J. Peter Donnelly, Nicole M.A. Blijlevens, Walter J.F.M. van der Velden
Infections in the Immunocompromised Host
General Principles
An intact defense system offers protection against most microbial aggressors through a complex interrelationship of protecting surfaces, cells, and soluble factors. Optimal nutritional status and normal organ function, together with granulocytes, macrophages, antibodies, complement, and other components of the cellular and humoral immune system, provide protection against potentially pathogenic microorganisms. The resident microbiota normally does not cause infection or alert the immune system, and it may protect against pathogens by competing for binding sites on the surfaces and for the available nutrients as well as in shaping the immune system.
Infection is a principal cause of morbidity and mortality of immunocompromised patients. Hence, a comprehensive understanding of the possible causes of infectious complications and the predisposing factors involved, as well as a comprehensive anti-infective strategy, is imperative when offering these patients care. The physician attending to immunocompromised patients has to be particularly aware of all potential risk factors for infection, including those related to the underlying disease and its treatment, because although susceptibility to infection is increased, timely diagnosis is difficult.
Deficiencies in Components of Host Defenses
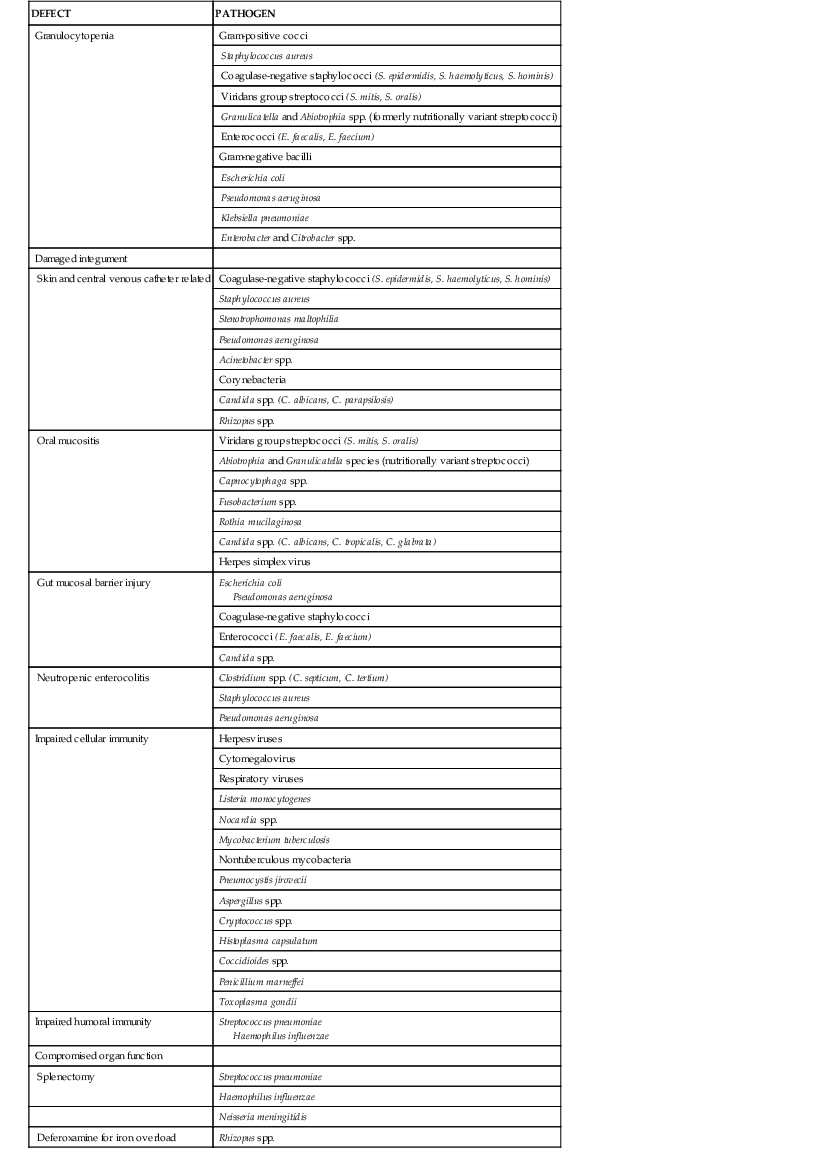
Appreciation of the predisposing risk factors is an essential but perplexing exercise because it suggests that each individual component plays an independent role. Certain organisms infect patients with specific defects, and these associations should be taken into account when selecting therapy. However, this is by no means always predictable. Theoretically, a specific deficiency increases the patient’s susceptibility to the very pathogens that are eradicated by that particular host defense mechanism (Table 309-1; see Chapters 5 to 9). Although a basic pattern is recognizable, the types and severity of infectious complications are often unpredictable. Single, isolated deficiencies are infrequently encountered, and malfunction of one part of the system often influences several other components. Moreover, therapeutic interventions and the underlying disease will disturb a range of defense mechanisms. Although the risks associated with granulocytopenia are well known, other toxicities, especially those affecting the mucosal barrier, are considered to be of greater importance than was previously the case. The advent of aggressive treatments has altered the classic concept of specific defects of host defense mechanisms in the various types of diseases because the effects of antineoplastic chemotherapy and irradiation are now seen as the primary factors determining the nature and extent of the defect. Also, transplantation (especially of stem cells) can cause defects in host immunity via graft-versus-host disease as well as immunosuppressive chemotherapy. Patients with impaired humoral immunity as manifested by defective opsonization and phagocytosis of bacteria will also be exposed to chemotherapy-induced neutropenia or deficient cellular immunity as a result of treatment with purine analogues or monoclonal antibodies for treating malignancies.
The genetic makeup of the host also has an impact on the risk for infection because functional changes due to polymorphisms in immune genes influence individual susceptibility for infection.1,2 The specific context of an immune deficiency determines which of the remaining components of the immune system will be most important. Therefore, variation in the genes that correspond to these components may become clinically important. Components of the innate immune system are believed to survive chemotherapy and contribute to immune defenses. Consequently, in the setting of cancer therapy and hematopoietic stem cell transplantation (HSCT), most gene polymorphisms associated with the risk for infection involve innate immune genes, especially those coding for pattern recognition receptors and cytokines.3–5
Cellular and Humoral Immunity
The host defense against pathogenic microorganisms encompasses innate and acquired immunity. The innate immune system comprises both cellular components, including monocytes, neutrophils, natural killer cells, and innate lymphoid cells, and humoral components, including complement, some antibodies (“natural” antibodies, perhaps directed against normal microbiota), antimicrobial peptides, and lysozyme. This mechanism is very effective in dealing with the vast majority of infectious agents. It has become clear that the innate immune system not only specifically recognizes various classes of microorganisms via pattern recognition receptors (microbe-associated molecular patterns) that sense conserved structures of the invading microorganisms but also initiates and modulates the subsequent adaptive responses delivered by T cells and B cells by their interaction with antigen-presenting cells (especially dendritic cells).
Granulocytes
In normal circumstances, neutrophils, sometimes accompanied by eosinophils, congregate at the site of inflammation and are followed by macrophages. Formation of this inflammatory exudate is the result of activation of humoral factors and normal function of the vascular endothelium (see Chapter 8). Meanwhile, in the peripheral blood, granulocytosis evolves as a consequence of release of the marrow reserve and increased granulocytopoiesis, which is regulated by hematopoietic growth factors such as interleukin (IL)-3, granulocyte-macrophage colony-stimulating factor, and granulocyte colony-stimulating factor.6
Virtually all cytotoxic drugs used in the treatment of malignant diseases have a deleterious effect on the proliferation of normal hematopoietic progenitor cells. Therefore, after obliteration of the mitotic pool and depletion of the marrow pool reserve, granulocytopenia ensues. Likewise, therapeutic radiation can induce clinically important granulocytopenia, depending on the dose rate, total dose given, and irradiated area of the body. Total-body irradiation, as used to prepare for HSCT, is the most obvious illustration of the possible negative impact of irradiation. Thus, profound neutropenia is an unavoidable consequence of the treatment of malignancy and may persist for 3 or 4 weeks or even longer. Granulocytopenia or a treatment-related decrease in the granulocyte count is probably the most important primary risk factor for infection. Fever develops in nearly all cases of profound granulocytopenia (i.e., a granulocyte count <100/mm3 for more than 3 weeks), whereas only one fifth of the febrile episodes in cancer patients occur when granulocyte counts are normal.7 Moreover, during iatrogenic granulocytopenia, the risk for infection and infection-related mortality increases proportionally with time.
Granulocytes that accumulate at the site of infection are of little use if they are unable to function normally. Antineoplastic drugs and irradiation each interfere with these nonproliferating cells and their function, resulting in decreased chemotaxis, diminished phagocytic capacity, and defective intracellular killing by granulocytes. Glucocorticosteroids seem to enhance granulocytopoiesis and mobilize the marginal and the marrow pool reserve, but these putative positive effects on neutrophilic granulocytes are offset by numerous disadvantages. These drugs curb the accumulation of neutrophils at the site of inflammation by reducing their adherent capacity and diminishing their chemotactic activity. Furthermore, they decrease phagocytosis and intracellular killing of microorganisms. The lack of functioning neutrophils deprives the host of a primary defense mechanism against invading microorganisms, which are consequently able to readily establish themselves, initiate local infection, disseminate unhindered, and eventually lead to fulminant sepsis and death unless treated promptly and effectively.
Macrophages
Normal macrophages have a limited capacity for killing ingested microorganisms, and various organisms are able to survive and replicate inside the cell, unless the macrophage becomes activated. Activation of macrophages is a complex process primarily under the control of cytokines (e.g., interferon-γ).
Specific Cellular Immunity (see Chapter 6)
Both antigen-specific and antigen-nonspecific cells contribute to the development of cellular immunity. The antigen-specific branch of cell-mediated immunity can be divided into two major categories. One category involves cytotoxic effector cells, which are able to lyse virus-infected or foreign cells including malignant cells. The second category involves subpopulations of helper T cells that mediate differentiated cytokine reactions (Th1, Th2, Th17) after antigen recognition.
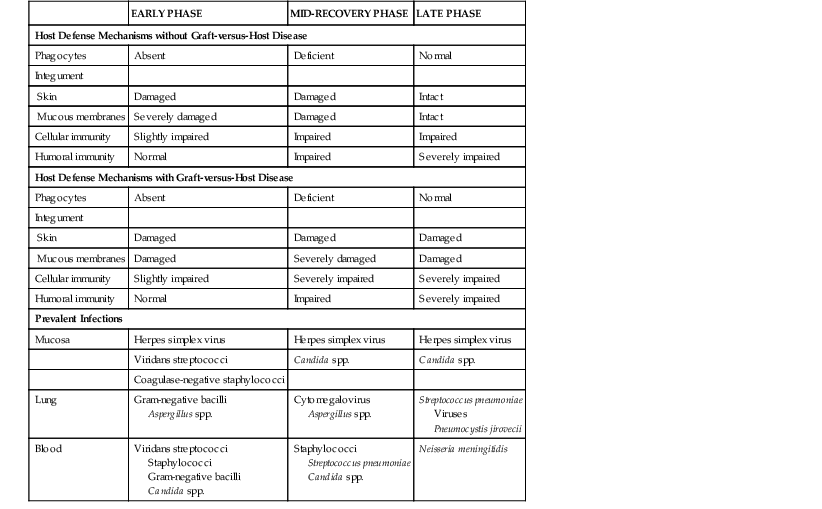
This fine-tuned system can easily be deregulated by congenital defects or defects acquired as a result of a disease or its treatment. Long-term cytotoxic therapy, extensive irradiation, and immunosuppressive drugs such as corticosteroids, azathioprine, cyclosporine, tacrolimus, sirolimus, and everolimus suppress cellular immunity. Some monoclonal antibodies, such as alemtuzumab, are being used as antitumor and immunosuppressive agents and can exert profound and prolonged effects on cellular immunity. Purine analogues, including fludarabine and cladribine, are particularly detrimental to cellular immunity and create a situation similar to acquired immunodeficiency syndrome. Likewise, malignant lymphomas, particularly Hodgkin’s disease, are associated with impaired cellular immunity. Allogeneic stem cell transplantation brings about a long-lasting dysfunction of T and B cells, especially in association with graft-versus-host disease and its treatment (Table 309-2). The coordination of cellular immunity is often lost, and when aided and abetted by suppressed humoral immunity, the paracrine mediators that are released go on to induce the sepsis cascade, which may culminate in multiorgan failure instead of arresting infection.8
Humoral Immunity
The humoral branch of the immune system, which is primarily responsible for clearing extracellular bacteria, involves the interaction of B cells with antigen and their subsequent proliferation and differentiation into antibody-secreting plasma cells (see Chapter 5). An important difference in antigen recognition by T cells and B cells is that the latter can recognize some antigens without the help of an antigen-presenting cell. The humoral system can identify a plethora of bacterial or viral microorganisms, as well as the soluble proteins that they release. When challenged by an antigen, immunoglobulins are produced that bind to the antigen. The specific functions of immunoglobulin G (IgG) and immunoglobulin M (IgM) include neutralization of the antigen as well as complement activation and opsonization, that is, enhancement of phagocytosis of the antigen by neutrophils and macrophages. Secretory immunoglobulin A (IgA), which is found on mucosal surfaces, is not an opsonin but nonetheless inhibits the motility of bacteria and prevents them from adhering to epithelial cells. The production of immunoglobulins is decreased in lymphoproliferative disorders such as chronic lymphocytic leukemia and multiple myeloma, whereas humoral immunity is generally well preserved in patients with acute leukemia. However, intensive irradiation and chemotherapy will lead not only to neutropenia but also, ultimately, to hypogammaglobulinemia. In particular, monoclonal antibodies such as rituximab deplete B-lymphocytes, inducing profound and long-lasting hypogammaglobulinemia and, consequently, infections.9
Cytokines and chemokines are indispensable for communication between innate and acquired immune and nonimmune cells in shaping effective antimicrobial immune reactions. Hence, interference by anticytokine antibodies and cytokine scavengers (e.g., infliximab) results in increased risk for infection in autoimmune diseases, transplantation, and cancer therapy.10–12
Spleen
The spleen is the principal organ for eliminating microbes that are not opsonized, such as encapsulated bacteria. Macrophages that occupy strategic positions within the organ are able to remove them. The primary immunoglobulin response also takes place in the spleen. Spleen-produced specific opsonizing antibodies are necessary for efficient phagocytosis of encapsulated bacteria. Splenectomy may result in a reduced level of the complement factor properdin and thereby lead to suboptimal opsonization,13 a decrease in functional tuftsin, and low levels of circulating IgM.14 The lack of opsonizing antibodies in serum against common encapsulated bacteria impairs the activity of all phagocytic cells, including granulocytes, monocytes, and macrophages. As a consequence, infections with Streptococcus pneumoniae and Haemophilus influenzae are often more severe in splenectomized patients, as well as in those who have had HSCT and are functionally asplenic. Opsonizing antibodies are also important for effective antibody-dependent cell-mediated cytotoxicity of natural killer cells.
Platelets
The protective role of platelets in healthy individuals is often underestimated but becomes obvious during treatment of malignant disease. Thrombocytopenia is an almost inevitable repercussion of intensive chemotherapy and irradiation, but decreased thrombocyte function is also a matter of concern. Thrombocytopathy is either disease related or caused by concurrent medication. The consequences of both increased susceptibility to infection and a decreased capacity to repair damaged tissues can be considerable and may have an impact on the eventual outcome of a treatment episode. Thrombocytopenia also appears to be an independent risk factor for bacteremia,15 and the incidence of major hemorrhage at autopsy of patients who die with or of an infection is striking.16
Physical Barriers: The Integument
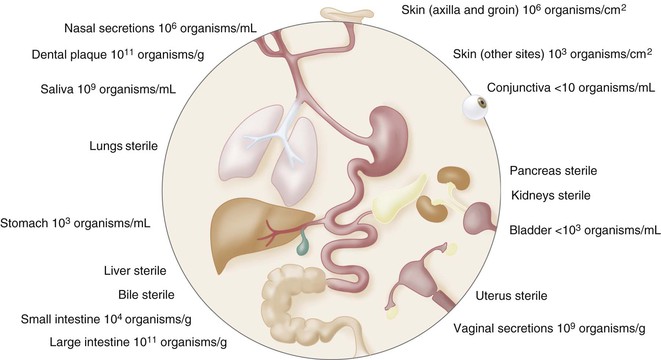
The skin, the respiratory tract (including the nasal cavity), the ears and conjunctiva, the alimentary tract, and the genitourinary tract are in contact with the environment (Fig. 309-1) and provide a first line of defense against microbial invasion. The skin and the mucosal surfaces of the alimentary and respiratory tracts form principal barriers against microbial invasion. These surfaces are normally colonized with a variety of microorganisms, including many different genera of bacteria and yeast that have an intimate association with a particular ecologic niche and help to maintain the function and integrity of this first line of defense. When intact and healthy, both the mucosa and the skin are capable of resisting colonization with the allochthonous organisms found in the immediate environment, as long as an ecologic balance is maintained within the indigenous microbial microbiota. Acidity plays a crucial role both in disinfecting the stomach and in regulating the microbial milieu of the vagina. The integrity of the mucosa, production of saliva and mucus, peristalsis, bile acids, digestive enzymes, and levels of defensins, trefoil factors, and secretory IgA also play an important role in maintaining a favorable microecology.17,18 Elimination of an inoculum is achieved by sneezing and coughing of microbes trapped in mucus, whereas flushing of the mouth and esophagus by saliva, as well as micturition and peristalsis, inhibits continuous intimate contact between a given surface area and unattached invasive microorganisms.
Skin
Healthy skin provides an effective barrier against invasion by microorganisms, mainly by remaining intact. Desquamation helps limit the opportunities for transient organisms to establish residence. Normally, very little water is present on the skin surface. Colonization with organisms sensitive to desiccation, such as gram-negative bacilli, is not favored. The skin also forms an acid mantle with a pH of 5.0 to 6.0, and its surface temperature is on average approximately 5° C lower than the core body temperature.19 Besides containing secretory IgA, sweat also possesses sufficient salt to create a high osmotic pressure. Organisms that can withstand these conditions and compete successfully for binding sites and nutrients include staphylococci, corynebacteria, and the lipophilic yeast Malassezia furfur.19 These organisms further modulate the microecology of the skin by releasing fatty acids from sebaceous secretions to produce a hydrophobic milieu as well as lactic and propionic acids, which help maintain a low pH. Many of the bacteria also elaborate bacteriocins that inhibit other microorganisms.
The composition of the skin microflora is influenced by general factors including climate, body location, age, sex, race, and occupation, as well as by the use of soaps, detergents, and disinfectants. Antibiotics secreted in sweat disturb the balance within the commensal microbiota and leave the surface vulnerable to colonization by exogenous gram-negative bacilli. Antibiotics also exert selective pressure on the skin microbiota and cause resistance to emerge, as has been observed during treatment with ciprofloxacin.20 Moreover, ciprofloxacin is excreted in sweat and induces resistance among skin staphylococci within a few days of exposure.21,22 β-Lactam antibiotics, including ceftazidime, ceftriaxone, cefuroxime, benzyl penicillin, and phenoxymethylpenicillin (penicillin V), can also be found in sweat, which might explain the ready selection of resistant staphylococci.23 Chemotherapy and irradiation can cause radical changes in healthy skin that cause hair loss, dryness, and loss of sweat production.
Needle punctures and catheters provide a ready means of access for microorganisms through the stratum corneum and into the bloodstream. When the skin is broken, the release of fibronectin is thought to assist colonization with Staphylococcus aureus, whereas other changes facilitate colonization with gram-negative bacilli such as Acinetobacter baumannii and enteric bacteria. Abraded skin can lead to local infection, which can be a reservoir that promotes further spread to entry sites of intravenous catheters. When the balance is lost between host defenses and commensal microbiota around hair follicles, the follicles can become inflamed and necrotic and form a potential nidus of infection. Clinical infection therefore results from breaks in the skin, loss of local immunity, and disturbances within the resident microbiota.
Vascular devices have gained widespread acceptance as a relatively safe form of long-term venous access, but regular use is associated with a marked increase in the incidence of bacteremia with coagulase-negative staphylococci, which frequently colonize the catheter lumen (see Chapter 302).24,25 These staphylococci are commonly resistant to aminoglycosides, trimethoprim-sulfamethoxazole, and penicillinase-resistant penicillins and may also be resistant to fluoroquinolones.26 Unless the catheter ends in an implanted port, skin commensal microbiota have potential access into the bloodstream. The hub is the most likely source of contamination leading to catheter colonization,27 and the risk increases with use.28 Infections related to the external surface of the catheter (exit site infections and tunnel infections) can result in serious soft tissue infection, most notably by S. aureus. Exit site infections occur much less frequently than does intraluminal contamination. The latter may be caused by a variety of bacteria, many of which have relatively low virulence. Once established, these infections can be very difficult to treat without removing the device, particularly those caused by Bacillus spp., Candida spp., and Pseudomonas aeruginosa.29–32,33
Alimentary Tract
Microbial Microbiota
Anaerobic bacteria predominate among the resident microbiota of the oral cavity and large intestine population and play a crucial role in maintaining a healthy commensal microbiota by providing the facility to withstand the establishment of exogenous organisms, which is known as colonization resistance.34 However, the microbial microbiota is not the only participant in the establishment and maintenance of colonization resistance. Recently, there has been renewed interest in the human commensal microbiota, which can be divided into microbiomes that are associated with health and disease. The various species also participate in training and shaping the immune response, maintaining it and keeping it healthy. In fact, the host-microbial interaction is far more complex than hitherto imagined, involving host pattern recognition receptors, bacteriocins, and lactic acid, to name but a few, as well as competitors for available nutrients and the release of host antimicrobial peptides.35–37
Dysbiosis
Many antibiotics also exert a negative influence on the commensal microbiota. Very susceptible bacteria such as the oral Neisseria spp. are suppressed by a wide range of antimicrobial agents, whereas oral viridans streptococci of the mitis group, such as Streptococcus mitis and Streptococcus oralis,38 and other unusual oral commensal microbiota, such as Rothia mucilaginosa and Capnocytophaga spp., are likely to be selected by antimicrobial agents, to which the bacteria are only marginally susceptible, if at all. In particular, penicillins, rifampin, clindamycin, macrolides, bacitracin, and vancomycin significantly impair colonization resistance, probably because they inhibit the gram-positive nonsporulating, lactic acid–producing bacilli such as bifidobacteria.39 Certain cephalosporins are also detrimental, whereas trimethoprim-sulfamethoxazole and the quinolones have been declared “friendly,” hence their frequent use as prophylaxis.39 Unexpectedly, imipenem used at higher doses has led to an increase in cases of diarrhea caused by Clostridium difficile.40 Newer antibiotics such as tigecycline with activity against anaerobic gram-positive bacteria may lead to increases in the numbers of patients with Candida. This change usually reflects marked perturbations of the gut ecology.41 However, this effect does not increase the risk for infection due to C. difficile.42 Chlorhexidine mouthwashes used to minimize plaque and gingivitis also influence the microflora.43–45
Because the normal commensal microbiota attach to the surfaces of the epithelium, their loss creates an ecologic vacuum that allows other organisms to establish colonization by occupying the vacant cell surfaces or by taking advantage of the surfeit of nutrients. Collapse of the ecology is invariably manifested by yeast overgrowth and colonization with nosocomial bacteria such as Klebsiella pneumoniae and P. aeruginosa46–49 and failure to detect viable anaerobes directly or indirectly.47,48 Examples of infectious complications associated with disturbance of the normal microbial equilibrium include the selection of previously uncommon species such as Enterococcus faecium and Clostridium septicum.
Dyspepsia is sufficiently commonplace for antacids such as histamine-2 (H2) blockers and proton pump inhibitors to be regularly prescribed. Reduced gastric acidity inadvertently destroys the natural barrier that prevents gastric and intestinal colonization by oral commensal microbiota, many of which are resistant to most of the antimicrobial agents used for prophylaxis in impaired hosts. When patients swallow large amounts of mucus as a result of severe mucositis, any oral commensal microbiota may survive passage to the bowel. Loss of the gastric barrier therefore effectively extends the area of potential sites for colonization to the full length of the alimentary tract, which may explain the pathogenesis of α-hemolytic (viridans group) streptococcal bacteremia.50,51,52 Viridans streptococci, usually S. mitis, can cause infections associated with life-threatening complications, including septic shock and pneumonitis and acute respiratory distress syndrome. High-dose cytarabine predisposes to these complications.51,53–55 Finally, the ecology of the bowel microbiota is markedly altered by gut damage induced by treatment with certain cytostatic agents such as cytarabine,56,57 by graft-versus-host disease,58 and by total-body irradiation.59 Various coagulase-negative staphylococci, including Staphylococcus epidermidis, are also present in the endogenous oral microbiota and gastrointestinal tract of neutropenic patients.60 Plasmid pattern analysis of coagulase-negative staphylococcal bloodstream isolates has shown that the mucosa is the origin of bacteremia in 70% of patients managed in a hematology ward of one center.61 Others have found that vascular catheters were the most likely source of coagulase-negative Staphylococcus bacteremia in patients with hematologic malignancies (see Chapter 302). However, there is clear evidence that the coagulase-negative staphylococci responsible for bacteremia can originate from both the mucosa of the oral cavity and gut, as well as the catheter.62,63
Mucosal Barrier Injury
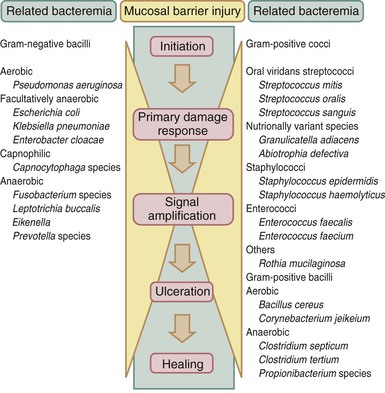
Injury to the mucosal barrier induced by chemotherapy and radiation therapy is probably the most substantial and earliest breach in the host defenses against infecting microorganisms. The process is more complex than just the direct effect of cytotoxic therapy on cells with a high mitotic index, such as epithelial cells of the mouth and gastrointestinal tract, and the indirect effect of local infections associated with evolving neutropenia.46 Sonis64 postulated that the pathobiology of mucositis involved five sequential but overlapping phases (Fig. 309-2), culminating in tissue damage that manifests clinically as mucositis. The initiation phase involves free radical generation and induction of apoptotic cell death induced by both DNA and non-DNA damage. The primary damage response phase occurs next, during which the master transcription factor, nuclear factor kappa B (NF-κB), leads to the upregulation of several genes resulting in the production of the proinflammatory cytokines (tumor necrosis factor-α, IL-1β, and IL-6) and then signal amplification. The net result is the ulceration phase, in which microorganisms and microbe-associated molecular patterns such as peptidoglycan and lipopolysaccharide can translocate the damaged physical barrier more easily and are able to activate tissue macrophages to produce more proinflammatory cytokines, thereby exacerbating the damage.65,66 In addition, damage-associated molecular patterns including endogenous ligands, the alarmin, high-mobility box group-1, ATP, and heat shock proteins released as a result of tissue damage all contribute to inflammation and tissue damage.64 Last, the healing phase occurs and is to a certain extent dependent on the rate of recovery of stem cells that are capable of repopulating the epithelium. Cytostatic chemotherapy regimens and irradiation injury induce varying degrees of mucosal barrier injury with differing dynamics, although the progression and decline of signs may be similar.67 Individual patients are also likely to vary in their susceptibility to mucosal damage, suggesting that there are genetic differences in the expression of proinflammatory cytokines or proteins that control stem cell apoptosis (e.g., TP53 and BCL2 along the gastrointestinal tract).