Intrinsic Host Factors
Hematologic Malignancies
Some hematologic malignancies are associated with increased frequency of infections even in the absence of treatment. For instance, the rate of mycobacterial disease seems to be increased in hairy cell leukemia and Hodgkin lymphoma. Multiple myeloma and chronic lymphocytic leukemia are associated with a high risk of encapsulated bacterial infections due to impaired B-cell immunity. Mutations in GATA2 are associated with myelodysplastic syndrome, acute leukemia, and Epstein-Barr virus (EBV)-associated malignancies, and they result in a particular immunodeficiency syndrome (MonoMac) characterized by viral, mycobacterial, and fungal infections in the setting of monocytopenia, B, natural killer, and CD4 lymphocytopenia.1
Solid Tumors
Tumor-related erosion of normal anatomic barriers or obstruction of the respiratory, biliary, and genitourinary tracts contributes to an increased risk of infection. The relief of obstruction remains the primary therapeutic maneuver, with ancillary antimicrobial therapy directed by a knowledge of the normal flora and its alterations in the presence of obstruction. The specific association of colon cancer with bacteremia and/or endocarditis caused by Streptococcus gallolyticus (formerly Streptococcus bovis) should still be noted. Breast tumors increase the risk of mastitis and abscess formation, usually by Staphylococcus aureus. Adrenal corticosteroid-producing tumors and ectopic adrenal corticotrophin hormone–secreting tumors are associated with an increased risk of bacterial and opportunistic infections.
Asplenia
Functional or surgical asplenia is a risk for overwhelming sepsis by encapsulated bacteria. Functional asplenia is present after splenectomy, splenic irradiation, and with chronic graft-versus-host disease (GVHD).2 The most common pathogen is Streptococcus pneumoniae, but other pathogens include Hemophilus influenzae and Neisseria meningitidis. Patients without a functional spleen who present with fever should be started promptly on antibiotics active against S. pneumoniae. The drug susceptibility of S. pneumoniae now is unpredictable, and local patterns should be taken into consideration. Pathogens associated with animals (Capnocytophaga canimorsus) and geographic risks (Babesia, Plasmodium) should be considered. The US Centers for Disease Control and Prevention (CDC) recommends that asplenic persons be immunized with the pneumococcal polysaccharide and meningococcal vaccines.3 The conjugated meningococcal vaccine is preferred in adults 55 years of age or younger because it confers longer lasting immunity than the polysaccharide vaccine. Immunization of adults with the H. influenzae type B vaccine is also recommended. Immunization is ideally performed at least 2 weeks in advance of splenectomy. If this is not feasible, immunization is still advisable after the procedure. Penicillin prophylaxis is advised in asplenic patients to prevent pneumococcal disease.
Treatment-Related Factors
Mucositis
Chemotherapy and radiation therapy impair mucosal immunity at several different levels. Chronic GVHD may further compromise mucosal immunity, including defective salivary immunoglobulin (Ig) secretion. Compromise of the epithelial lining may result in invasion by local flora, and bacteremia and candidemia may result. Severe mucositis is a known risk factor for viridans-group streptococcal infections,4 but many pathogens, including oral anaerobes, may cause invasive disease in this setting.
Chemotherapy-Induced Neutropenia
Neutropenia is a major risk factor for infections in cancer patients. Lack of granulocytes facilitates bacterial and fungal infections, and blunts the inflammatory response, allowing infections to progress much faster. The source of bacterial infections is most commonly the commensal flora of the gastrointestinal tract. Fungal infections may be related to prior colonization in the case of Candida (skin or gastrointestinal flora) or inhalation in the case of molds. Risk of fungal diseases increases with the duration of neutropenia. Aspergillosis is uncommon during neutropenic periods of <10 days, but its incidence increases in direct proportion to the length of neutropenia after 14 days.5
Hematopoietic Stem Cell Transplantation
Autologous and allogeneic hematopoietic stem cell transplantation (HSCT) present different infectious disease problems. Autologous transplant may be considered a form of intensive chemotherapy. As such, it is typically associated with a few days or weeks of neutropenia and mucositis, followed by a few weeks or months of defective T-cell–mediated immunity. The duration of the defect in T-cell immunity varies depending on the type of cancer (longer in hematologic malignancies), the manipulation of the stem cells preinfusion (longer with T-cell depletion), and the age of the recipients (shorter immunodeficiency in children). Autologous transplants have fewer infections than allogeneic transplants, but T-cell depletion results in risk for cytomegalovirus (CMV) and other opportunistic infections similar to allogeneic HSCT recipients.6
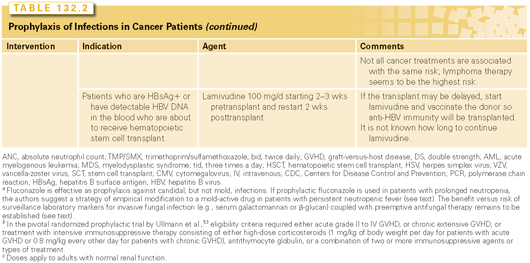
Allogeneic transplant is a more complex procedure. The character and duration of increased risk for infection varies with conditioning, human leukocyte antigen (HLA) matching, source and dose of transplanted cells, and GVHD prophylaxis. Infections after HSCT tend to follow a timeline associated with different predominant immune defects: neutropenia early on, then GVHD disease and its treatment. Active GVHD requires treatment with corticosteroids and other immunosuppressive agents and is associated with increased risk of infection.7 The specific role of CMV reactivation (which often accompanies GVHD) is also important, not only as a potential disease process, but as a predisposition to bacterial and fungal infections.8 Defects in cell-mediated immunity persist for several months even in uncomplicated allogeneic HSCT recipients, predisposing to opportunistic infections. Allografts from alternative donors (e.g., HLA-matched unrelated donors, haploidentical family donors, umbilical cord blood) may result in higher risk of infection due to higher risk of GVHD, decreased T-cell function, and delayed immune reconstitution.9 Recommendations for monitoring and prophylaxis are outlined in Table 132.2.


Immunomodulatory Agents and Infectious Risk
Corticosteroids
High-dose corticosteroids have profound effects on the distribution and function of neutrophils, monocytes, and lymphocytes. Corticosteroids blunt fever and local signs of infection. Patients treated with corticosteroids have impaired phagocytic function (increasing risk of bacterial and fungal infections) and cell-mediated immunity (increasing risk of infections like herpes zoster and Pneumocystis jirovecii). The incidence of infectious complications increases when the adult equivalent of prednisone 20 mg per day is administered for longer than 4 to 6 weeks.10
Fludarabine
Fludarabine is a lymphotoxic analogue of adenine, primarily affecting CD4+ lymphocytes. The combination of fludarabine and corticosteroids results in a uniform depression of CD4+ cells that may persist for several months after completion of therapy, resulting in opportunistic infections like P. jirovecii pneumonia (PCP) or listeriosis, sometimes more than a year after treatment. Mycobacterial and herpes virus infections have also been described.
Interleukin-2
Patients receiving high dose interleukin (IL)-2 for malignancy have an increased risk of bacterial infections, mainly S. aureus and coagulase-negative staphylococci, possibly related to indwelling catheters. High-dose, continuous infusion IL-2 causes a profound but reversible defect in neutrophil chemotaxis that may account for the increased frequency of infections. Prophylactic oxacillin led to a reduction in central venous catheter–associated staphylococcal bacteremia in IL-2 recipients in one randomized trial.11
Monoclonal Antibodies
Monoclonal antibodies (mAb) that target specific cellular steps or factors have made it possible to manipulate disease pathways in such a way that it has been possible to use “targeted” cancer therapies with astonishing clinical responses.12 However, many of these agents interfere with natural immunity and have therefore increased rates of both common and uncommon infections.13 As with many other immunosuppressive drugs, establishing causality is a challenge; only those associated with a probable increase in the risk of infection are described in the following (see Table 132.1).
