Transplantation increasingly is being used as treatment for children with end-stage organ diseases, hematopoietic rescue from therapy used to treat malignancies, and as cure for primary immune deficiencies. This article reviews some of the major concepts regarding infections that complicate pediatric transplantation, highlighting differences in epidemiology, evaluation, treatment and prevention for children compared with adult recipients.
Transplantation increasingly is being used as treatment for children with end-stage organ diseases, hematopoietic rescue from therapy used to treat malignancies, and as a cure of primary immune deficiencies. The numbers of transplant procedures performed on children is substantially less than those performed on adults, with recipients under the age of 18 years accounting for only 7.7% of all solid organ transplants performed in the United States. Because the numbers are limited, data on specific infections more often are based on retrospective reviews from single institutions and less rigorously defined than data for adults. In addition, data on infections from adult studies often are extrapolated to assist with the management of pediatric patients. Although this approach is reasonable in many cases, it may be less reliable for situations where the underlying disease influences the risk for infection and where age of the recipient has substantial impact upon the risk for infectious complications. This article reviews some of the major concepts of infections that complicate pediatric transplantation, highlighting differences of epidemiology, evaluation, treatment and prevention for children compared with adult recipients.
Solid organ transplantation
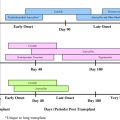
Over the last 20 years, increasing numbers of children have undergone transplantation of kidney, liver, heart, lungs, pancreas, and intestines with survival continuing to improve over time. For example, children who undergo heart transplantation and survive the first year have a median survival well over 15 years. Although both patient and graft survival varies by type of organ and by age of the recipient, observed survival for pediatric solid organ transplantation (SOT) recipients is often as good, if not better than that of adults. For example, in the United States, pediatric kidney transplant recipients have a survival over 97% compared with adult 3-year survival of 91%. Infections, however, remain a major cause of morbidity and mortality in these patients. To understand the types of infections that might occur after transplantation, it is useful to consider several sets of key principles related to infectious complications. The first principle is that the type of infection present in an SOT recipient is predicted by the time period after transplantation in which the patient presents. In general, the pattern and timing of infections are similar in both adults and children. Although the actual breakpoints of a time line are indistinct, one can consider the general timing of presentation after SOT to help predict the types of infections that might be occurring in a given child based on stereotypical patterns:
The early period (0 to 4 weeks) is one in which postoperative bacterial surgical infections predominate.
The middle period (generally 1 to 6 months) is the time wherein opportunistic infections and reactivation of latent infections in the recipient or from the donor are prominent.
The late period (usually after 6 months) is a period when community-acquired viruses and infections associated with chronic graft dysfunction predominate.
This concept, put forward in the early era of SOT, is generally true today. Not only does it inform the evaluation of fevers in a patient after SOT, but it likewise has led to the tailoring of preventive strategies for specific time periods and patients.
The second major principle informing the understanding of infectious complications of SOT is that there is a defined set of key risk factors that predispose to infection in these children. These risk factors can be categorized as those present before transplantation, those relating to the transplant procedure itself, and postoperative risk factors that are influenced most heavily by the immunosuppression required to prevent rejection. A careful examination of these risk factors identifies the major differences in types and outcome of infection between pediatric and adult recipients.
Pretransplant Risk Factors
The age of the child at the time of undergoing SOT is an important factor that influences the types of infections they may experience after transplantation. Age impacts upon the chance of having had prior exposure to infectious agents. The presence of prior infection can have both negative and positive influences on the transplant recipient. For example, older candidates are more likely to have encountered pathogens that establish lifelong latent infection such as herpes simplex virus (HSV), cytomegalovirus (CMV), tuberculosis, as well as certain endemic fungi. This can be negative, as these microbes can reactivate under the pressure of immune suppression with significant clinical consequences. Accordingly, strategies have been developed to screen for or prevent reactivation of these potential pathogens following transplantation. On the positive side, disease associated with reactivation of latent pathogens (or even reinfection with a new strain of a given latent pathogen) tends to be less aggressive than that associated with primary infection occurring after SOT, as the person has some baseline immunity. In some cases (eg, Epstein-Barr virus [EBV]), this pre-existing immunity provides a high degree of protection against clinical disease after transplantation.
