans, produce a leukotoxin that destroys polymorphonuclear leukocytes and macrophages and is believed to be a key virulence factor.18 Host and environmental factors, such as smoking, malnutrition, underlying disease such as diabetes mellitus, and certain genetic factors may play an even bigger role.20 In particular, patients with neutrophil defects (such as Chédiak-Higashi syndrome, agranulocytosis, and cyclic neutropenia) have a higher incidence of periodontal disease.21 Other factors include various hormonal effects that may exacerbate disease activity during puberty, menstruation, and pregnancy. Two major predisposing factors are poor oral hygiene and increasing age.22 In contrast to its role in dental caries, dietary carbohydrate intake does not appear to have a significant role in the pathogenesis of periodontal disease.
An excessive inflammatory host response to commensal oral microflora or failure to downregulate this immune response may also be present in some patients with destructive forms of periodontal disease.23 Thus, host-mediated tissue injury as a consequence of microbial infection, rather than the infection itself, has become a major focus in the study of the pathogenesis of periodontitis and its possible link to coronary atherosclerosis and heart disease.24
Mucosal Immunity of the Oral Cavity
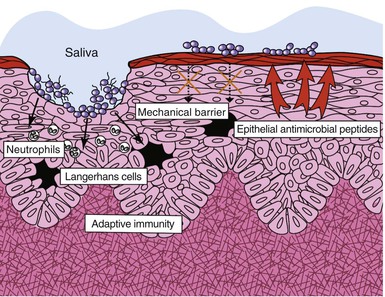
The oral cavity has three major host defenses against bacterial invasion: the oral mucosa as a physical barrier, nonspecific (innate) immunity, and adaptive (acquired) immunity (Fig. 65-2).25 The oral mucosa consists of a layer of interconnected epithelial cells containing mainly keratinocytes resting on a basal membrane. The oral epithelium constantly undergoes cellular renewal and turnover. Microorganisms seeking to colonize mucosal surfaces must develop a strategy to counteract the constant turnover of the epithelial cell layer.
Keratinocytes also have an innate system for recognizing pathogenic microbes by the activation of Toll-like receptors (TLRs). TLRs function as pathogen-recognition receptors that interact with conserved domains on microorganisms (so-called pathogen-associated molecular patterns [PAMPs]).26 Activation of TLRs results in a cascade of signaling pathways, ultimately leading to the upregulation of various proinflammatory (e.g., interleukin [IL]-1α, IL-6, IL-12, tumor necrosis factor-α [TNF-α]) and anti-inflammatory (e.g., IL-10, transforming growth factor-β [TGF-β]) cytokines and chemokines (e.g., RANTES [regulated on activation, normal T-cell expressed and secreted], macrophage inflammatory protein-1 [MIP-1], IL-8), which are crucial for nonspecific defense in the oral cavity. Phagocytic cells such as leukocytes and macrophages are abundant in the lamina propria and serve as the first line of defense against pathogenic microbes. Keratinocytes also produce a variety of antimicrobial peptides, including histatins and β-defensins, which have broad antibacterial and antifungal properties. Human β-defensin (hBD)-1 is expressed constitutively in epithelial tissues, whereas hBD-2 and hBD-3 are expressed in response to bacterial stimuli or inflammation. Commensal and pathogenic bacteria have been found to use different signaling pathways in hBD-2 induction; this finding suggests that epithelial cells from different body sites may use common signaling mechanisms to distinguish between commensal and pathogenic bacteria.16
In addition, the lamina propria within the mucous membrane contains a full complement of immunocompetent cells that are responsible for adaptive immunity. These cells, which include lymphocytes, macrophages, dendritic cells, natural killer (NK) cells, and eosinophils, constitute a common mucosal immune system known as mucosa-associated lymphoreticular tissue (MALT). Dendritic cells within MALT (Langerhans cells) process foreign antigens for presentation to and activation of T cells, as well as differentiation of B cells into immunoglobulin-secreting plasma cells. The primary immunoglobulin secreted at these sites within salivary and other exocrine glands is secretory immunoglobulin A (sIgA), whose major function is bacterial agglutination, inhibition of bacterial adherence, toxin neutralization, and antigen exclusion at the mucosal surface.27 IgA also downregulates the proinflammatory cytokine response by binding with the crystallizable fragment alpha receptor (FcαR receptor) on phagocytic cells. A number of oral microorganisms implicated in periodontitis, including P. gingivalis, P. intermedia, Prevotella melaninogenica, Capnocytophaga spp., S. sanguis, and S. mitis, are found to secrete IgA proteases.28 It has been suggested that cleavage of IgA by microbial IgA proteases may impair local mucosal immunity of the host. It remains to be seen whether similar or other defects of host resistance can be identified in different forms of destructive odontogenic infections.