Rituximab and Ofatumumab. There are now multiple anti-CD20 mAbs available for clinical use. Those approved in oncology are rituximab, a chimeric murine-human mAb that targets CD20 on mature B-lymphocytes, and ofatumumab, an entirely human mAb that targets different CD20 epitopes. Most evidence regarding infectious complications refer to rituximab, but it is likely that similar risks will apply to the newer anti-CD20 mAbs. These agents are used against a variety of B-cell malignancies, as preemptive treatment of EBV-associated lymphoproliferative disorder and also as immunomodulating drugs in the treatment of GVHD.
Rituximab results in rapid and profound depletion of B cells that can last up to several months. In most patients, serum Ig levels remain largely stable, as plasma cells do not express CD20. Some patients, however, develop persistent hypogammaglobulinemia, although its contribution to infection risk is unclear.14 Initial trials suggested that rituximab had minimal effect on the occurrence of infections; however, more recent meta-analyses have reported a higher relative risk of infections. The increase in the risk infection seems to be more prominent in the setting of repeated administration and in patients with underlying immune defects or concomitant significant immunosuppression.15 Hepatitis B virus (HBV) reactivation has been consistently associated with rituximab treatment, including reports of fulminant hepatitis and death in patients who experience hepatitis B flare, particularly when used in combination therapy (e.g., rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone [R-CHOP]). Also a reverse seroconversion phenomenon has been described, with loss of protective HBV surface antibodies and reactivation.16 Most reactivation occurs within 6 months of therapy, but there are cases up to a year after treatment. The US Food and Drug Administration (FDA) has issued a black-box warning regarding hepatitis B reactivation and rituximab and ofatumumab (http://www.fda.gov/Drugs/DrugSafety/ucm366406.htm). It is recommended to assess the HBV status in all patients before rituximab or ofatumumab by hepatitis B surface antigen and hepatitis B core antibody and consider pharmacologic suppression of HBV with lamivudine or lamivudine and tenofovir, and follow all patients for signs of hepatitis B while they are receiving either drug and for several months thereafter, as reactivation has been described well after completion of therapy. Periodic determination of hepatitis B DNA while on treatment should be considered.
Another opportunistic infection reported with both rituximab and ofatumumab is progressive multifocal leukoencephalopathy (PML), also called JC encephalopathy. This is a demyelinating disease caused by JC virus, a ubiquitous virus that is acquired during infancy by most people. Since the initial approval of rituximab, there have been close to 100 cases of PML associated with its use.17 Most cases have been reported in patients with hematologic malignancies; the role of rituximab in the development of PML is not well understood. A few reports of PCP following the administration of rituximab are far from conclusive, given that most patients had received other immunosuppresive therapies. It is unclear whether PCP prophylaxis should be recommended.18 Other rare infections that have been described in the setting of rituximab use are enteroviral meningoencephalitis, CMV disease, disseminated varicella zoster virus (VZV), refractory babesiosis, parvovirus B19, and nocardiosis.
Alemtuzumab. Alemtuzumab (Campath-1H) is a humanized mAb that targets and lyses cells expressing CD52, a glycoprotein abundantly expressed on most B- and T-lymphocytes, macrophages, and natural killer cells. This results in profound and sustained deficits in cellular and humoral immunity. CD4 and CD8 cell count nadir within weeks and may remain at <25% of baseline for approximately 9 months. Alemtuzumab is increasingly used for a variety of hematologic malignancies. Infections associated with its use include reactivation of latent viruses (herpesvirus like CMV, EBV, VZV, and human herpesvirus [HHV]-6, as well as adenovirus and JC virus), fungal infections (PCP, cryptococossis, disseminated histoplasmosis, aspergillosis, mucormycosis), and mycobacterial infections (both tuberculosis [TB] and nontuberculous mycobacteria).19 The incidence of these opportunistic infections varies with the dose and combination with other immunosuppressants. All patients receiving alentuzumab should receive PCP and herpesvirus prophylaxis for a minimum of 2 months after therapy or until CD4 counts are ≥200 cells/μl (Campath package insert). Given the high incidence of CMV reactivation and disease, prophylaxis or preemptive therapy is warranted.
Daclizumab and Basiliximab. Daclizumab and basiliximab are humanized and chimeric mAbs, respectively, that target CD25, the α chain of the IL-2 receptor complex expressed on activated T-lymphocytes. They competitively inhibit IL-2 binding and prevent IL-2–mediated activation of lymphocytes and cytokine release. In hematology/oncology, they are used for steroid-refractory GVHD after allo-HSCT.20 Infections described in this setting are common bacterial infections, viral reactivations (CMV, BK virus, adenovirus, HSV, RSV, influenza), EBV-associated posttransplant lymphoproliferative disease (PTLD), invasive fungal infections, as well as legionella, nocardia, nontuberculous mycobacteria, TB, and toxoplasmosis.21 The specific contribution of daclizumab to the infectious risk is impossible to quantify.
Tumor Necrosis Factor-α Inhibitors: Adalimumab, Infliximab, Etanercept, Certolizumab Pegol, and Golimumab
Infliximab, adalimumab, and golimumab are mAbs directed against interferon-α; certolizumab pegol is a pegylated Fab fragment of a humanized anti–tumor necrosis factor (TNF)-α mAb; and etanercept is a soluble receptor for TNF-α.13 These agents are potent immunosuppressants, but their use in oncology is very limited, mainly GVHD. TNF-α is a cytokine that plays a central role in macrophage and phagosome activation and granuloma formation and maintenance. TNF-α blockade results in increased risk of infection, particularly granulomatous infections such as TB and histoplasmosis, but also multiple others.22,23 The risk of serious infections appears to be lower with etanercept, than with the other TNF-α blockers. Several cases of severe HBV reactivation in patients with positive surface antigen at the start of treatment have been reported. Patients receiving anti-TNF-α therapy should be screened for TB (latent or active), HBV, and hepatitis C virus before starting treatment.24
Cetuximab. Cetuximab is chimeric human-murine IgG1 that targets epidermal growth factor receptor. It is used in the treatment of epidermal growth factor receptor–expressing metastatic colorectal cancer and in advanced or metastatic squamous cell carcinoma of the head and neck. Its use has been associated with slight increase in the risk of serious bacterial infections, mainly pneumonia.25 There is one case report of a disseminated Mycobacterium chelonae infection.26
Brentuximab Vedotin. Brentuximab vedotin is an antibody-drug conjugate consisting of a chimeric IgG1 antibody that targets CD30 and the microtubule-disrupting agent monomethyl auristatin E. Brentuximab vedotin results in death of CD30+ cells. It is approved for use in relapsed Hodgkin lymphoma and relapsed systemic anaplastic large cell lymphoma.27 Its use has been associated with a high frequency of upper respiratory tract infections. More relevant has been the reports of some cases of PML.28,29
Bevacizumab. Bevacizumab, an inhibitor of vascular endothelial growth factor, widely used for the treatment of metastatic colon cancer, non–small-cell lung cancer, and other tumors, has been associated with increased risk of gastrointestinal perforation.30 Anecdotal reports of localized aspergillosis and fusariosis could be related to the potential for reduced angiogenesis to contribute to the pathogenesis of invasive mold infections.31,32
Infections may be prevented by avoidance of exposure, immunization, and chemoprophylaxis. A comprehensive guideline cosponsored by the Center for International Blood and Marrow Research, the National Marrow Donor program, the European Blood and Marrow Transplant Group, the American Society for Blood and Marrow Transplantation, the Canadian Blood and Marrow Transplant Group, the Infectious Diseases Society of America (IDSA), the Society for Healthcare Epidemiology of America, the Association of Medical Microbiology and Infectious Disease Canada, and the CDC makes evidence-based recommendations for HSCT that may be applicable to other cancer patients.33 The National Comprehensive Cancer Network publishes and continuously updates broad guidelines for prophylaxis.34,35 A summary is provided in Table 132.2.
In general, antimicrobial prophylaxis reduces the number of episodes of the targeted infection. The question frequently is whether this reduction translates into a survival benefit or a clinically meaningful outcome (e.g., sepsis, hospital admission, shortened hospitalization) that outweighs the toxicities or secondary effects (e.g., generation and spreading of resistant organisms). The number of subjects that need to be treated to prevent one episode of infection and the severity of the infection should be considered.