The age of a child at the time of SOT impacts on the likelihood that he or she will have acquired immunity against potential pathogens from natural infection. Certain pathogens appear to specifically cause infections in younger SOT recipients. For instance, children who receive a heart transplant before 2 years of age have been noted to be at increased risk for recurrent Streptococcus pneumoniae disease including bacteremia, even when they are older and have normal splenic function. It is hypothesized that this may occur because of lack of normal antibody function. Children receiving a transplant before 1 year of age often will not have had exposures to common respiratory pathogens until after transplantation. Respiratory syncytial virus (RSV), influenza, and parainfluenza have been shown to be more severe in the very young transplant recipient who is less than a year of age compared with older individuals.
Age also impacts on the likelihood that children undergoing SOT will have had the opportunity to receive their full compliment of immunizations. Accordingly, young children who receive a transplant before receiving their routine primary vaccinations will be at higher risk for vaccine-preventable infections. In addition, the authors have found that even with increasing age children who have underlying diseases that require transplantation often miss the opportunity to receive their age-appropriate vaccinations because of time spent in the hospital or primary attention being diverted from routine childhood care (Michaels MG, unpublished observation, 2009).
A second pretransplant factor influencing the risk of infectious complications following SOT is the underlying cause of organ dysfunction. The causes of organ failure in children are typically different than adults. Accordingly, this leads to differences in the risk of infection between pediatric and adult SOT recipients. For example, hepatitis C virus (HCV) is a leading cause of liver disease requiring transplantation in adults, but is rare in the pediatric population. For this reason, recurrent HCV infection following liver transplantation is not typically an issue in pediatric recipients. On the other hand, children are more likely to have congenital anomalies (eg, Biliary atresia) as a cause of end-stage liver disease. These malformations may predispose to recurrent episodes of infection (eg, cholangitis) that might predispose to infection with multidrug-resistant bacteria following the organ transplant. Congenital heart defects that require neonatal transplantation do so at a time when the child is particularly vulnerable to bacterial infections. For example, neonates requiring heart transplantation are at increased risk for mediastinitis caused by gram-negative bacteria compared with older children and adults. Small bowel transplantation in childhood is more often associated with neonatal catastrophic events such as necrotizing enterocolitis from prematurity or complicated gastroschisis. These children have been hospitalized for significant periods of their life and have numerous prior intravascular line associated infections and exposures to antibiotics. Accordingly, they often are colonized with bacteria that have resistance to multiple antibiotics.
Intraoperative Factors
In contrast to adult SOT recipients, pediatric patients more often receive organs from adult donors, with a resultant discrepancy in their size because of the relative paucity of pediatric donors. This size discrepancy can lead to an increased risk for anastomotic complications with the potential consequence of leakage, thrombosis, or necrosis, or the potential to leave the body cavity (abdomen or chest) open for a prolonged period of time. In these cases, the child is at an increased risk of bacteria and yeast infections postoperatively.
Children are also at increased risk for donor-associated transmission of pathogens, as they more frequently lack immunity to this set of organisms. In particular, children are much more likely to be EBV seronegative before transplant, placing them at an increased likelihood of being in a mismatched state and at a marked increased risk of developing post-transplant lymphoproliferative disorders (PTLDs) compared with adult recipients. This in large part explains the much higher frequency of EBV/PTLDs observed in children compared with adults. This is also true for CMV; accordingly primary CMV infections also are seen much more frequently in pediatric recipients compared with adult recipients.
Post-transplant Risk Factors
As with adult recipients, immunosuppression is the major post-transplant risk factor for infection in children undergoing SOT. Unlike adults, this immunosuppressive therapy can have a substantial impact on growth and development, including the developing immune system. Children requiring higher levels of immune suppression because of rejection are at increased risk for developing more severe infection. This is true not only for common transplant associated pathogens (eg, CMV, EBV), but also for community-acquired viral pathogens such as RSV, parainfluenza, and influenza. Because children are more likely to be immunologically naïve to community pathogens, disease attributable to these agents tends to be more severe in children than adults.