Finally, saliva also acts as an important source of antimicrobial activity against oral pathogens.29 Mechanically, saliva serves to coat the teeth and contribute to the protective pellicle. In addition, it flushes the oral cavity, clearing away bacteria and their by-products, as well as food debris that may aid bacterial growth and colonization. The buffering capacity of saliva contributes to the maintenance of salivary pH. Numerous chemical constituents that inhibit bacterial growth, such as lysozyme, lactoferrin, defensins, and the peroxidase system, are found within the saliva.30 Lysozyme is able to lyse bacteria by catalyzing breakdown of the bacterial cell wall in a manner akin to the action of penicillin. It is also active against gram-negative bacteria in the presence of complement and antibody, and it disrupts the lipopolysaccharide coat in the cell wall.31 Lactoferrin sequesters iron from the environment, thus inhibiting the growth of various facultative and aerobic bacteria that are dependent on iron for metabolism. Salivary lactoperoxidase and myeloperoxidase are generated by polymorphonuclear leukocytes within the gingival crevices and have potent bactericidal properties. Lactoperoxidase generates a hypothiocyanate (HOSCN) molecule that is toxic to bacteria.32
Anatomic Considerations
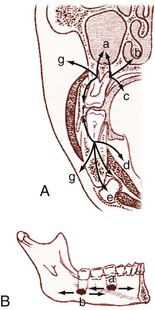
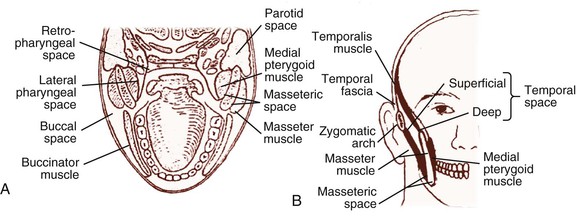
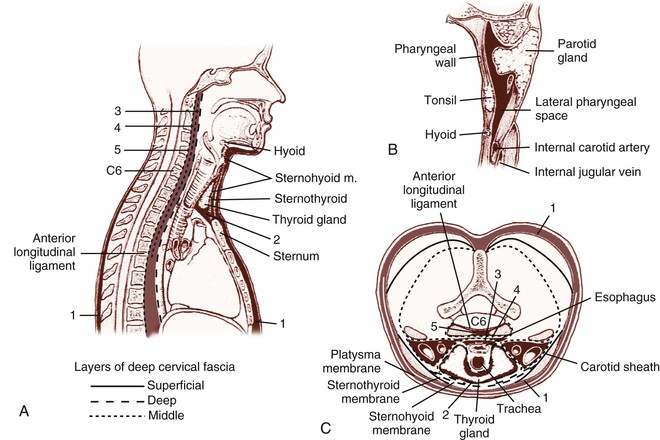
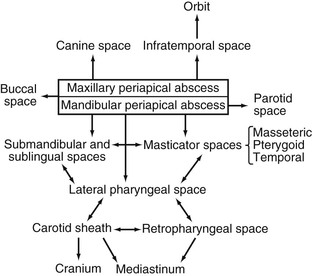
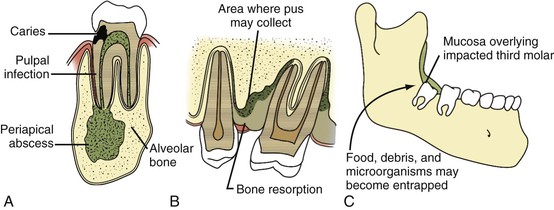
Soft tissue infections of odontogenic origin tend to spread along planes of least resistance from the supporting structures of the affected tooth to various potential spaces in the vicinity. Accumulated pus, therefore, must generally perforate bone at the site where it is thinnest and weakest before it extends into the periapical areas or deeper fascial spaces. In the mandible, this is usually in the region of the molar teeth on the lingual aspect and, more anteriorly, on the buccal aspect. In the maxilla, the bone is weakest on the buccal aspect throughout and relatively thicker on the palatal aspect. If pus perforates through either the maxillary or the mandibular buccal plate, it does so intraorally if inside the attachment of the buccinator muscle to the maxilla or mandible and extraorally if outside this muscle attachment (Fig. 65-3A).33 When a mandibular infection perforates lingually, it does so in the sublingual space if the apices of the involved teeth lie above the attachment of the mylohyoid muscle (e.g., mandibular incisor, canines, premolars, first molars) and in the submandibular space if the apices lie below the attachment of this muscle (e.g., second and third molars) (see Fig. 65-3B). Thus, these local anatomic barriers of bone, muscle, and fascia predetermine the routes of spread, the extent, and the clinical manifestations of many orofacial infections of odontogenic origin. The clinically important “fascial spaces” most often involved are illustrated in Figures 65-4 and 65-5. These are potential spaces between layers of fascia normally bound together by loose connective tissue. The breakdown of these attachments by a spreading infective process results in a fascial space infection. These spaces intercommunicate with one another to varied degrees, and the potential pathways of extension from one space to another are illustrated in Figure 65-6. A thorough understanding of the potential anatomic routes of infection not only provides valuable information on the nature and extent of infection but also suggests the optimal surgical approach for effective drainage.
Clinical Manifestations and Management
Orofacial Odontogenic Infections
Odontogenic infections originate in either the dental pulp or the periodontium (Fig. 65-7). The most common site is the dental pulp, and the most common infections are dentoalveolar. Deep fascial space infections are rare. Their clinical manifestations and management are briefly reviewed here. Antimicrobial therapy is further discussed later in the section “Therapeutic Considerations” (Tables 65-2 and 65-3).
TABLE 65-2
Antimicrobial Regimens for Various Odontogenic and Nonodontogenic Orofacial Infections
| CLINICAL ENTITY | COMMON CAUSATIVE ORGANISMS | ANTIMICROBIAL REGIMENS |
| Odontogenic | ||
| Supragingival dental plaque and dental caries prevention | Streptococcus mutans, other streptococci, Actinomyces spp. | Fluoride-containing toothpaste or oral rinses (e.g., sodium fluoride 1.1% or stannous fluoride 0.4%) two or three times daily with or without |
| Fluoride-containing varnishes (e.g., sodium fluoride 5%) applied three or four times yearly | ||
| with or without | ||
| Chlorhexidine 0.12% oral rinses | ||
| Acute simple gingivitis | Streptococci, Actinomyces spp., oral spirochetes | Penicillin G, 2-4 MU IV q4-6h (or penicillin V, 500 mg q8h) plus metronidazole, 500 mg PO or IV q8h |
| or | ||
| Ampicillin-sulbactam, 1.5-3 g IV q6-8h | ||
| or | ||
| Amoxicillin-clavulanate, 500 mg PO q8h | ||
| or | ||
| Clindamycin, 450 mg PO q6-8h or 600 mg IV q6-8h | ||
| Acute necrotizing ulcerative gingivitis (ANUG), or Vincent’s angina | Prevotella intermedia, Fusobacterium spp., Tannerella forsythensis, Treponema denticola, other oral spirochetes | Metronidazole, 500 mg PO or IV q8h |
| or | ||
| Amoxicillin-clavulanate, 500 mg PO q8h | ||
| or | ||
| Ampicillin-sulbactam, 1.5-3 g IV q6h | ||
| or | ||
| Clindamycin, 450 mg PO q6h or 600 mg IV q6-8h | ||
| Early-onset, “aggressive,” or “localized juvenile” periodontitis | Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Treponema denticola, Prevotella intermedia | Doxycycline, 200 mg PO or IV q12h (only in patients ≥8 yr of age) |
| or | ||
| Metronidazole, 500 mg PO or IV q8h | ||
| Adult or “established “ periodontitis | Treponema denticola, other oral spirochetes, black-pigmented Bacteroides spp. (Porphyromonas gingivalis and Prevotella melaninogenica), Tannerella forsythensis | Topical application of minocycline microspheres (Arestin) |
| or | ||
| Topical application of doxycycline hyclate periodontal extended-release liquid (Atridox) | ||