Prevention of Bacterial Infections
Antibacterial Prophylaxis in Afebrile Neutropenic Patients
Several meta-analyses support a beneficial effect in fever, documented infections, and overall mortality by administering fluoroquinolone prophylaxis to patients who are neutropenic for ≥7 days.36–39 In high-risk neutropenic patients, prophylactic levofloxacin led to a reduction in infections.40 In lower-risk neutropenic patients (e.g., those with solid tumors and short-duration neutropenia), prophylaxis showed a modest but significant reduction in febrile episodes.41 The potential risks of quinolone prophylaxis regarding selection for resistant organisms and Clostridium difficile colitis are important questions that have made some experts stay away from recommending levofloxacin prophylaxis except in special circumstances,42 but the most recent American Society of Clinical Oncology guidelines still endorse their use when neutropenia is expected to last 7 days or more.43
Prophylaxis against Pneumococcal Infection
Pneumococcal prophylaxis should be considered in asplenic patients and in allogeneic HSCT recipients with chronic GVHD. The Working Party of the British Committee for Standards in Haematology recommend lifelong prophylactic antibiotics in patients who have had a splenectomy, and particularly in the first 2 years, in children up to age 16, and in patients with other immune impairment.44 Among allogeneic HSCT recipients, pneumococcal disease typically occurs in the later transplant period, from 3 months to years after transplant. Chronic GVHD is the major risk factor. Vaccination is recommended as in asplenic patients. Penicillin prophylaxis is also recommended.33 Some experts recommend starting penicillin prophylaxis in all HSCT recipients 3 months after transplant, whereas others reserve it for patients with active chronic GVHD. Alternative agents may be considered based on local susceptibility patterns, although the risk/benefit ratio is not well defined.
Prevention of Fungal Infections
Candida spp. and molds are the major fungal pathogens in patients with cancer. Systemic antifungal prophylaxis may be mold-active (voriconazole, posaconazole, echinocandins, amphotericin B) or not (fluconazole). Candida spp. are part of our endogenous flora; the major risk factors for candidemia in patients with cancer are chemotherapy-related mucositis and central venous catheters. Patients who have undergone gastrointestinal surgeries complicated by anastomotic leaks, total parenteral nutrition, and prolonged admission in the intensive care unit are also risk factors for invasive candidiasis. In contrast, the risk of invasive mold infections is principally related to the duration of neutropenia, and, in allogeneic HSCT recipients, amount and duration of corticosteroids used to treat GVHD. The most common mold infections are aspergillosis and mucormycosis, but multiple opportunistic molds can infect neutropenic cancer patients (Fusarium, Paecylomyces, dematiaceous molds)
Antifungal prophylaxis should be risk-based: different patient groups are at risk for different pathogens, and these should be targeted accordingly.45 Most cancer patients do not require antifungal prophylaxis. Prolonged neutropenia increases the risk of candidiasis, and this is prevented by the use of fluconazole 400 mg/day46,47 (which may result in colonization by azole-resistant Candida strains).48 Itraconazole has similar efficacy and may reduce the risk of aspergillosis, but is poorly tolerated and has higher toxicity and worse drug interactions.49,50 Micafungin, an echinocandin with activity against Candida and Aspergillus, was as effective as fluconazole in preventing candidiasis and showed a favorable trend to prevent aspergillosis in a randomized, double-blinded study in HSCT recipients.51 Prevention of aspergillosis has been shown most convincingly with posaconazole, with a survival benefit demonstrated in neutropenic patients52 and decreased incidence of aspergillosis in transplant patients with GVHD.53 A randomized trial comparing voriconazole and fluconazole prophylaxis in allogeneic HSCT recipients showed a trend toward reduction of aspergillosis cases in voriconazole recipients, but no difference in overall or fungal infection–free survival.54 A systematic review and meta-analysis suggests that, overall, mold-active prophylaxis significantly reduces invasive aspergillosis (IA) and invasive fungal infection (IFI)-related mortality, but does not affect overall mortality and has more adverse effects than fluconazole.55
In summary, fluconazole is a safe and well-studied agent to prevent invasive candidiasis, but it has no activity against molds. When the risk of aspergillosis is significant, a mold-active drug should be considered, but different institutions may choose different agents.56 The mold-active azoles (i.e., itraconazole, voriconazole, posaconazole) are potent inhibitors of certain cytochrome P-450 isoenzymes to a greater degree than fluconazole. This may lead to reduced clearance of other drugs, such as calcineurin inhibitors, cyclophosphamide metabolites,57 and vinca alkaloids with potentially serious adverse effects.58 Careful monitoring of drug-drug interactions and appropriate dose modifications are required.
Prevention of Pneumocystis jirovecii Pneumonia
Defective T-cell immunity is the principal risk factor for PCP. Recommendations for prophylaxis in the non-AIDS setting are based on the expected level of risk of PCP and consensus criteria rather than on randomized clinical trials. The traditional groups of cancer patients at risk have been acute lymphocytic leukemia and allogeneic HSCT. After allo-HSCT, prophylaxis is recommended until at least day 180 after transplant, and longer if immunosuppressive agents are continued.59 PCP prophylaxis should be considered in patients with cancer who receive prolonged high-dose steroids (i.e., equivalent of prednisone 20 mg daily for ≥1 month). Other candidates for prophylaxis include alemtuzumab recipients (package insert recommends prophylaxis until at least 2 months after completion of alemtuzumab and CD4 count ≥200/mcL, whichever occurs later), patients with chronic lymphocytic leukemia receiving fludarabine, and patients with gliomas receiving temozolomide and radiation or corticosteroids.
The most effective agent is trimethoprim/sulfamethoxazole (TMP/SMX). A variety of dosages seem to be effective (from one double-strength tablet daily, to one double-strength tablet twice daily 2 days per week). When TMP/SMX cannot be administered because of marrow intolerance or hypersensitivity reaction, second-line agents include dapsone (50 mg twice daily or 100 mg orally daily), inhaled pentamidine (300 mg every 4 weeks), and atovaquone (1,500 mg daily) (see Table 132.2). All second-line agents are less effective than TMP/SMX, with the difference increasing with the degree of immune compromise.
Prophylaxis of Viral Infections
Prevention of Herpes Simplex Virus and Varicella Zoster Virus
Among seropositive patients, the incidence of herpes simplex virus (HSV) reactivation following induction chemotherapy for leukemia or conditioning for HSCT is 70% to 80%. HSV and VZV infections are common after alemtuzumab, bortezomib,60 and lenolidomide.61 Antiviral prophylaxis (acyclovir, valacyclovir, or famciclovir) against HSV is advised in patients receiving chemotherapy for acute leukemia, in all HSV-seropositive allogeneic HSCT recipients, and in some autologous HSCT recipients at high risk for mucositis during the neutropenic period. Prophylaxis is recommended until mucositis resolves and engraftment takes place.34 It is also advised in myeloma patients treated with bortezomib or lemolidomide.60,61 For alemtuzumab recipients, the package insert recommends at least 2 months after completion of therapy and CD4 count ≥200/mcL, whichever occurs later.
Nosocomial transmission of VZV is well documented. Patients with chickenpox, disseminated zoster, and immunocompromised patients with dermatomal zoster should be placed under contact and respiratory isolation. In the absence of varicella zoster immune globulin, oral acyclovir for 2 weeks after exposure has been used successfully for postexposure prophylaxis. The two available live attenuated varicella vaccines are Varivax (used to induce immunity in persons who have not been exposed to varicella; Merck, Inc., Whitehouse Station, NJ) and Zostavax (for older immunocompetent persons with prior exposure to varicella to augment immunity to prevent shingles; Merck, Inc.). Neither vaccine should be used in highly immunosuppressed persons because of the risk of viral disease. Household contacts of immunocompromised patients and health-care workers with no history of varicella and seronegative for VZV should receive the Varivax vaccine to prevent infection by wild-type varicella.33 If a rash occurs following vaccination, direct contact with immunocompromised persons should be avoided.
Prevention of Cytomegalovirus Disease
CMV is a very important pathogen after allo-HSCT, but CMV disease is uncommon in other cancer patients. It has been reported mainly in patients receiving alemtuzumab (although asymptomatic reactivation without disease seems to be much more frequent) and occasionally in patients receiving therapy for acute leukemia. CMV infection happens early in life and results in viral latency. Most adults (60% to 80%) are infected, as proven by the detection of CMV-specific IgG in their serum. A transplant recipient is at risk for reactivation (if he or she is CMV seropositive) or for primary infection (from the allograft if the donor is CMV seropositive) or from blood products.62
Prevention of Cytomegalovirus Disease After Allogeneic Hematopoietic Stem Cell Transplantation
Prevention of Primary Cytomegalovirus Infection. To prevent CMV primary infection, CMV-seronegative HSCT recipients receiving allografts from CMV-seronegative donors should receive transfusion products from only CMV-negative donors or, if this is not feasible, leukocyte-depleted blood products.63
Prevention of Cytomegalovirus Disease Following Reactivation. In case of preexisting CMV infection, CMV disease may be prevented by one of two approaches: prophylaxis or preemptive management. In prophylaxis, antiviral agents are administered for a variable period to all allogeneic HSCT recipients in which either the donor or recipient is CMV seropositive. In preemptive management, active surveillance of the patients at risk is followed by initiation of antiviral agents following detection of CMV reactivation.
Prophylaxis for Cytomegalovirus. Ganciclovir prophylaxis for CMV-seropositive allogeneic transplant recipients was highly effective at suppressing CMV during the early transplant period, but was associated with higher rates of neutropenia, bacterial and opportunistic infections, and late CMV disease, without improvement in overall survival.64,65
Preemptive Therapy for Cytomegalovirus. CMV infection may be diagnosed early by detection of the CMV pp65 antigen in peripheral blood leukocytes or by measuring CMV DNA by polymerase chain reaction (PCR). A single positive CMV antigenemia or two consecutive positive PCR results are triggers for preemptive antiviral therapy. When compared in a randomized controlled trial, preemptive therapy and prophylaxis resulted in equivalent overall survival, but with different complications: universal prophylaxis had less early CMV disease but more invasive fungal infections, more ganciclovir use, and more late CMV disease. Preemptive therapy (using pp65 antigenemia) resulted in more CMV disease by day 100.66 The preemptive approach is more commonly used. Foscarnet can also be used preemptively, with similar results but different toxicities (renal toxicity with foscarnet, myelosuppression with ganciclovir). Valganciclovir an orally administered prodrug of ganciclovir is another alternative, although it may result in increased exposure to ganciclovir and potentially more toxicity.67 Similar CMV recommendations have been made for patients treated with alemtuzumab.34
Prevention of Viral Hepatitis
Three etiologic agents of viral hepatitis are a matter of significant concern in cancer patients. Hepatitis B and C have special importance because of their etiologic role in hepatobiliary cancer and the enormous populations affected by with these viruses.
Hepatitis B
Carriers of HBV, even if they are asymptomatic or have undetectable HBV DNA levels, are at risk for hepatitis B flare after immunosuppressive therapy or cytotoxic chemotherapy, particularly with rituximab and high-dose corticosteroids. All patients should be screened for hepatitis B status before receiving cytotoxic chemotherapy by determining hepatitis B surface antigen (HBsAg) and anti–hepatitis B core antibody. Seronegative patients should be vaccinated if possible. HBsAg-positive patients, regardless of their HBV DNA level, should treated with lamivudine or some other of the nucleoside/nucleotide analogues currently available during the duration of chemotherapy and for up to 12 months thereafter.68 It is unclear what the duration and best method of monitoring are. Treatment of active hepatitis B during cytotoxic chemotherapy is daunting and should be attempted in consultation with an expert in the use or combinations of these newer agents.
Hepatitis C
Chronic hepatitis C infection has a broad worldwide distribution and thus would be expected to occur at baseline in a substantial portion of the cancer patient population. There is little evidence to support concerns for recrudescence of latent disease in patients who are screened and not viremic at baseline, whether by natural evolution or as the result of therapy. The inflammatory disease associated with chronic hepatitis C is variable and has been associated with clinically relevant worsening, predominantly in patients with therapy for hematologic malignancy, but occasionally in solid tumor patients.69,70 As with hepatitis B, interferon-based therapy has prohibitive toxicities in this context, but the evolution of true antiviral agents may make consultation with an expert in the use of these drugs an important consideration.
Hepatitis E
Hepatitis E, a predominantly water-borne virus, has caused chronic infection in both solid and hematologic transplant patients. No specific antiviral therapies are available, but acquisition and recrudescence should be considered in patients receiving chemotherapy who develop new or worsening hepatic inflammation.71–73
DIAGNOSIS AND MANAGEMENT OF INFECTIOUS DISEASES SYNDROMES
Fever is single oral T ≥38.3°C or sustained T ≥38°C for 1 hour.74 Slightly different definitions may have been used in different trials over the years. Neutropenia is defined as an absolute neutrophil count (ANC) of <500 cells/mm3 or an ANC that is expected to decrease to <500 cells/mm3 during the next 48 hours.74 In neutropenic patients, fever should be considered evidence of infection and treated accordingly.
Bacterial infections may progress quickly in the absence of granulocytes and appropriate antibiotics must be started immediately. Empirical administration of broad-spectrum antibiotics to neutropenic febrile patients showed early on to result in improved outcomes75 and remains the standard of care. Numerous professional organiztions have issued guidelines for the management of fever during neutropenia in adults and children, inpatient and outpatient.34,43,74,76,77 The initial evaluation consists of a complete history and physical examination. Important elements of the history include prior infections or known colonization with resistant pathogens, as well as timing of the fever in relation to manipulation of the central venous catheter if one is present. Special attention should be given to the mouth, skin, catheter exit site, and perianal region during the physical exam.
Initial laboratory studies should include complete blood count and differential, serum chemistry including liver function tests, and at least two sets of blood cultures. Other cultures (urine, sputum, stool if diarrhea) should not delay the administration of antibiotics. An initial chest radiograph in the absence of symptoms or signs of pulmonary infection is of low yield in ambulatory adult patients with fever and neutropenia.
Risk Stratification
One of the key current concepts in the management of neutropenic fever involves risk stratification: not all patients who develop fever during chemotherapy-induced neutropenia have the same risk of a poor outcome, and this has practical implications for management (choice of antibiotics, inpatient versus outpatient setting). Most adult guidelines endorse the use of the Multinational Association for Supportive Care in Cancer (MASCC) index.78 The pediatric guidelines support the concept of risk stratification, but emphasize the importance of using strategies that have been validated locally.77 The MASCC index was designed as a tool to identify adult patients at low risk of complications.78 To obtain a MASCC score, points are allocated and added up. Points are given for burden of illness (no or mild symptoms 5, severe symptoms 3), absence of hypotension (5), absence of chronic obstructive pulmonary disease (4), solid tumor OR no previous fungal infection (4), absence of dehydration (3), outpatient status (3) and age <60 years (2). The points are added up, and patients with a score of ≥21 points (of 26 possible) are “low risk” and can be considered for oral therapy. The index has been validated in multiple settings and performs well, although it may do so better in solid tumors than in hematologic malignancies.79 The IDSA guidelines include “expert” clinical criteria derived from clinical trials: patients with neutropenia expected to last ≥7 days, clinically unstable or with significant comorbidities, or high intensity chemotherapy are all “high risk” and should be hospitalized for intravenous antibiotics.74 Similarly, the American Society of Clinical Oncology guidelines present a list of conditions that makes the outpatients high-risk independently of their MASCC score.43
For “low-risk” patients, it is still important to make sure they can tolerate oral agents and have adequate access to medical care before deciding to treat them as outpatients with oral antibiotics. The antibiotics used for adults have been ciprofloxacin + amoxicillin/clavulanic acid80 and moxifloxacin monotherapy.81 Quinolones should not be used for treatment if patients were receiving them for prophylaxis. In penicillin-allergic patients, the combination of ciprofloxacin + clindamycin is recommended.43
Neutropenic Fever Syndromes
The practical management of fever during neutropenia is easier if every episode is categorized as one of the following: first fever, persistent fever, recrudescent fever, and fever at the time of myeloid recovery.82,83 Although overlap may exist, these four syndromes represent useful clinical categories that should be managed differently.
First Episode of Fever
Most episodes of fever during neutropenia are supposed to be infectious in origin. Infection, however, is not always documented. The percentages are roughly as follows: fever of unknown origin, 50% to 60%; microbiologically documented infection (frequently bacteremia), 10% to 20%; and clinically documented infection (e.g., typhlitis or cellulitis without any pathogen being isolated), 20% to 30%.74 The majority of documented infections during neutropenia are caused by the patient’s own endogenous bacterial flora. In the absence of localizing symptoms, physical examination findings, or positive cultures, several antibiotic regimens may be used. For high-risk patients, most of the guidelines recommend to start monotherapy with a beta-lactam with activity against Pseudomonas aeruginosa (piperacillin-tazobactam, imipenem, meropenem, cefepime, ceftazidime),34,74,77 with the important caveat that some form of combination therapy should be chosen in patients who are clinically unstable and/or if there is suspicion (or high risk) of infection caused by resistant gram-negative (second gram-negative agent should be added) or gram-positive bacteria (vancomycn or linezolid should be added). The antipseudomonal beta-lactam of choice depends on local resistance patterns; meta-analyses suggest that, unless there is high prevalence of resistant bacteria that make its use unadvisable, piperacillin-tazobactam may offer the best combination of high efficacy and low toxicity.84 The routine use of combination therapy (typically beta-lactam + aminoglycoside) was shown to be associated with higher toxicity and not superior outcome.85,86 The so-called de-escalation approach suggests starting with broader coverage (based on local susceptibility patterns this may consist, for instance, of carbapenem + colistin ± another agent) then de-escalate appropriately.76 All the guidelines recommend not including vancomycin routinely in the initial regimen and not adding it empirically for persistent fever. The details regarding when to actually use vancomycin are more variable. The IDSA strongly recommends adding vancomycin in cases of hemodynamic instability, pneumonia, clinically evident catheter–related infection, severe mucositis, and known colonization with methicillin-resistant S. aureus (MRSA), although the quality of the evidence is low.74 Our own approach is presented in Figure 132.1 and Table 132.3. Regarding the use of alternative agents for gram-positive bacteria, linezolid and vancomycin had similar efficacy in a randomized trial where there were no cases of vancomycin-resistant enterococci (VRE), but linezolid resulted in delayed neutrophil and platelet recovery.87 Regardless of the initial regimen actually chosen, modifications may be required between 30% and 50% of the time, depending on clinical evolution and/or microbiologic results (see Table 132.3). If fever resolves following initiation of empirical therapy, the assumption is that an infection is responding to antibiotics. The standard recommendation is to continue antibiotics until resolution of neutropenia. If the neutropenia persists, older studies showed that 2 weeks may be adequate duration of treatment.88 More recent data, mainly in children, suggest shorter duration of antibiotic treatment may be associated with similar outcomes as long as the antibiotics are restarted promptly if fever recurs, but it is important to pay attention to the patient population included in these trials.76


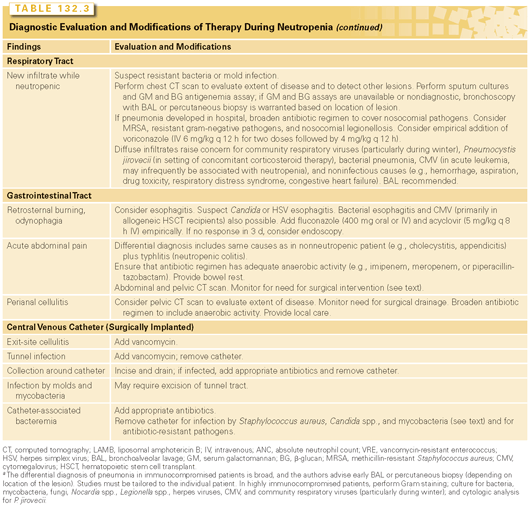
Persistent Fever
The average time to defervescence in neutropenic fever is 4 days. Modifications of the antimicrobial regimen are not recommended during this period in the absence of new clinical or microbiologic findings. However, the persistence of fever beyond 4 days (96 hours) on broad-spectrum antibiotics identifies a population of patients with higher likelihood of IFI. The majority of persistently febrile neutropenic patients do not harbor an identifiable fungal infection, but several autopsy series in the 1970s documented occult fungal infection as a common cause of death in persistently febrile neutropenic patients.89 Delay in initiating antifungal therapy was associated with treatment failure and death,90 and empirical use of amphotericin B seemed to help.91 Two randomized trials established the benefit of adding amphotericin B (one after 7, the other after 4 days of persistent fever) compared with continuing the antibacterial regimen in terms of preventing fungal infection, although they were not powered to demonstrate a survival advantage.92,93 Of note, these studies were performed before there was systemic antifungal prophylaxis and identified Candida spp. as the principal fungal pathogen in this situation. Initiation of amphotericin B after 4 to 7 days of fever became standard of care, and most randomized controlled trials have focused on the choice of antifungal agent (liposomal amphotericin B,94 voriconazole,95 caspofungin96,97). To reduce the perceived unnecessary use of empirical antifungal therapy with its attendant toxicity and cost, an alternative approach to empirical antifungal therapy has been proposed and called “preemptive antifungal therapy.”45 The goal is to use the currently available diagnostic modalities (computed tomography [CT], serum galactomannan, and/or β-D-glucan) to postpone starting antifungal therapy until IFI is more likely. By design, this approach means that patients receiving “preemptive” antifungals are more likely to actually have an IFI than patients receiving “empirical” therapy by the time the antifungal agent is started.98 The results of this approach are encouraging,99–101 and it has been endorsed by the IDSA.74 The details vary according to risk category of the patient, specific antifungal prophylaxis given, and diagnostic modalities available. The basic concept is that a persistently febrile neutropenic patient with no clinical findings, normal CT scans of chest and sinuses (where most mold infections would start), and repeatedly negative fungal serologic markers (β-D-glucan and galactomannan) is unlikely to have an IFI, and antifungal coverage may be safely delayed. When these diagnostic options are not available, we support the addition of empirical antifungal therapy, typically caspofungin or liposomal amphotericin B, after 5 days of fever.95
Recrudescent Fever
Recurrent fever refers to a new episode of fever that takes place after the initial episode has resolved with antimicrobial therapy when the patient remains neutropenic.82 This is a common occurrence in clinical practice in cases of prolonged neutropenia, and it has not been adequately studied. The one systematic investigation of this syndrome analyzed data on 836 neutropenic patients who had had a first fever that responded to antimicrobials and then had remained afebrile for 4 days.102 The important result of this analysis is that fungal infections were just as common as breakthrough bacterial infections, suggesting the appropriate management of this situation is to modify both the antibacterial and the antifungal part of the regimen, as well as perform a thorough diagnostic workup. Unfortunately, this scenario is not addressed separately from persistent fever in the guidelines.
Fever After Resolution of Neutropenia
In most cases, an undiagnosed fever that has persisted during neutropenia will resolve around the time of myeloid recovery. In a minority of patients, fever appears or worsens at this time. The three likely causes of this situation are superinfection, immune reconstitution syndrome,103 and engraftment syndrome.104 Other noninfectious causes should also be considered (e.g., drug fever, transfusion reactions, and deep venous thrombosis). A systematic, thorough diagnostic workup is more appropriate than empirical therapeutic modifications. Blood and urine cultures, complete blood cell count, serum chemistry, and liver enzyme levels should be obtained. An elevated alkaline phosphatase should prompt consideration of hepatosplenic candidiasis (which is uncommon in patients receiving yeast-active antifungal prophylaxis). A chest X-ray should be obtained. Pulmonary infiltrates identified after neutrophil recovery have a favorable prognosis and may be managed conservatively.105 In contrast to the neutropenic period, empirical antibiotics can be discontinued after resolution of neutropenia in patients who are stable with fever without apparent source.
Fungal Infections in Patients with Cancer
Pathogenic fungi include yeasts and molds. Candida spp. are yeasts that form part of the normal flora; they gain access to the bloodstream through disruption of anatomic barriers (mucositis or indwelling catheters). Molds are ubiquitous soil inhabitants whose conidia or spores are inhaled on a regular basis. Aspergillosis is the most common mold infection in cancer patients, but other pathogenic fungi (Mucor, Rhizopus, Fusarium, and Scedosporium spp.) have become more common over the past 20 years. In-depth practice guidelines for the management of the different fungal diseases have been issued by the IDSA.106,107
Candidiasis
Oropharyngeal and Esophageal Candidiasis. Cytotoxic chemotherapy, corticosteroids, and antibiotics predispose to oral candidiasis. The most common presentation is white adherent plaques on the palate, buccal mucosa, tongue, or gingiva. A wet mount or Gram stain showing pseudohyphae establishes the diagnosis. Therapy includes local treatments such as clotrimazole troches or oral fluconazole. Esophageal candidiasis causes odynophagia. The differential diagnosis includes HSV, CMV (principally in HSCT recipients), and bacteria. Initial therapy should be with fluconazole. Options for fluconazole-resistant mucosal candidiasis include an echinocandin, voriconazole, posaconazole, or amphotericin B formulation.
Candidemia. Candida species are the fourth most common cause of nosocomial bloodstream infections in the United States. Clinical findings vary from asymptomatic to full-blown septic shock. The species should be identified. Candida albicans is the most common, and it is usually susceptible to fluconazole, but the proportion of nonalbicans Candida has been increasing. Candida tropicalis is highly virulent in neutropenic hosts but is susceptible to most agents. Candida krusei is always resistant to fluconazole, and Candida glabrata has variable susceptibility. Candida parapsilosis is mostly associated with vascular catheters and is usually susceptible to fluconazole but relatively resistant to echinocandins. A minority of Candida lusitaniae and Candida guilliermondii isolates are resistant to amphotericin B.
It is recommended to remove all intravascular catheters in patients with candidemia.106 In neutropenic patients, candidemia may arise from defects in the gut mucosa rather than the catheter, so the need for catheter removal has been questioned.108 In general, we remove the catheter where feasible, but sometimes attempt to conserve it when vascular access is limited and thrombocytopenia is present. We immediately remove all intravenous catheters in the setting of clinical instability, lack of resolution of fever within 2 to 3 days, or persistent candidemia after 2 days of appropriate antifungal therapy.
Chronic Disseminated Candidiasis. Chronic disseminated candidiasis (also called hepatosplenic candidiasis) is a complication of intensive chemotherapy regimens, such as those used as induction therapy for acute leukemia. The typical picture is persistent fever after neutrophil recovery with elevation of liver enzymes (particularly alkaline phosphatase). Numerous target lesions in the liver and spleen become apparent by CT scan, ultrasonography, or magnetic resonance imaging (MRI). A liver biopsy is required for a definitive diagnosis. Blood and biopsy cultures are almost always negative, but the pathology is diagnostic. We recommend surgical liver biopsy when the diagnosis is in question. Chronic disseminated candidiasis that is controlled with antifungal therapy is not a contraindication to subsequent chemotherapy or HSCT.109
Therapy for Invasive Candidiasis. An echinocandin (caspofungin, micafungin, or anidulafungin) or a lipid formulation of amphotericin B is recommended for candidemia in neutropenic patients.106,109 In patients receiving an azole who develop breakthrough candidemia, an echinocandin is advised. Azoles (generally, fluconazole) can be used as step-down oral agents or as initial therapy in certain patients at lower risk for mortality and serious complications. Voriconazole does not have a demonstrated advantage over fluconazole, but can be considered for treatment of C. krusei (which is intrinsically fluconazole-resistant) and when a mold-active agent is otherwise warranted (e.g., as prophylaxis in a high-risk patient). We reserve amphotericin B products for unusual complicated cases, such as meningitis and endocarditis, in which data to support optimal therapy are lacking. Some authorities still prefer a lipid formulation of amphotericin B in neutropenic patients, particularly in the presence of hemodynamic instability.
Invasive Aspergillosis
Risk Factors and Clinical Manifestations. Prolonged and persistent neutropenia is a critical risk factor for IA.5 Most cases, however, take place during episodes of active GVHD treated with high doses of corticosteroids. Other risk factors for aspergillosis after allogeneic HSCT include T-cell–depleted transplant, lymphopenia, CMV disease, and respiratory virus infections.110,111 Aspergillosis can involve virtually any organ in the immunocompromised host, but sinopulmonary disease is the most common. Angioinvasion of hyphae leading to vascular thrombosis and tissue infarction and coagulative necrosis is characteristic. Clinical manifestations are quite variable. During neutropenia, persistent fever, chest pain, and a pleural rub are common, although nonspecific, signs. When steroids are the only risk factor, the manifestations may be subtle, and sometimes a pulmonary infiltrate is found in the absence of symptoms.
Diagnosis of Invasive Aspergillosis. The most common finding on chest CT in early invasive pulmonary aspergillosis in patients with neutropenia and HSCT recipients is the presence of one or more well-circumscribed nodules. These may be inapparent on chest radiographs (Fig. 132.2). Other characteristic findings include the “halo sign,” a haziness surrounding a nodule or infiltrate representing alveolar hemorrhage, and the “crescent sign” (cavitation that usually coincides with neutrophil recovery). These signs reflect different stages of hemorrhagic infarction secondary to angioinvasive organisms. Sequential CT scans of neutropenic patients with IA demonstrated that halo signs were common at diagnosis but decreased during the first week of infection as the frequency of the “air crescent sign” increased. The median volume of lesions increased during the first week of therapy and remained stable during the second week.112 Bronchoalveolar lavage (BAL) cultures have approximately 50% sensitivity in focal pulmonary lesions, and definitive diagnosis often requires an invasive procedure and is usually made only when the disease is advanced. The use of galactomannan antigen seems to be much more sensitive.113,114 Isolation of an Aspergillus sp. from sputum or BAL should be presumed to represent invasive disease in neutropenic patients.

Both the serum Aspergillus galactomannan115 and β-D-glucan116 assays, immunoassays that detect fungal antigens in peripheral blood, have been accepted as diagnostic adjuncts of invasive fungal infections in the revised European Organisation for Research and Treatment of Cancer/Mycosis Study Group consensus criteria.117 PCR-based detection is considered investigational.118 These immunoassays have three potential uses: (1) as a diagnostic adjunct, (2) as a surveillance tool in high-risk patients (e.g., allogeneic HSCT recipients) to detect early aspergillosis prior to clinically overt disease, and (3) in monitoring response to antifungal therapy.119 The sensitivity of the galactomannan assay is significantly reduced by concomitant mold-active antifungal agents, and false-positive results may be more common in allogeneic HSCT recipients or with concomitant piperacillin/tazobactam and other antibiotics.120 Meta-analysis suggest that the galactomannan assay has a sensitivity of 60% to 80% and specificity of 80% to 90% for proven IA, but emphasize the results are fairly heterogeneous.115,119 A rising serum galactomannan correlates with failure of antifungal therapy, and decreasing galactomannan correlates with a positive outcome in patients with IA.121
The serum β-D-glucan assay has recently received FDA approval as a diagnostic adjunct. In patients with acute myeloid leukemia and myelodysplastic syndrome, the assay was highly sensitive and specific in detecting early invasive fungal infections, including candidiasis, fusariosis, trichosporonosis, and aspergillosis.116 It does not detect mucormycosis.
Therapy for Invasive Aspergillosis. Voriconazole is currently the treatment of choice of IA,107 because in a randomized controlled trials it was more effective than amphotericin B (successful outcomes: 53% versus 32%) and was associated with significantly improved survival (71% versus 58%).122 Voriconazole appears to have comparable safety and efficacy in children with invasive mold infections compared to adults, although the doses in children must be higher.123 Three different lipid formulations of amphotericin B are available: amphotericin B colloidal dispersion, amphotericin B lipid complex, and liposomal amphotericin B. The lipid formulations have less nephrotoxicity than conventional amphotericin B deoxycholate, and are therefore more suitable for long-term administration. A randomized controlled trial has shown that liposomal amphotericin B at a dose of 3 mg/kg per day is at least as effective, and less toxic, than 10 mg/kg per day for IA.124
Posaconazole is a broad-spectrum azole, so far only available in oral form, which has been used successfully as salvage therapy for a variety of IFIs refractory to standard therapy. Of note, 5 to 7 days are required before therapeutic serum levels are reached.125 The overall success rate of posaconazole in patients with IA refractory or intolerant of standard therapy was 42%.126 Monitoring levels of both posaconazole and voriconazole may be reasonable, particularly when there are questions about absorption or when the response to treatment is inadequate.127 Echinocandins have not been evaluated as initial monotherapy for IA in controlled trials. Caspofungin as salvage therapy in patients with IA led to a favorable response in 37 of 83 patients (45%).128
There has been significant interest in combination antifungal therapy pairing an echinocandin with either an amphotericin B preparation or a mold-active azole. The rationale is that echinocandins target a site (the β-glucan constituent of the fungal cell wall) distinct from the polyenes and azoles that target the fungal cell membrane. In vitro and animal models data have been encouraging, and uncontrolled clinical studies suggest minimal toxicity and potentially improved outcome of aspergillosis. The question of combination is far from settled, but many experts recommend its use, at least early in the course of treatment.109
Patients who recover from an episode of IA are at risk for relapse of infection during subsequent immunosuppression. Secondary prophylaxis with a mold-active agent is advised for the entire period of immunosuppression.
Mucormycosis (Formerly Zygomycosis)
Risk factors for mucormycosis include diabetic ketoacidosis, protein-calorie malnutrition, iron overload, desferrioxamine (but not other iron chelators), corticosteroid therapy, and prolonged neutropenia.129 Mucormycosis typically manifests as rhinocerebral or pulmonary disease following inhalation of spores. In rhinocerebral disease, fever, facial pain, and headache are common. Contiguous extension may lead to orbital involvement with proptosis and extraocular muscle paresis, involvement of hard palate, and spread to the brain. The use of voriconazole prophylaxis may contribute to increased incidence of mucormycosis.130 Therapy for zygomycosis involves amphotericin B lipid formulation (5 mg/kg per day) plus early and aggressive surgical debridement in cases of localized cutaneous or sinus disease. Voriconazole and echinocandins are not active against zygomycetes. Posaconazole has shown promising results as salvage therapy.131
Less Common Molds
Fusarium, Paecilomyces, Scedosporium, and several dematiaceous molds are increasingly important causes of mortality in leukemia and in allogeneic HSCT recipients.132 It is recommended to obtain infectious diseases expertise for the management of these infections. The likelihood of infection by a Fusarium spp. is increased by the presence of disseminated cutaneous lesions and isolation of a mold from blood culture. Different species of Fusarium have different susceptibilities, and it has been proposed to start empirical voriconazole and amphotericin B until the results of testing are available.109 Scedosporium apiospermum and Scedosporium are usually treated with voriconazole. Dematiaceous (dark-walled) molds like Alternaria, Curvularia, and Phaeoacremonium can cause subcutaneous infection in immunocompetent persons following traumatic inoculation; pneumonia, central nervous system (CNS) disease, and dissemination can occur in severely immunocompromised persons. Itraconazole, voriconazole, or posaconazole are appropriate initial therapy.
Immune Augmentation
The optimal treatment of fungal infections requires attempting to optimize the host’s ability to fight infection. Corticosteroids should be tapered or discontinued if possible, therapeutic levels of the antifungal agents achieved anatomic obstacles, if present, removed. The use of colony-stimulating factors (CSF) and granulacyte transfusions is frequently a consideration.
Colony-Stimulating Factors
Granulocyte-colony stimulating factor (G-CSF) has shown to reduce the incidence of febrile neutropenia and is recommended by European133 and American134 professional organizations when the risk of neutropenic fever is ≥20% or if the patient has experienced febrile neutropenia in previous cycles. Multiple randomized clinical trials of prophylactic CSFs have shown benefit in reducing the time to neutrophil recovery and duration of fever and hospitalization. Meta-analyses confirm its efficacy in HSCT135 and breast cancer,136 although reductions in mortality has not been shown.
The use of CSFs as treatment of established infection continues to be experimental. The rationale to use CSFs for established infections (as opposed to prophylaxis) stems from the quantitative and qualitative effects of these agents on phagocytic cells. Randomized trials have not shown a benefit of CSFs as adjunct therapy for patients with newly diagnosed fever and neutropenia. Although the benefit of any CSF for established infections is unproven, some experts may consider them in selected cases of profound neutropenia (ANC <100/mcL) and/or with serious documented infections.
Granulocyte Transfusions
The rationale for granulocyte transfusions is to provide support for the neutropenic patient with a life-threatening infection by augmenting the number of circulating neutrophils until autologous myeloid regeneration occurs.137 In the 1970s, apheresis technology for harvesting large numbers of donor granulocytes became available. Controlled trials of granulocyte transfusions as adjuvant therapy in neutropenic patients at the time produced mixed results. In the 1980s, the enthusiasm for granulocyte transfusions waned as more effective antibiotics became available, survival from serious bacterial infections improved, and recombinant growth factors reduced the duration of neutropenia. In addition, concerns about the toxicity of granulocyte transfusions, including acute pulmonary reactions,138 HLA alloimmunization (which could render patients refractory to platelet transfusions and potentially impair myeloid engraftment following HSCT), and transfusion-associated infections (particularly CMV), outweighed the perceived benefits.
Today, the impetus to reexamine the role of granulocyte transfusions stems largely from improvements in donor mobilization methods (G-CSF). Currently, the mean absolute neutrophil yield per collection is in the range of 8 × 1010 cells, resulting in higher posttransfusion neutrophil counts that are sustained for 24 to 30 hours following transfusion. The qualitative functions of G-CSF– and steroid-mobilized neutrophils are intact based on in vitro testing. Published single-center experience suggests this procedure may have tolerable safety and can sometimes be helpful,139,140 but whether it provides overall benefit remains controversial.141
Catheter-Related Infections and Bacteremia
Bacteremia is a common complication of antineoplastic cytotoxic chemotherapy. Common sources are the intravenous catheter and the digestive tract. To maximize the detection of bacteremia, it is important to obtain adequate volume of blood.142 The recommendation of the American Society of Microbiology is between 10 ml and 30 ml of blood per culture.143 When possible, we recommend sampling all the lumens of multilumen catheters.144 The importance of peripheral blood cultures obtained by venipuncture (as opposed to blood drawn from preexisting central lines) is related to the ability to determine the source of bacteremia by using a quantitative or semiquantitative method, rather than to sensitivity.145
Guidelines from professional societies provide extensive reviews of the available evidence for the prevention and management of intravascular catheter-related infection.146–148 Organized systems (bundles) that place and manage vascular access should be a part of all cancer center programs.149 Programs have demonstrated benefit in ambulatory populations, and we believe that this justifies the extension to the inpatient setting.150
Several types of catheter-related infections have been defined: exit site infection, tunnel infection (or pocket infection in the case of ports), and catheter-related bacteremia. Bacteremia may or may not be present in the first two types.151 Exit and tunnel (or pocket) infections are clinical diagnoses based on whether pain, erythema, and tenderness extend >2 cm from the exit site (tunnel infection) or not (exit site infection). Infected short-term catheters should be removed. Tunneled and implanted devices require more thorough investigation. Although some times exit site infections can be treated with systemic antibiotics and local care, signs and symptoms of infection that may reflect associated vascular thrombosis or involvement of a tunneled catheter track >2 cm central to the exit site suggest the need for immediate removal.152 Purulence from the exit site may be present, although in neutropenic patients, local erythema and tenderness may be the only signs of infection, making it difficult to distinguish from sterile inflammation associated with mild trauma.
Catheter-related bacteremia or fungemia may occur with or without signs of localized infection. Determining whether an episode of bacteremia is associated to the catheter may be difficult, and it has important implications for management. Differential time to positivity (catheter culture positive at 2 hours earlier than peripheral blood) is the most commonly used tool.153–156 When used as a gold standard, it provides further justification for a modification of existing diagnostic standards for central line–associated blood stream infection.157 Frequently, however, there is no peripheral blood culture to determine differential time to positivity. In these situations, “host criteria” and “organism criteria” are used to estimate the likely source of bacteremia. Some gram-positive bacteria, such as coagulase-negative Staphylococcus spp. and Corynebacterium spp. are common blood culture contaminants, but they may also represent true bacteremia in the presence of intravenous catheters. The likelihood of true bloodstream infection caused by these organisms is increased when they are isolated from more than one blood culture. On the other hand, isolation of S. aureus from a single blood culture should be considered evidence of true bacteremia and treated accordingly.
Neutropenia, gastrointestinal GVHD, and severe mucositis with diarrhea secondary to the treatment of solid tumors158 are “host criteria” that affect the assessment of infection source. These factors are associated with different microbiology than that of bacteremias that originate in intravascular catheters.159,160
Mucosal-barrier injury–associated bacteremias are the proposed events that are related to specific populations and “organism criteria.” Organisms include Candida spp., Enterococcus spp., Enterobacteracieae, viridans streptococci, and certain anaerobes (Bacteroides spp., Clostridium, Fusobacterium, Prevotella, Peptostreptococcus, Veillonella). This concept applies to patients with grade 3-4 gut GVHD, severe diarrhea, or neutropenia. Although these diagnostic criteria have been developed primarily as reporting tools for attribution of hospital-related events to preventable causes,161,162 they support the need for a conceptual change.157,163
The standard approach for central line–associated blood stream infection is removal of the catheter and treatment of the patient with systemic antibiotics.151 For permanent catheters in hemodynamically stable patients, catheter salvage may be considered.164 Microbiologic factors are important in deciding whether a catheter can be saved. Coagulase-negative staphylococci are successfully treated in >93% of cases, although recurrence of infection is markedly increased with catheter retention.165 Attempts to salvage catheters infected with S. aureus usually fail and frequently result in complications.166 Similarly, catheters infected with Candida or mycobacteria should be removed.151,166 Antibiotic lock techniques are sometimes effective, almost always with concomitant systemic therapy, but failure may occur because the catheter to be salvaged needs to be used, thus limiting the dwell time of antibiotic lock solutions.167–169 The catheter should be removed if the blood cultures remain positive 3 days after starting appropriate therapy or in case of recurrent bacteremia.
Skin Lesions and Soft Tissue Infections
Consider infectious and noninfectious causes for these lesions. Some noninfectious causes include drug reactions (including chemotherapy-induced hand-foot syndrome), Sweet syndrome, erythema multiforme, vasculitis, leukemia cutis, and (in the case of allogeneic transplant) GVHD.170 Early biopsy of skin lesions for histology and culture is recommended.171
Infections of the skin can either be localized or manifestations of systemic infection. Ecthyma gangrenosum is the most characteristic skin lesion associated with systemic P. aeruginosa infection, but can also be caused by S. aureus, enteric gram-negative bacilli infection, and molds including Aspergillus, zygomycetes, and Fusarium. Ecthyma gangrenosum begins as a raised erythematous papule or nodule that progresses to a bluish-black necrotic lesion within 12 to 24 hours. A central area of necrosis surrounded by erythema is typical. Pathologically, ecthyma gangrenosum is a necrotizing process in which masses of bacteria are often observed within the vessel wall and infiltrating white cells are absent.
A needle aspirate of the lesion showing gram-negative bacilli establishes the diagnosis of invasive infection, but a negative aspirate does not rule it out. Parenteral antibiotics with activity against P. aeruginosa, MRSA, and anaerobes (e.g., carbapenem + vancomycin ± a second agent against gram-negative bacteria) should be instituted emergently, pending culture results. The presence of a necrotizing process indicates surgical debridement is required.172 Sometimes surgical exploration is necessary to determine the extent of the infection.
Gram-positive bacteria that cause skin and soft tissue infections include Streptococcus (group A and B) and S. aureus. Besides P. aeruginosa, other gram-negative bacilli with propensity to cause dermatologic infections include Stenotrophomonas maltophilia (mucocutaneous ulcerations, primary cellulitis, metastatic nodular cellulitis, and ecthyma gangrenosum), Aeromonas hydrophila, and Vibrio vulnificus (septicemia with secondary cellulitis with hemorrhagic bullae after ingestion of contaminated seafood, most common in patients with underlying liver disease, or primary cellulitis with bacteremia when an open wound is exposed to seawater). Clostridium spp. are gram-positive anaerobes that may cause deep soft tissue infection involving the fascia and muscle. In neutropenic patients, the typical presentation is disseminated soft tissue infection with Clostridium septicum bacteremia. Typically, a small dusky or purplish lesion on the leg or abdominal wall rapidly expands, and as infection progresses, the lesions may become necrotic, bullous, and hemorrhagic. Systemic toxicity including fever, malaise, and mental status changes occur early. Because the infection occurs in the deep soft tissue, tenderness and evidence of vascular compromise typically precede the development of cellulitis. A rapidly progressive deep soft tissue infection with gas formation suggests clostridial myonecrosis or polymicrobial necrotizing fascitis. Needle aspiration characteristically shows the organism in the setting of a mild or absent inflammatory response. Extensive surgical debridement may be life-saving if initiated early, but the mortality rate is high.173 Metronidazole plus an antipseudomonal cephalosporin (such as ceftazidime or cefepime) or single-agent therapy with imipenem, meropenem, or piperacillin/tazobactam are reasonable regimens.
The characteristic skin lesions of disseminated candidiasis are raised erythematous discrete papules, measuring about 0.5 to 1 cm in diameter. In their earliest form, the lesions resemble those of heat rash. They are usually not tender. Concurrent myalgias raise the possibility of Candida myositis. The yeast is cultured from skin lesions in only about half the cases. Blood cultures are typically positive. Similar lesions may appear with disseminated trichosporonosis.174
Cutaneous infection by molds may be primary or result from systemic infection. The hematogenous lesions of Aspergillus and Fusarium usually begin as discrete subcutaneous nodules that may be tender, whereas traumatic inoculation appears as ulcerations. If a primary cutaneous lesion is isolated and surgically resectable, it has an excellent prognosis. In the neutropenic patient, the likelihood of systemic infection is high, and therefore systemic antifungal therapy is warranted. Clinically, these lesions resemble ecthyma gangrenosum. Histologically, hyphae are present with angioinvasion and infarction. Primary cutaneous fusariosis has a varied appearance, including cellulitis, paronychia, onychomycosis resembling dermatophyte infection, as well as papular and nodular lesions.175
HSV and VZV can involve the skin. Oral or genital ulcers should be sampled to rule our HSV by immunofluorescence or PCR. Multiple well-circumscribed disseminated cutaneous ulcers can be manifecstations of disseminated HSV. Disseminated HSV disease should be treated with intravenous acyclovir (10 mg/kg every 8 hours). Acyclovir-resistant HSV is occasionally observed in HSCT recipients. When acyclovir resistance is suspected, the decision to switch therapy (generally to foscarnet) should be made on clinical grounds as antiviral susceptibility testing of the isolate at a reference laboratory takes weeks. VZV presents with maculopapular or vesicular lesions, often but not always in a dermatomal distribution. Secondary bacterial infections, usually due to streptococci or S. aureus, may occur. Visceral involvement of VZV can manifest as hemorrhagic pneumonia, encephalitis, retinal necrosis, hepatitis, and small bowel disease. Both viruses can be detected in the blood by PCR in case of disseminated infection; this may be particularly helpful if VZV presents with pain only, with no apparent rash. Intravenous acyclovir is the established treatment for primary varicella or disseminated zoster in immunocompromised patients.
Respiratory Tract Infections
Community-Acquired Respiratory Viruses
Community respiratory viruses include influenza, parainfluenza, respiratory syncytial virus, human metapneumovirus, adenoviruses, rhinoviruses, and coronaviruses. Most respiratory viruses typically cause self-limited infection in healthy persons, but are important causes of morbidity and mortality in immunocompromised patients with hematologic malignancies and in HSCT recipients.176 Immunocompromised persons with symptoms of respiratory infection should be evaluated for respiratory viruses, among other etiologies, and contact and respiratory precautions should be established. There are no significant differences in clinical presentation that allow predicting the causative virus in any individual case. Rapid immunodiagnostic tests (i.e., enzyme-linked immunosorbent assay) are insensitive: they are useful only to “rule in” these infections but a negative result cannot be used to rule out the disease. PCR is the most sensitive test.
Influenza.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