The general requirement for immunosuppression also results in decreased immunogenicity of vaccinations that are provided following SOT. Although there are inadequate studies addressing the use of vaccines in pediatric SOT recipients, it is very likely that those children requiring higher levels of immune suppression are less likely to experience the full benefit of these vaccinations. Accordingly, they will be at increased risk of developing vaccine-associated diseases compared with both adults and children requiring lower levels of immunosuppression after transplant. Further, live virus vaccines remain generally contraindicated after transplant. Although a growing number of transplant centers will administer varicella vaccine and measles-mumps-rubella vaccine after SOT, the numbers of children studied are small, and potential risks of vaccine-associated disease are not inconsequential.
Finally, young children, whether immunosuppressed or not, are more likely than adults to share potentially infectious secretions with one another. Children who undergo SOT therefore are more likely to have infectious exposures from their siblings, playmates, and classmates. Their imperfect hygienic practices create an increased risk for exposure to community-acquired pathogens compared with adults.
Prevention
Recommendations for the prevention of infection following SOT are generally similar for both pediatric and adult organ transplant recipients. Along with routine serologic screening for human immunodeficiency virus (HIV), hepatitis B virus (HBV), HCV, and syphilis, pediatric transplant centers should evaluate immunity to EBV, CMV, and HSV. In addition, organ candidates who are old enough to have been immunized or have had wild-type infection should have antibody measured against measles and varicella. Results of serologic assays should be interpreted, taking into account the potential of passive antibody presence from the mother (children under 12 to 15 months of age) or from blood products.
One important opportunity to improve post-transplant outcome can be accomplished during the pretransplant evaluation of potential pediatric candidates of SOT. A history of vaccination should be evaluated as early as possible in these candidates, and accelerated schedules should be encouraged to give them as many protective vaccines as possible before transplantation. Primary vaccine series can be started as early as 6 weeks of age and subsequent primary immunizations given every 4 weeks. Varicella vaccination and measles-mumps-rubella vaccination should be given at least a month before transplantation.
Following SOT, children with herpes simplex seropositivity should be given acyclovir prophylaxis until the maximal period of immunosuppression has past (generally several months). The decision to use prophylaxis against CMV versus virologic monitoring of patients at risk to inform pre-emptive antiviral therapy is similar to decisions used for adult recipients. In contrast to adults, however, children are more likely to be at risk of developing primary CMV infection. Accordingly, many pediatric transplant centers will use chemoprophylaxis against CMV, as many experts believe this is the preferred strategy for high-risk mismatched patients (seronegative recipients of seropositive donors). Finally, because most children are EBV seronegative, many centers have employed strategies of EBV viral load monitoring to inform preemptive reductions in immune suppression in an effort to prevent complications associated with this pathogen.
Hematopoietic stem cell transplantation
Replacement of the bone marrow in children can be as a rescue measure after intense chemotherapy or radiation required to eradicate certain malignancies, or for replacement of a deficient bone marrow as seen with primary immune deficiencies, primary bone marrow failure, inborn errors of metabolism, or an assortment of genetic disorders. Overall, there are fewer differences in the infections seen between pediatric and adult hematopoietic stem cell transplantation (HSCT) than that for SOT, since both adults and children are rendered immunologically naïve in preparation for the transplant. The source of cells can be autologous (from the individual’s own cells) or allogeneic (from another person), and it can be from bone marrow (related or unrelated), peripheral blood that has been stimulated to be enriched for stem cells, or from cord blood. The risk for infection varies with both the underlying reason for HSCT and with the type of donor used, as well as how the cells have been prepared. For example, people undergoing autologous HSCT will have risks for infection before engraftment but fewer risks afterwards. Patients receiving HSCT with a T-cell depleted stem cell product may have less risk of graft-versus-host disease (GVHD) but some increased risk of infection. Cord blood transplants may take longer to engraft, with concomitant increased risks for infection during the pre-engraftment period. In addition to these risk factors, both GVHD and the medications to prevent or treat GVHD put the child at risk for infections. Finally, infections following HSCT can be classified according to specific time periods after transplantation similar to SOT recipients. These time periods include the pretransplantation period, pre-engraftment period (0 to 1 month), postengraftment period (1 to 3 months), and the late post-transplantation period (>3 months). Children and adults have specific defects in host defenses that vary during these periods and predispose to infection. The presence of indwelling catheters or mucositis that may occur secondary to radiation or chemotherapy interrupts the normal anatomic barriers creating defects in this important host defense that may be present anytime following transplantation but tend to predominate in the early periods.
Pretransplantation Period
Children come to HSCT with various underlying diseases, differing exposures to chemotherapy, and variable histories of prior infections and amount of immunosuppression. Infections that are present in these children before transplantation, whether because of neutropenia or bone marrow dysfunction from immune or genetic disorders, or the presence of invasive catheters, should be recognized and addressed prior to transplantation. During the pretransplant period, children undergoing autologous HSCT are at similar risk for infection as those undergoing allogeneic transplantation, as the major risks during this period are neutropenia and breaks in the normal barriers of the skin and oropharyngeal and gastrointestinal (GI) mucous membranes. Infection with circulating community viral infections (RSV, influenza, adenovirus) can have an important negative impact. Accordingly, having infection control policies to prevent the spread of viruses to these children is imperative.
Pre-engraftment Period
During this period, neutropenia and breeches in the normal anatomic barriers of the body comprise the greatest risk factors for infection. Bacterial infections predominate, with bacteremia being the most commonly documented. Both gram-positive and gram-negative organisms occur. Gram-negative bacilli increase in frequency when the mucosal lining of the GI tract is interrupted. Oral mucositis predisposes to the presence of S viridans, which can be associated with antibiotic resistance. Likewise, the presence of extended-spectrum β-lactamase production in the gram-negative bacilli is being noted increasingly. Fungal infections occurring during this phase most often are caused by Candida species, but Aspergillus subspecies are increased in frequency with prolonged neutropenia. More recently, mucormycosis also has been identified in those who have had prolonged neutropenia before HSCT.
Viral infections also occur during the pre-engraftment period. Although reactivation of HSV is observed commonly after HSCT in adults, it is less frequent in children, who are less frequently seropositive before transplantation. As noted in the section concerning prevention, children who have had prior infection with HSV should receive prophylaxis to prevent reactivation. Nosocomial or community exposures to circulating viral pathogens represent an important potential source of infection for these children. There is growing evidence that community-acquired viruses cause increased morbidity and mortality for HSCT recipients during this time period. Adenovirus is a particularly important viral pathogen that may present earlier in children, although it typically presents after engraftment.
Postengraftment Period
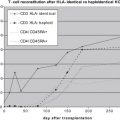
The predominant defect in host defenses in the early phase after engraftment is altered cell-mediated immunity. Infectious risks are potentiated by the presence of GVHD. This risk is especially accentuated 50 to 100 days after HSCT, when host immunity is lost and donor immunity is not yet established. Opportunistic pathogens predominate during this time period. Without the use of appropriate prophylaxis Pneumocystis jiroveci pneumonia presents in this phase early after engraftment. Aspergillus is a prominent cause of fungal infection during this period; in addition, other opportunistic mycoses also are being recognized increasingly. Hepatosplenic candidiasis frequently presents during the postengraftment period, although seeding likely occurred during the neutropenic phase. Reactivation of Toxoplasma gondii , a rare cause of disease among pediatric HSCT recipients, also may present after engraftment.
CMV is one of the most important causes of morbidity and mortality among HSCT recipients, and it typically presents during this early postengraftment phase; it is covered in detail in an article on CMV in this journal. Although primary infection from the donor can cause disease, the most prominent problems from CMV after HSCT are caused by reactivation in an HSCT recipient whose donor was naïve to the virus. For this reason, the older HSCT patient is at greater risk by virtue of more likely having acquired CMV before transplantation. Disease risk from CMV after HSCT also is increased in recipients of donors who are unrelated, or T-cell depleted and children whose course is complicated by GVHD. Similar to adults, children with CMV disease present with fevers, with or without associated symptoms including hepatitis, esophagitis, or gastroenteritis, and life-threatening interstitial pneumonitis. Asymptomatic shedding or viremia also can occur. Prophylaxis and monitoring with institution of pre-emptive treatment have helped to decrease the risk of serious fatal disease.
Adenovirus is the second most important viral infection during this period in children undergoing HSCT, causing disease in approximately 30% of stem cell recipients. Similar to CMV, children receiving grafts from HLA-matched donors or cord blood cell transplants have an increased risk for disease, along with those who had total body irradiation. Polyomaviruses, such as BK virus, are recognized as a cause of hemorrhagic cystitis and renal dysfunction following HSCT. Nosocomial acquisition of circulating community-acquired pathogens likewise can occur during this time period.
Late Post-transplantation Period
Late after HSCT, in the absence of GVHD, infections are less of a problem. When present, however, chronic GVHD significantly affects the humoral and cell-mediated immune function and causes a breakdown of some anatomic barriers such as is seen with chronic GVHD of the GI tract or lungs. Encapsulated bacteria such as S pneumoniae and Haemophilus influenzae have been noted to cause disease during this period. Viral infections, in particular reactivation of varicella–zoster virus (VZV), also accounts for infections during this time period. Fungal infections are less frequent during the late post-transplantation time period.
Prevention
Similar to preventive strategies for children who are going to undergo SOT, children being evaluated for HSCT should have a thorough history reviewed of their past infections and their risk for infections. Also similar to SOT, many of the decisions on prophylaxis have been derived from studies in adult recipients. Serology should be obtained to determine the prior presence of latent or persistent viruses such as members of the herpesvirus family (HSV, VZV, CMV, EBV), HIV, HBV, HCV, hepatitis A virus (HAV), and syphilis. Many centers also will screen for antibody against T gondii, because it can reactivate in seropositive individuals. Similar to adults, the use of prophylaxis is advised to prevent reactivation of specific viruses such as HSV. For those with past disease, acyclovir (either intravenously or orally depending on the patient’s clinical status) should be used during the highest periods of immunosuppression. A study in adults suggests a year of prophylaxis is beneficial without adverse side effects. This therapy is also useful for prophylaxis against reactivation of varicella. Although prophylaxis against CMV is efficacious, ganciclovir (the best studied medication) is toxic to the bone marrow. Accordingly, many centers opt to use a stringent monitoring protocol and institute pre-emptive treatment when the viral load is positive. Children who are negative for CMV before HSCT also should receive leukocyte-reduced blood products in an effort to avoid exposure to and infection with this pathogen.
Fungal prophylaxis and bacterial prophylaxis usually are instituted during the period of neutropenia with variation on the specific type of drug based on the infectious disease history of the individual child and the type of HSCT he or she is receiving (eg, autologous vs allogeneic). Intravenous immunoglobulin use is somewhat controversial. However, it is used at many centers through the early engraftment phase or when there is significant chronic GVHD. Trimethoprim sulfamethoxazole is the mostly widely employed prophylaxis against P jiroveci pneumonia (PCP). Because of myelosuppression associated with its use, it usually is given before HSCT and then held until engraftment, at which time it is reinstituted.
Vaccination is particularly important among children undergoing HSCT as they may not have even had their full complement of primary vaccines before beginning chemotherapy. The timing to restart vaccination after HCST is based on the risk of disease, the ability for the immune system to respond to the antigen, and the safety of the vaccine. These issues are impacted upon by the type of transplant and donor’s immunity, the presence of ongoing GVHD, and the use of passive immunoglobulin. In general, the recipient will acquire the immunity that is found in the donor. Antibody titers to vaccine antigens, however, tend not be long-lasting, and repeat vaccination is warranted. Live virus vaccines are the ones that have the potential to cause serious vaccine-associated disease when given to an immunosuppressed host. Recommendations do exist to repeat or reissue non-live vaccines at 12, 14, and 24 months after HSCT. Studies in children who have undergone HSCT show that vaccinations against measles, mumps, and rubella as a single vaccine have been given safely to children 2 years after HSCT if they do not have chronic GVHD or receipt of ongoing immunosuppressive medication. While these recommendations are set forward, some experts believe that rather than having one guideline for all HSCT recipients, recommendations should be individualized based on the ability of a particular recipient to mount a response.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree