Recent shifts in the epidemiology of invasive fungal infections (IFIs) among transplant and oncology populations have led to new recommendations on treatment; however, they have also brought new controversies. New pharmacologic therapies are being studied and guidelines for management of several IFIs have been changed accordingly. More information is being discovered about unique genetic factors that put some transplant recipients at greater risk than others for fungal infection. The role of immunomodulation continues to be investigated, and the delicate balance of maintaining some immune integrity while assuring protection of the graft remains critical. For transplant and oncology patients, the diagnosis and management of IFIs remain challenging, and improving outcomes depends on continued progress in all of these arenas. This article highlights recent advances and important factors to consider when treating transplant and oncology patients with IFIs.
Invasive fungal infections (IFIs) in oncology and transplant populations have been associated with significant morbidity and mortality. Research in this area remains in flux; as epidemiologic patterns shift, more is being learned about optimal treatment and the unique risks that predispose these special populations to such potentially devastating infections. This article highlights recent advances and important factors to consider when treating transplant and oncology patients with IFIs.
Epidemiology of IFIs
Despite high associated morbidity and mortality, the epidemiology of IFIs in high-risk populations has not previously been well defined. Incidence estimates have been primarily based on single-center, retrospective studies. The Transplant Associated Infections Surveillance Program (TRANSNET), a network of 23 transplant centers in the United States, prospectively studied the epidemiology of IFIs among solid-organ and stem cell transplant populations over a 5-year period (March 2001 to March 2006) and provided the first true approximation of the burden of fungal disease among transplant populations in the United States. Based on TRANSNET data, the overall incidence of IFIs in the hematopoietic stem cell transplant (HSCT) population was 3.4%, somewhat lower than previous estimates (DP Kontoyiannis, unpublished data, July 2009). In addition, invasive aspergillosis (IA) surpassed invasive candidiasis (IC) as the most common IFI encountered in the HSCT population: Aspergillus accounted for 43% of infections and Candida accounted for 28%, followed by other or unspecified molds including Fusarium and Scedosporium (16%), and zygomycetes (8%). Pneumocystosis, endemic fungal infections, and cryptococcosis were rarely encountered in the HSCT population. Consistent with previous reports, mortality was high and 1-year survival was low for HSCT patients with IFI. Fusarium infections and IA were associated with the lowest 1-year survival (6% and 25%, respectively); however, survival among patients with zygomycosis (28%) and IC (34%) was not substantially better.
Among solid-organ transplant (SOT) recipients, Candida infections were significantly more common than Aspergillus infections. This distribution held true for all solid-organ groups except lung transplant recipients. In lung transplant recipients, Aspergillus was the most common fungal pathogen, and, when coupled with other molds, invasive mold infections were responsible for 70% of IFIs (PG Pappas, unpublished data, July 2009). This distribution has also been shown in other studies of SOT recipients. Less common overall, but seen more frequently than in the HSCT population, were infections due to Cryptococcus and endemic fungi, causing 8% and 5% of IFIs, respectively. Zygomycetes were responsible for 2% of infections (PG Pappas, unpublished data, July 2009). The mortality associated with IFIs in the SOT population is high, but lower overall than in HSCT and oncology patients.
There are no recent, multicenter studies describing the incidence and clinical outcome of IFIs among the general oncology population, and it is difficult to obtain an accurate estimate of the frequency of fungal infections in this population from the published literature because most reports do not provide sufficient information regarding the patients’ underlying disease. In general, compared with patients with solid tumors, patients with hematologic malignancies are at increased risk for fungal disease and response to IFI treatment is lower. A 1992 international autopsy survey of patients with cancer identified fungal infections in 25% of patients with leukemia, 12% with lymphoma, and 5% with solid tumors. Overall, Candida was the most common fungal pathogen, responsible for 58% of fungal infections, whereas 30% of fungal infections were caused by Aspergillus . A more recent single-center survey of autopsies performed on patients with hematologic malignancy confirmed the increased risk for IFI among patients with leukemia. Consistent with trends among transplant populations, the prevalence of IFI remained high and constant throughout the study period (1989–2003); although the rate of IC decreased, the prevalence of invasive mold infections increased.
Types of IFIs
Aspergillus
Aspergillus fumigatus is the most frequent species of Aspergillus causing clinical disease, perhaps due to specific virulence factors unique to the organism. However, other species, most commonly Aspergillus flavus , Aspergillus terreus , and Aspergillus niger , are also implicated in invasive infections in humans. A terreus has been associated with amphotericin B resistance and a higher mortality than other Aspergillus species, although the data to support this claim were primarily gleaned from patients treated with amphotericin B as initial therapy and before use of triazoles as first-line treatment of IA.
In immunocompromised hosts, Aspergillus most commonly presents as invasive pulmonary aspergillosis, often with subsequent dissemination. In lung transplant recipients, Aspergillus may also cause tracheobronchitis and bronchial anastomotic infection. However, pulmonary infections can present with fever, hemoptysis, cough, dyspnea, reduction in pulmonary function, pleuritic chest pain, respiratory failure, and altered mental status, and the immunosuppressed patient may have few, or only subtle, clinical signs and symptoms present early in the course of infection. The distinction between colonization and infection with Aspergillus can be difficult. For example, Aspergillus can be recovered from the lower respiratory tract of many patients after lung transplant, but, based on a review of the literature, progression from colonization to infection in lung transplant recipients is rare. In contrast, recovery of Aspergillus from lower respiratory tract specimens in patients with hematologic malignancy or undergoing HSCT has a high positive predictive value for invasive disease.
Candida
The overall decrease in Candida infections and the shift from Candida albicans to non- albicans Candida as the most common infecting Candida species in the past 2 decades are notable. Data from Brazil collected between 1997 and 2003 document that 79% of episodes of candidemia in patients with hematological malignancies, and 52% in those with solid tumors, were caused by non- albicans Candida ( P = .034). Similarly, between 2001 and 2007 at MD Anderson Cancer Center, non- albicans Candida species were responsible for 75% of IC cases occurring in patients with hematologic malignancy or undergoing HSCT. The routine use of azole prophylaxis in high-risk cancer populations has contributed to the decreased incidence of IC in these populations, and likely accounts in part for the increasing frequency of infections caused by non- albicans Candida . Although C albicans remains the most frequently isolated Candida species among SOT recipients, a shift toward more non- albicans Candida infections also seems to be occurring in this population.
Infections due to Candida can manifest as candidemia, peritonitis, empyema, endopthalmitis, esophagitis, and urinary tract or anastomotic infections. In lung transplant recipients, Candida can also cause tracheobronchitis. Presenting clinical signs may be fever, leukocytosis, and, less commonly, hypothermia.
Hyaline Hyphomycetes
The other molds responsible for IFIs in immunosuppressed patients are a heterogeneous group of organisms. More than 30 non- Aspergillus hyalohyphomycetes have been implicated in human disease, including species of Acremonium , Fusarium , Paecilomyces , and Scedosporium . These organisms are typically opportunistic, causing invasive disease following environmental exposures. Several of the non- Aspergillus hyalohyphomycetes are unique in their capability to sporulate in vivo, which permits recovery of the organisms from the bloodstream and dissemination to other organs, particularly skin.
Recently, a shift toward more non- Aspergillus mold infections has been noticed in SOT recipients. In a prospective multicenter study, 53 invasive mold infections were reported from liver and heart transplant recipients. Pathogens included Aspergillus species in 70%, non- Aspergillus hyalohyphomycetes in 9%, phaeohyphomycetes in 9%, zygomycetes in 6%, and other or unidentified molds in 6% of patients. Dissemination was significantly more likely with infection due to a non- Aspergillus mold compared with Aspergillus .
Zygomycetes
Zygomycetes cause devastating invasive disease in a variety of different hosts. In one review of 929 reported cases of zygomycosis, 36% were seen in patients with diabetes mellitus, 7% in SOT recipients, and 5% in bone marrow transplant recipients. Among the bone marrow transplant group, slightly more than half (52%) had pulmonary zygomycosis, with 16% having infection in the sinuses. Outcome from zygomycosis varied based on the underlying condition, site of infection, and use of antifungal therapy. For patients with underlying malignancy, overall mortality was 66%. Other studies cite mortalities up to 80% among those with hematologic malignancies. The incidence of zygomycosis seems to be increasing in oncology centers and in HSCT populations specifically, possibly related to the use of voriconazole prophylaxis.
Pneumocystis jiroveci
The risk of Pneumocystis jiroveci infection (previously Pneumocystis carinii ) in HSCT and SOT recipients can be as high as 5% to 15% without prophylaxis. In the era of routine P jiroveci prophylaxis, transplant recipients who develop infection typically do so after stopping their prophylactic regimen. Similarly, patients with cancer who develop Pneumocystis infection typically do so in the absence of prophylaxis. Pneumocysti s has a worldwide distribution and the organism that infects humans has been recognized as unique and distinct from that infecting animals ; humans seem to acquire Pneumocystis only from other humans, but active pneumonia does not seem to be required for transmission to occur. Serologic data indicate that most humans are infected with Pneumocystis within the first 2 to 4 years of life. Immunocompromised patients develop disease as a consequence of reinfection with a new strain, or possibly from reactivation of latent infection. However, it is believed that most cases of P jiroveci pneumonia develop following acquisition of a new strain shortly before clinical symptoms manifest.
Particular attention was given to P jiroveci infection in SOT recipients in the 1980s given to high rates of infection in heart-lung transplant recipients. However, in the era of routine prophylaxis for at least 6 months following the transplant procedure in all solid-organ groups, Pneumocystis infections in the SOT population are rare. In one retrospective review of 32,757 kidney recipients transplanted between 2000 and 2004, the cumulative incidence was 0.4%. Patients receiving sirolimus as part of their immunosuppressive regimen had an increased risk of developing P jiroveci pneumonia that was associated with increased risk of graft loss and death. The underlying mechanism by which sirolimus predisposes to P jiroveci infection is as yet undefined; however, it may ultimately be linked with the ability of sirolimus to cause interstitial pneumonia, a known side effect of the drug.
Cryptococcus
Cryptococcus neoformans and Cryptococcus gatti represent the main pathogenic species in the genus Cryptococcus . Although cryptococcosis has been most commonly encountered in the HIV-infected population, a multicenter study reporting 306 cases of cryptococcosis in patients who are not infected with HIV found 0.7% of total cases occurred in HSCT recipients, 18% in SOT recipients, 9% in patients with hematologic malignancies, and 9% in patients with other malignancies. Other studies in the United States have found similarly low rates of cryptococcal infection in the HSCT population, most likely because of the use of routine fluconazole prophylaxis following HSCT. The overall mortality for cryptococcosis in the non-HIV population was 30%, attributable mortality 12%, and hematologic malignancy as an underlying diagnosis was associated with decreased survival.
Cryptococcus infection most commonly involves the lungs and central nervous system, but cutaneous infection and disseminated disease also occur. In one study, heart transplant patients were more likely than other solid-organ groups to develop cryptococcosis, but kidney transplant recipients were most likely to have disseminated disease. This study also showed that serum cryptococcal antigen was not always helpful in identifying isolated pulmonary Cryptococcus infection; 82% of patients with cryptococcal pneumonia had a negative serum cryptococcal antigen.
Endemic Fungi
Endemic fungi, including Histoplasma capsulatum , Blastomyces dermatitidis , and Coccidioides immitus , are present in the soil in certain geographic regions, and inhalation of conidia leads to systemic infection. Disease may manifest after primary exposure or through reactivation of a latent focus when there is a decrease in cell-mediated immunity. Pulmonary involvement is common but clinical symptoms are nonspecific and may be subacute in onset.
Although endemic mycoses are rarely encountered in cancer and transplant populations, immunosuppression (defined as hematologic malignancy or treatment with immunosuppressive medications) has been identified as a risk for developing histoplasmosis. Among immunosuppressed patients with histoplasmosis, 74% had fatal or disseminated infections, compared with 7% of patients who were not immunosuppressed. Histoplasmosis is the most frequent endemic mycosis reported in the SOT population and it has been transmitted to SOT recipients via the transplanted allograft. Information regarding B dermatitidis in transplant populations remains limited to individual case reports and small case series. The largest series included 11 cases in SOT recipients; infection occurred a median of 26 months after SOT and rejection did not precede any case. B dermatitidis pneumonia was frequently complicated by acute respiratory distress syndrome and accordingly high mortality (67%). Even in endemic regions, C immitus infection is rarely encountered in the HSCT population, and most descriptions are in SOT recipients. As with the other endemic mycoses in the immunosuppressed population, dissemination is common, mortality is high (up to 72%), and infection can be transmitted via donated organs.
Types of IFIs
Aspergillus
Aspergillus fumigatus is the most frequent species of Aspergillus causing clinical disease, perhaps due to specific virulence factors unique to the organism. However, other species, most commonly Aspergillus flavus , Aspergillus terreus , and Aspergillus niger , are also implicated in invasive infections in humans. A terreus has been associated with amphotericin B resistance and a higher mortality than other Aspergillus species, although the data to support this claim were primarily gleaned from patients treated with amphotericin B as initial therapy and before use of triazoles as first-line treatment of IA.
In immunocompromised hosts, Aspergillus most commonly presents as invasive pulmonary aspergillosis, often with subsequent dissemination. In lung transplant recipients, Aspergillus may also cause tracheobronchitis and bronchial anastomotic infection. However, pulmonary infections can present with fever, hemoptysis, cough, dyspnea, reduction in pulmonary function, pleuritic chest pain, respiratory failure, and altered mental status, and the immunosuppressed patient may have few, or only subtle, clinical signs and symptoms present early in the course of infection. The distinction between colonization and infection with Aspergillus can be difficult. For example, Aspergillus can be recovered from the lower respiratory tract of many patients after lung transplant, but, based on a review of the literature, progression from colonization to infection in lung transplant recipients is rare. In contrast, recovery of Aspergillus from lower respiratory tract specimens in patients with hematologic malignancy or undergoing HSCT has a high positive predictive value for invasive disease.
Candida
The overall decrease in Candida infections and the shift from Candida albicans to non- albicans Candida as the most common infecting Candida species in the past 2 decades are notable. Data from Brazil collected between 1997 and 2003 document that 79% of episodes of candidemia in patients with hematological malignancies, and 52% in those with solid tumors, were caused by non- albicans Candida ( P = .034). Similarly, between 2001 and 2007 at MD Anderson Cancer Center, non- albicans Candida species were responsible for 75% of IC cases occurring in patients with hematologic malignancy or undergoing HSCT. The routine use of azole prophylaxis in high-risk cancer populations has contributed to the decreased incidence of IC in these populations, and likely accounts in part for the increasing frequency of infections caused by non- albicans Candida . Although C albicans remains the most frequently isolated Candida species among SOT recipients, a shift toward more non- albicans Candida infections also seems to be occurring in this population.
Infections due to Candida can manifest as candidemia, peritonitis, empyema, endopthalmitis, esophagitis, and urinary tract or anastomotic infections. In lung transplant recipients, Candida can also cause tracheobronchitis. Presenting clinical signs may be fever, leukocytosis, and, less commonly, hypothermia.
Hyaline Hyphomycetes
The other molds responsible for IFIs in immunosuppressed patients are a heterogeneous group of organisms. More than 30 non- Aspergillus hyalohyphomycetes have been implicated in human disease, including species of Acremonium , Fusarium , Paecilomyces , and Scedosporium . These organisms are typically opportunistic, causing invasive disease following environmental exposures. Several of the non- Aspergillus hyalohyphomycetes are unique in their capability to sporulate in vivo, which permits recovery of the organisms from the bloodstream and dissemination to other organs, particularly skin.
Recently, a shift toward more non- Aspergillus mold infections has been noticed in SOT recipients. In a prospective multicenter study, 53 invasive mold infections were reported from liver and heart transplant recipients. Pathogens included Aspergillus species in 70%, non- Aspergillus hyalohyphomycetes in 9%, phaeohyphomycetes in 9%, zygomycetes in 6%, and other or unidentified molds in 6% of patients. Dissemination was significantly more likely with infection due to a non- Aspergillus mold compared with Aspergillus .
Zygomycetes
Zygomycetes cause devastating invasive disease in a variety of different hosts. In one review of 929 reported cases of zygomycosis, 36% were seen in patients with diabetes mellitus, 7% in SOT recipients, and 5% in bone marrow transplant recipients. Among the bone marrow transplant group, slightly more than half (52%) had pulmonary zygomycosis, with 16% having infection in the sinuses. Outcome from zygomycosis varied based on the underlying condition, site of infection, and use of antifungal therapy. For patients with underlying malignancy, overall mortality was 66%. Other studies cite mortalities up to 80% among those with hematologic malignancies. The incidence of zygomycosis seems to be increasing in oncology centers and in HSCT populations specifically, possibly related to the use of voriconazole prophylaxis.
Pneumocystis jiroveci
The risk of Pneumocystis jiroveci infection (previously Pneumocystis carinii ) in HSCT and SOT recipients can be as high as 5% to 15% without prophylaxis. In the era of routine P jiroveci prophylaxis, transplant recipients who develop infection typically do so after stopping their prophylactic regimen. Similarly, patients with cancer who develop Pneumocystis infection typically do so in the absence of prophylaxis. Pneumocysti s has a worldwide distribution and the organism that infects humans has been recognized as unique and distinct from that infecting animals ; humans seem to acquire Pneumocystis only from other humans, but active pneumonia does not seem to be required for transmission to occur. Serologic data indicate that most humans are infected with Pneumocystis within the first 2 to 4 years of life. Immunocompromised patients develop disease as a consequence of reinfection with a new strain, or possibly from reactivation of latent infection. However, it is believed that most cases of P jiroveci pneumonia develop following acquisition of a new strain shortly before clinical symptoms manifest.
Particular attention was given to P jiroveci infection in SOT recipients in the 1980s given to high rates of infection in heart-lung transplant recipients. However, in the era of routine prophylaxis for at least 6 months following the transplant procedure in all solid-organ groups, Pneumocystis infections in the SOT population are rare. In one retrospective review of 32,757 kidney recipients transplanted between 2000 and 2004, the cumulative incidence was 0.4%. Patients receiving sirolimus as part of their immunosuppressive regimen had an increased risk of developing P jiroveci pneumonia that was associated with increased risk of graft loss and death. The underlying mechanism by which sirolimus predisposes to P jiroveci infection is as yet undefined; however, it may ultimately be linked with the ability of sirolimus to cause interstitial pneumonia, a known side effect of the drug.
Cryptococcus
Cryptococcus neoformans and Cryptococcus gatti represent the main pathogenic species in the genus Cryptococcus . Although cryptococcosis has been most commonly encountered in the HIV-infected population, a multicenter study reporting 306 cases of cryptococcosis in patients who are not infected with HIV found 0.7% of total cases occurred in HSCT recipients, 18% in SOT recipients, 9% in patients with hematologic malignancies, and 9% in patients with other malignancies. Other studies in the United States have found similarly low rates of cryptococcal infection in the HSCT population, most likely because of the use of routine fluconazole prophylaxis following HSCT. The overall mortality for cryptococcosis in the non-HIV population was 30%, attributable mortality 12%, and hematologic malignancy as an underlying diagnosis was associated with decreased survival.
Cryptococcus infection most commonly involves the lungs and central nervous system, but cutaneous infection and disseminated disease also occur. In one study, heart transplant patients were more likely than other solid-organ groups to develop cryptococcosis, but kidney transplant recipients were most likely to have disseminated disease. This study also showed that serum cryptococcal antigen was not always helpful in identifying isolated pulmonary Cryptococcus infection; 82% of patients with cryptococcal pneumonia had a negative serum cryptococcal antigen.
Endemic Fungi
Endemic fungi, including Histoplasma capsulatum , Blastomyces dermatitidis , and Coccidioides immitus , are present in the soil in certain geographic regions, and inhalation of conidia leads to systemic infection. Disease may manifest after primary exposure or through reactivation of a latent focus when there is a decrease in cell-mediated immunity. Pulmonary involvement is common but clinical symptoms are nonspecific and may be subacute in onset.
Although endemic mycoses are rarely encountered in cancer and transplant populations, immunosuppression (defined as hematologic malignancy or treatment with immunosuppressive medications) has been identified as a risk for developing histoplasmosis. Among immunosuppressed patients with histoplasmosis, 74% had fatal or disseminated infections, compared with 7% of patients who were not immunosuppressed. Histoplasmosis is the most frequent endemic mycosis reported in the SOT population and it has been transmitted to SOT recipients via the transplanted allograft. Information regarding B dermatitidis in transplant populations remains limited to individual case reports and small case series. The largest series included 11 cases in SOT recipients; infection occurred a median of 26 months after SOT and rejection did not precede any case. B dermatitidis pneumonia was frequently complicated by acute respiratory distress syndrome and accordingly high mortality (67%). Even in endemic regions, C immitus infection is rarely encountered in the HSCT population, and most descriptions are in SOT recipients. As with the other endemic mycoses in the immunosuppressed population, dissemination is common, mortality is high (up to 72%), and infection can be transmitted via donated organs.
Timing of IFIs
IFI Timeline: HSCT
Time to development of IFI after transplantation varies according to type of fungal infection, type of transplant, and the use/duration of antifungal prophylaxis. As shown in Fig. 1 , the timeline for IFIs following HSCT is typically broken into 3 periods, early onset (≤40 days after HSCT), late onset (41–180 days after HSCT), and very late onset (>180 days after HSCT). In the TRANSNET cohort, 66% of Candida infections among autologous HSCT recipients occurred within the first 30 days (DP Kontoyiannis MD, unpublished data, July 2009). Similarly, in a single-center study of 655 allogeneic HSCT recipients transplanted between 1994 and 1997 and receiving routine fluconazole prophylaxis, the median time to development of candidemia was day 28 after transplant. A recent multicenter report of IFIs occurring between 2004 and 2007 reported the median timing of IC after HSCT to be 77 days; IC tended to occur earlier after autologous HSCT (median 28 days) compared with allogeneic HSCT (median 108 days). In general, early-onset IC following HSCT is influenced by the presence of neutropenia and mucosal injury (mucositis), whereas later onset is more often seen in allogeneic HSCT recipients owing to the development of graft-versus-host disease (GVHD) and the need for chronic central venous catheters.
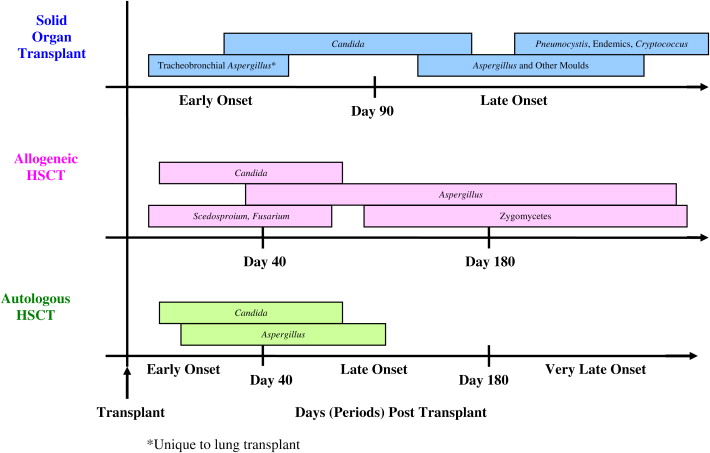
Aspergillus and other mold infections tend to occur later after HSCT. In a single-center study of allogeneic HSCT recipients transplanted between 1993 and 1998, 30% of IA diagnoses (N = 187) were early, 53% late, and 17% very late onset following the procedure. In the more recent TRANSNET cohort, 50% of IA cases among autologous HSCT recipients were early onset and 24% occurred more than 120 days after onset, whereas 22% of cases among allogeneic HSCT recipients were early onset and 47% occurred more than 120 days after transplant (DP Kontoyiannis, MD, unpublished data, July 2009). In general, IA occurs more frequently and is encountered later after allogeneic HSCT compared with autologous HSCT. Late IA has been associated with a higher mortality, possibly because of increased fungal burden accompanying a delay in diagnosis and the cumulative burden of immunosuppression in patients with chronic/refractory GVHD.
The timing of non- Aspergillus mold infections such as zygomycetes, Fusarium , and Scedosporium seems to be organism specific. One large study of more than 5500 HSCT recipients showed that the majority (56%) of zygomycete infections occurred more than 90 days after transplant, and GVHD was associated with zygomycete infection. However, Scedosporium infections were more likely to occur within the first 30 days after transplant. Similarly, nearly half (46%) of patients with fusariosis were neutropenic at the time of diagnosis, and the median time from transplant to diagnosis was 64 days.
IFI Timeline: SOT
The timeline for infections following SOT has traditionally been divided into 3 phases: the first month, months 2 to 6, and more than 6 months after the transplant procedure. Recent data regarding the epidemiology of IFIs following SOT suggest that the timing of fungal infections may no longer conform precisely with these risk windows.
Historically, infections due to Candida occurred early after SOT, typically during the transplant hospitalization. However, TRANSNET data showed a somewhat later time to onset, with median time to diagnosis of IC of 103 days (PG Pappas MD, unpublished data, July 2009). In addition, a recent Australian study of candidemia in SOT recipients found that 54% of infections developed greater than 6 months after transplant, most of them in renal transplant recipients. Nearly all these patients were hospitalized at the time of diagnosis because of complications from various bacterial infections, and had been receiving broad-spectrum antibiotics.
Most Aspergillus infections historically occurred within the first year following SOT. Tracheobronchial or anastomotic Aspergillus infections typically occurred within the first 90 days after transplant, compared with invasive pulmonary aspergillosis that tended to occur later. Most experts agree that the risk for IA is high enough immediately following lung transplant to warrant antifungal prophylaxis, and American Society of Transplantation guidelines recommend continuing prophylaxis following lung transplantation at least until bronchial anastomosis remodeling is complete. A 2006 international survey of lung transplant centers revealed that 69% (30/43) used universal antifungal prophylaxis during the immediate posttransplant period as the anastomosis was healing, most commonly an aerosolized formulation of amphotericin B alone or in combination with itraconazole. The median durations of prophylaxis with aerosolized amphotericin B and itraconazole were 30 and 90 days, respectively. In the current era of routine prophylaxis in high-risk organ transplant recipients, nearly one-half of Aspergillus infections in SOT recipients occur late (>90 days after SOT) and, as in the HSCT population, late-onset IA has been associated with a higher mortality compared with early-onset infection.
Cryptococcus and the endemic mycoses tend to occur even later in the posttransplant period. In one study of SOT recipients with cryptococcosis, the median time to diagnosis in lung, heart, and kidney transplant recipients was 210, 450, and 630 days, respectively. In the TRANSNET cohort, median time to diagnosis of cryptococcosis was 575 days. Similarly, the median time to diagnosis of the endemic mycoses was 343 days (PG Pappas MD, unpublished data, July 2009), and P jiroveci infections are most often seen after routine prophylaxis is stopped, typically more than 180 days after transplant.
Risk factors developing IFIs
Unique Risks for IFIs in HSCT Recipients
Many factors affect a patient’s individual risk for fungal disease, including those associated with the host, the transplanted graft, and complications of the procedure. The influence of each factor fluctuates throughout the posttransplant course, creating a dynamic timeline. Host (eg, older age) and transplant variables (eg, human leukocyte antigen mismatch) tend to influence IFI risk early, whereas complications of the transplant procedure (eg, GVHD and cytomegalovirus [CMV] disease) tend to predominate later. Certain biologic factors, such as malnutrition, iron overload, diabetes mellitus, and cytopenias, are influential throughout the posttransplant course. Risk factors specific to early-onset IA have been identified as aplastic anemia, myelodysplastic syndrome, cord-blood transplantation, delayed neutrophil engraftment, and CMV disease. Risks for late-onset IA were multiple myeloma, neutropenia, GVHD, and CMV disease. Iron overload has been shown to be a risk factor for severe bacterial infections in autologous HSCT recipients, and also associated with IA and zygomycete infections. Diabetes mellitus, voriconazole prophylaxis, and malnutrition have also been identified as risks for zygomycosis.
Only a subset of patients who are at risk will actually develop IFI. This fact has led to a growing interest in host genetic differences that may contribute to the individual’s risk of developing IFI. Recently, studies in HSCT populations have shown that polymorphisms in Toll-like receptor 4 and genetic variations within the plasminogen allele may influence susceptibility to IA after transplant. More research is needed into host genetic influence on the risk of fungal disease following transplant.
Unique Risks for IFIs in Oncology Patients
In patients with acute leukemia, the risk for IC in published reports varies considerably. This is related to the status of leukemia (newly diagnosed, postremission, relapsed, or refractory to treatment), duration of neutropenia, and the types of antineoplastic agents used. Based on a study of patients from Brazil with cancer and with candidemia between 1997 and 2003, in comparison with patients with solid tumors, neutropenia and corticosteroid use were more frequent in the hematologic malignancy group. Only 22% of patients with solid tumors were neutropenic before candidemia. The presence of ileus and the use of anaerobicides were independent risk factors for candidemia in patients with solid cancers. Compared with candidemic patients without cancer, central venous catheters and gastrointestinal surgery were independently associated with candidemia in patients with solid tumor.
Unique Risks for IFIs in SOT Recipients
Rejection and exogenous immunosuppressive agents, particularly high-dose steroids and antilymphocyte antibody treatment, lead to increased risk for IFIs in the SOT population. However, within organ transplant groups, the risk for IFI is strongly influenced by medical and surgical factors including technical complexity. For example, prolonged operative time requiring multiple blood transfusions, reperfusion organ injury during transplantation, or multiple simultaneous organ transplants have all been associated with the development of fungal infections. One study associated prolonged ischemia time with the development of IA in lung transplant recipients. Liver transplant recipients have been shown to be at higher risk for IFIs if there is fulminant hepatic failure, a need to undergo retransplantation, or renal failure. Unique risks for renal transplant recipients include diabetes mellitus or need for prolonged hemodiaylsis before transplant. Factors predisposing to IFI, primarily IC, in pancreas transplant recipients include older donor age, enteric (vs bladder) drainage, pancreas after kidney transplant (vs pancreas alone), the development of posttransplant pancreatitis, retransplantation, and preoperative peritoneal dialysis.
Infection with certain viruses following SOT has also been associated with the development of IFIs. The most frequently implicated virus is CMV. In a prospective study of liver transplant recipients, 36% of patients with CMV disease developed IFIs within the first year after transplant, compared with 8% of those without CMV disease. CMV prophylaxis seems to result in fewer IFIs, which further supports the association.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