| Nonodontogenic | ||
| Gangrenous stomatitis (noma) | Fusobacterium nucleatum, Borrelia vincentii, Prevotella melaninogenica, other oral anaerobes | Penicillin G, 2-4 MU IV q4-6h plus metronidazole, 500 mg PO or IV q8h |
| or | ||
| Ampicillin-sulbactam, 1.5-3 g IV q6-8h | ||
| or | ||
| Amoxicillin-clavulanate, 500 mg PO q8h | ||
| or | ||
| Clindamycin, 450 mg PO q6-8h or 600 mg IV q6-8h | ||
| Severe oral mucositis in immunocompromised hosts | Viridans and other streptococci, Bacteroides spp., Peptostreptococcus spp., and other oral anaerobes, facultative gram-negative bacilli | Topical chlorhexidine (0.1%) mouth rinses TID plus one of the following: |
| Cefotaxime, 2 g IV q6h | ||
| or | ||
| Ticarcillin-clavulanate, 3.1 g IV q4h | ||
| or | ||
| Piperacillin-tazobactam, 3.375 g IV q6h | ||
| or | ||
| Imipenem, 500 mg IV q6h | ||
| or | ||
| Meropenem, 1 g IV q8h | ||
| Sialadenitis and suppurative parotitis | Staphylococcus aureus,* Streptococcus viridans and other streptococci, Bacteroides spp., Peptostreptococcus spp., and other oral anaerobes | Nafcillin, 2 g IV q4h, or vancomycin, 1 g IV q12h plus either |
| Metronidazole, 0.5 g IV q6h | ||
| or | ||
| Clindamycin, 600 mg IV q6h |
* For Staphylococcus aureus infections in which methicillin-resistant S. aureus (MRSA) is suspected, replace nafcillin with vancomycin, 1 g IV q12h; in immunosuppressed hosts, cefotaxime or ceftriaxone or imipenem, as described in the table, can be added.
IV, intravenously; MU, million units; PO, orally; TID, three times a day.
TABLE 65-3
Initial Empirical Antimicrobial Regimens for Suppurative Infections of the Head and Neck
| INFECTION | USUAL CAUSATIVE ORGANISMS | ANTIBIOTIC REGIMENS, NORMAL HOST* |
| Suppurative orofacial odontogenic infections, including Ludwig’s angina | Streptococcus viridans and other streptococci, Peptostreptococcus spp., Bacteroides spp., and other oral anaerobes | Penicillin G, 2-4 MU IV q4-6h plus metronidazole, 0.5 g IV q6h |
| or | ||
| Ampicillin-sulbactam, 2 g IV q4h | ||
| or | ||
| Clindamycin, 600 mg IV q6h | ||
| or | ||
| Cefoxitin, 1-2 g IV q6h | ||
| Lateral pharyngeal or retropharyngeal space infections | ||
| Odontogenic | S. viridans and other streptococci, Staphylococcus spp., Peptostreptococcus spp., Bacteroides spp., and other oral anaerobes | Penicillin G, 2-4 MU IV q4-6h plus metronidazole 0.5 g IV q6h |
| or | ||
| Ampicillin-sulbactam 2 g IV q4h | ||
| or | ||
| Clindamycin 600 mg IV q6h | ||
| Rhinogenic | Streptococcus pneumoniae, Haemophilus influenzae, viridans and other streptococci, Bacteroides spp., Peptostreptococcus spp., and other oral anaerobes | One of the following: (1) Penicillin G, 2-4 MU IV q4-6h, or levofloxacin, 500 mg IV q24h, or ciprofloxacin, 750 mg IV q12h |
| plus | ||
| Metronidazole, 0.5 g IV q6h, or clindamycin, 600 mg IV q6h | ||
| or | ||
| (2) Moxifloxacin, 400 mg IV q24h | ||
| Otogenic | Same as for rhinogenic space infections | Same as for rhinogenic space infections |
| Suppurative cervical adenitis and infected embryologic cysts | Streptococcus pyogenes, Peptostreptococcus spp., Fusobacterium spp., oral anaerobes | One of the following: (1) Penicillin G, 2-4 MU IV q4h, plus metronidazole, 500 mg IV q6h |
| or | ||
| (2) Ampicillin-sulbactam, 2 g IV q4h | ||
| or | ||
| (3) Clindamycin, 600 mg IV q6h | ||
| or | ||
| (4) Cefoxitin, 1-2g IV q6h | ||
| Suppurative thyroiditis | S. aureus, S. pyogenes, S. pneumoniae, Haemophilus influenzae, Streptococcus viridans and other streptococci, oral anaerobes | Nafcillin, 2 g IV q4-6h, or vancomycin, 1 g IV q12h plus either |
| Metronidazole, 500 mg IV q6h | ||
| or | ||
| Clindamycin, 600 mg IV q6h | ||
| Cervicofacial actinomycosis | Actinomyces israelii, Arachnia propionica, Actinobacillus actinomycetemcomitans | One of the following: (1) Penicillin G, 2-4 MU IV q4-6h |
| or | ||
| (2) Doxycycline, 200 mg PO or IV q12h | ||
| or | ||
| (3) Clindamycin, 450 mg PO q6h or 600 mg IV q6h | ||
| Human or animal bites | S. pyogenes, S. aureus, Eikenella corrodens, oral anaerobes Pasteurella multocida | One of the following: (1) Ampicillin-sulbactam, 2 g IV q4h |
| or | ||
| (2) Amoxicillin-clavulanate, 500 mg PO q8h | ||
| or | ||
| (3) Moxifloxacin, 400 mg IV or PO q12h | ||
| Maxillofacial trauma, postsurgical wound infections | S. aureus, S. pyogenes, Peptostreptococcus spp., other oral anaerobes, Pseudomonas aeruginosa, Enterobacteriaceae spp. | Nafcillin, 2 g IV q4h, or vancomycin, 1 g IV q12h plus one of the following: |
| (1) Ticarcillin-clavulanate, 3.1 g IV q4h | ||
| or | ||
| (2) Piperacillin-tazobactam, 3.375 g IV q6h | ||
| or | ||
| (3) Imipenem-cilastatin, 500 mg IV q6h | ||
| or | ||
| (4) Meropenem, 1 g IV q8h | ||
| or | ||
| (5) Moxifloxacin, 400 mg IV or PO q24h | ||
| or | ||
| (6) Tigecycline, 100 mg IV, then 50 mg IV q12h | ||
| Suppurative jugular thrombophlebitis (Lemierre syndrome) | Fusobacterium necrophorum; same as for odontogenic space infections | Same as for odontogenic space infections |
| Suppurative cavernous sinus thrombosis | Same as for odontogenic, rhinogenic, or otogenic space infections | Same as for odontogenic, rhinogenic, or otogenic space infections |
| Mandibular osteomyelitis | Same as for odontogenic space infections | Clindamycin, 600 mg IV q6h |
| or | ||
| Moxifloxacin, 400 mg PO or IV q24h | ||
| Extension of osteomyelitis from prevertebral space infection | Staphylococcus aureus,† facultative gram-negative bacilli | Either nafcillin, 2 g IV q4h, or vancomycin, 1 g IV q12h plus |
| Either tobramycin, 1.7 mg/kg IV q8h, or ciprofloxacin, 400 mg IV q12h |
* For immunocompromised hosts, consider replacing penicillin G with one of the following: cefotaxime, 2 g IV q4h; ceftriaxone, 1 g IV q12h; or cefepime, 2 g IV q12h. Other regimens to consider are ticarcillin-clavulanate, 3.1 g IV q64h; piperacillin/tazobactam, 3.375 g IV q6h; imipenem, 500 mg IV q6h; meropenem, 1 g IV q8h; moxifloxacin, 400 mg IV q24h; or tigecycline, 100 mg IV, then 50 mg IV q12h.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree