This article examines the clinical manifestations of and risk factors for cytomegalovirus (CMV). Prevention of CMV infection and disease are also explored. Antiviral resistance and management of CMV are examined.
Human cytomegalovirus (CMV) is a betaherpesvirus in the same family as human herpesvirus-6 and -7. CMV is a large virus including approximately 200 proteins. CMV has been found in a wide range of cells, including endothelial cells, epithelial cells, blood cells including neutrophils, and smooth muscle cells. The presence of CMV in these cells may be caused by active replication within the cell, phagocytosis of CMV proteins, or abortive (incomplete) replication, and likely contributes to dissemination and transmission. As the other herpesviruses, CMV remains in the human body after primary infection for life. Little is known about the site or mechanisms of CMV latency and persistence. Several studies indicate that cells of the granulocyte-monocyte lineage carry CMV and these might be one site for latency and persistence. Transplantation of solid organs clearly can transmit CMV, so it is possible that cells other than those mentioned can harbor and transmit the virus. Whether the infected cell type in these organs is blood cells, macrophages, or other cell types, however, has not been clarified.
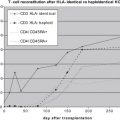
T-cell mediated cellular immunity is the most important factor in controlling CMV replication. CMV induces a strong CD8+ cytotoxic T-lymphocyte (CTL) response, and the proportion of circulating CD8+ T cells in healthy individuals that are specific for CMV antigens ranges from 10% to 40% increasing with age. Several CMV proteins are targeted by the CD8+ T-cell response including IE-1, IE-2, and pp65. Lack of CMV-specific CD8+ CTL responses predisposes to CMV infection, whereas reconstitution of CMV-specific CD8+ CTL responses after hematopoietic cell transplantation correlates with protection from CMV and improved outcome of CMV disease. After hematopoietic stem cell transplantation (HSCT), CMV-specific CD4+ responses are associated with protection from CMV disease. The lack of CMV-specific CD4+ cells is associated with late CMV disease and death in patients who have undergone HSCT. The role of humoral immunity in controlling CMV replication is not clear. Although antibodies to gB and gH can neutralize the virus in cell culture, they do not seem to prevent primary infection in adults, but rather may function to limit disease severity.
The innate immune system also seems to be involved in controlling CMV replication. CMV triggers cellular inflammatory cytokine production on binding to the target cell, mediated in part by the interaction of gB and gH with toll-like receptor 2. Polymorphisms in toll-like receptor 2 have been associated with CMV infection after liver transplantation. In humans, natural killer cell responses increase during CMV infection after renal transplantation, and a deficiency in natural killer cells is associated with severe CMV infection (among other herpesviruses). The genotype of the donor-activating killer immunoglobulin-like receptor, which regulates NK cell function, has recently been demonstrated to influence the development of CMV infection after allogeneic HSCT. Finally, polymorphisms in chemokine receptor 5 and interleukin-10 have been associated with CMV disease, whereas polymorphisms in monocyte chemoattractant protein 1 are associated with reactivation after allogeneic HSCT.
Diagnostic methods
The serologic determination of CMV-specific antibodies (IgG and IgM) is important for determining a patient’s risk for CMV infection after transplantation but cannot be used for the diagnosis of CMV infection or disease. Growth of CMV in tissue culture takes several weeks, making this technique obsolete for diagnosis of CMV in HSCT recipients. The shell vial (rapid culture/DEAFF) technique, in which monoclonal antibodies are used to detect CMV immediate-early proteins in cultured cells, is not sensitive enough to use for routine blood monitoring, but is highly useful on bronchoalveolar lavage (BAL) fluid in the diagnosis of CMV pneumonia.
The detection of the CMV pp65 in peripheral blood leukocytes (antigenemia) is a rapid and semiquantitative method of diagnosing CMV infection. A positive CMV pp65 assay is predictive for the development of invasive disease in transplant patients.
Polymerase chain reaction (PCR) is the most sensitive method for detecting CMV. Quantitative PCR (qPCR) relies on the amplification and quantitative measurement of CMV DNA, while at the same time maintaining high specificity. High levels of DNA in blood (whole blood or plasma) is a good predictor of CMV disease in HSCT recipients. Although PCR has been used on BAL fluid, viral-load cut-offs have not been defined, and although the sensitivity and negative predictive values are very high, the specificity and positive predictive values are not known.
The detection of CMV mRNA by nucleic acid sequence-based amplification on blood samples is similarly useful as DNA qPCR or pp65 antigenemia for guiding preemptive therapy after HSCT. This method is less frequently used, however, compared with the other techniques.
The presence of characteristic CMV “owl’s eye” nuclear inclusions in histopathology specimens is useful in the diagnosis of invasive CMV disease. This method has relatively low sensitivity, but can be enhanced by use of immunohistochemical techniques.
Clinical manifestations
CMV infection is defined as the detection of CMV, typically by DNA PCR, pp65 antigenemia, or mRNA nucleic acid sequence-based amplification, from plasma or whole blood in a CMV-seronegative patient (primary infection) or a CMV-seropositive patient (reactivation of latent or persistent virus or superinfection with another strain of CMV). International definitions of CMV disease, requiring the presence of symptoms and signs compatible with CMV end-organ involvement together with the detection of CMV using a validated method in the appropriate clinical specimen, have been published. Almost any organ can be involved in CMV disease. Fever is a common manifestation, but may be absent in patients receiving high-dose immunosuppression.
CMV pneumonia is the most serious manifestation of CMV in HSCT recipients with a mortality of more than 50%. CMV pneumonia often manifests with fever, nonproductive cough, hypoxia, and infiltrates commonly interstitial on radiography. The diagnosis of CMV pneumonia is established by detection of CMV by shell-vial, culture, or histology in BAL or lung biopsy specimens, in the presence of compatible clinical signs and symptoms. Pulmonary shedding of CMV is common, and CMV detection in BAL from asymptomatic patients who underwent routine BAL screening at day 35 after HSCT was predictive of subsequent CMV pneumonia in only approximately two thirds of cases. The presence of CMV in a BAL specimen in the absence of clinical evidence of CMV disease is not proof of CMV pneumonia, but the patient needs to be carefully followed. The relevance of PCR testing on BAL fluid is doubtful because there are little data correlating CMV DNA detection by PCR in BAL fluid with CMV pneumonia. Because of the high negative predictive value afforded by its high sensitivity, however, a negative PCR result can be used to rule out the diagnosis of CMV pneumonia. It is possible that qPCR on BAL might provide additional information, allowing this technique to be used for the diagnosis of CMV pneumonia in the future.
CMV can affect the entire gastrointestinal (GI) tract. Ulcers extending deep into the submucosal layers are seen on endoscopy, but can be macroscopically confused with other disorders including graft-versus-host disease (GVHD) and adenovirus disease. The diagnosis of GI disease relies on detection of CMV in biopsy specimens by culture or histology and can occur in the absence of CMV detection in the blood, even by PCR. CMV and GVHD are also frequently seen concomitantly, making the assessment of each disorder’s contribution to the symptomatology difficult.
Retinitis is relatively uncommon after transplantation, although its incidence seems to be increasing. Decreased visual acuity and blurred vision are early symptoms, and approximately 60% of patients have involvement of both eyes. Untreated, the risk for loss of vision on the affected eye is high. Other manifestations including hepatitis and encephalitis do occur, but are rare.
Clinical manifestations
CMV infection is defined as the detection of CMV, typically by DNA PCR, pp65 antigenemia, or mRNA nucleic acid sequence-based amplification, from plasma or whole blood in a CMV-seronegative patient (primary infection) or a CMV-seropositive patient (reactivation of latent or persistent virus or superinfection with another strain of CMV). International definitions of CMV disease, requiring the presence of symptoms and signs compatible with CMV end-organ involvement together with the detection of CMV using a validated method in the appropriate clinical specimen, have been published. Almost any organ can be involved in CMV disease. Fever is a common manifestation, but may be absent in patients receiving high-dose immunosuppression.
CMV pneumonia is the most serious manifestation of CMV in HSCT recipients with a mortality of more than 50%. CMV pneumonia often manifests with fever, nonproductive cough, hypoxia, and infiltrates commonly interstitial on radiography. The diagnosis of CMV pneumonia is established by detection of CMV by shell-vial, culture, or histology in BAL or lung biopsy specimens, in the presence of compatible clinical signs and symptoms. Pulmonary shedding of CMV is common, and CMV detection in BAL from asymptomatic patients who underwent routine BAL screening at day 35 after HSCT was predictive of subsequent CMV pneumonia in only approximately two thirds of cases. The presence of CMV in a BAL specimen in the absence of clinical evidence of CMV disease is not proof of CMV pneumonia, but the patient needs to be carefully followed. The relevance of PCR testing on BAL fluid is doubtful because there are little data correlating CMV DNA detection by PCR in BAL fluid with CMV pneumonia. Because of the high negative predictive value afforded by its high sensitivity, however, a negative PCR result can be used to rule out the diagnosis of CMV pneumonia. It is possible that qPCR on BAL might provide additional information, allowing this technique to be used for the diagnosis of CMV pneumonia in the future.
CMV can affect the entire gastrointestinal (GI) tract. Ulcers extending deep into the submucosal layers are seen on endoscopy, but can be macroscopically confused with other disorders including graft-versus-host disease (GVHD) and adenovirus disease. The diagnosis of GI disease relies on detection of CMV in biopsy specimens by culture or histology and can occur in the absence of CMV detection in the blood, even by PCR. CMV and GVHD are also frequently seen concomitantly, making the assessment of each disorder’s contribution to the symptomatology difficult.
Retinitis is relatively uncommon after transplantation, although its incidence seems to be increasing. Decreased visual acuity and blurred vision are early symptoms, and approximately 60% of patients have involvement of both eyes. Untreated, the risk for loss of vision on the affected eye is high. Other manifestations including hepatitis and encephalitis do occur, but are rare.
Risk factors
Allogeneic HSCT Recipients
In allogeneic HSCT recipients, the most important risk factors for CMV disease are the serologic status of the donor and recipient. CMV-seronegative patients receiving stem cells from a CMV-seronegative donor (D-/R-) have a very low risk of primary infection if CMV-safe blood products are used. Approximately 30% of seronegative recipients transplanted from a seropositive donor (D+/R-) develop primary CMV infection. Although the risk of CMV disease is low because of preemptive treatment of CMV infection, mortality caused by bacterial and fungal infections in these patients is higher than in similarly matched D-/R- transplants (18.3% vs 9.7%, respectively), possibly because of the immunosuppressive effects of CMV or its therapy.
Without prophylaxis, approximately 80% of CMV-seropositive patients experience CMV infection after allogeneic HSCT. Current preventive strategies have decreased the incidence of CMV disease, which had historically occurred in 20% to 35% of these patients. Although a CMV-seropositive recipient is at higher risk for transplant-related mortality than a seronegative recipient, the impact of donor serostatus on nonrelapse mortality and survival when the recipient is seropositive remains controversial. This combination, however, has been reported as a risk factor for delayed CMV-specific immune reconstitution, repeated CMV reactivations, late CMV recurrence, and development of CMV disease.
Other risk factors for CMV infection after allogeneic HSCT include the use of high-dose corticosteroids, T-cell depletion, acute and chronic GVHD, and the use of mismatched or unrelated donors. The use of sirolimus for GVHD prophylaxis seems to have a protective effect against CMV infection, possibly because of the inhibition of cellular signaling pathways that are co-opted by CMV during infection for synthesis of viral proteins. The use of nonmyeloablative conditioning regimens generally has been reported to result in a lower rate of CMV infection and disease early after HSCT compared with standard myeloablative regimens. By 1 year after HSCT, however, the risks of CMV infection and disease are comparable. Umbilical cord blood transplantation (CBT) is an increasingly used technology for HSCT. Because most infants are born without CMV infection, the transplanted allograft is almost always CMV-negative. Among CMV-seropositive recipients who do not receive antiviral prophylaxis, the rate of CMV infection after CBT is 40% to 80%, with one study reporting 100%. When patients receive prophylaxis with high-dose valacyclovir after CBT, it does not seem that CBT entails a significantly greater risk of CMV infection and disease than does peripheral blood stem cell or bone marrow transplantation.
Alemtuzumab is an anti-CD52 monoclonal antibody that results in CD4+ and CD8+ lymphopenia that can last for up to 9 months after administration. Patients who received alemtuzumab experienced a higher rate of CMV infection compared with matched controls not receiving alemtuzumab.
Late CMV Infection After Allogeneic HSCT
Today, with the use of preemptive ganciclovir therapy, CMV disease has become a more significant problem after day 100 following allogeneic HSCT. The risk varies a lot between different centers, presumably because of factors related to patient and donor selection and the choices of transplantation modalities used at the different centers (stem cell source, GVHD prophylaxis and treatment, conditioning regimens). Late CMV infection is strongly associated with nonrelapse mortality. Several factors predict the development of late CMV disease and extended monitoring and antiviral therapy are warranted in patients with risk factors to reduce the risk.
Autologous HSCT
After autologous HSCT, approximately 40% of seropositive patients develop CMV infection. Although CMV disease is rare after autologous HSCT, the outcome of CMV pneumonia is similar to that after allogeneic HSCT. Risk factors for CMV disease after autologous HSCT include CD34+ selection, high-dose corticosteroids, and the use of total-body irradiation or fludarabine as part of the conditioning regimen.
Prevention of CMV infection and disease
CMV serology should be assessed as early as possible when a patient is considered a candidate for HSCT and safe blood products should be used in CMV-seronegative candidates to reduce the risk for primary CMV infection. To reduce the risk for transmission of CMV, blood products from CMV-seronegative donors or leukocyte-reduced, filtered blood products should be used. Recipients who are CMV seronegative before allogeneic HSCT should ideally receive a graft from a CMV-negative donor. Weighing the factor of donor CMV serostatus compared with other relevant donor factors, such as HLA-match, is difficult. No data exist indicating whether HLA-matching is more important compared with CMV serostatus in affecting a good outcome for the patient. For lesser degrees of mismatch, (allele-mismatches or mismatches on HLA-C, DQ, or DP), the CMV serostatus of the donor should be considered in the selection process.
Intravenous immunoglobulin (IVIG) is not reliably effective as prophylaxis against primary CMV infection. Likewise, the effect of immunoglobulin on reducing CMV infection in seropositive patients is modest. The prophylactic use of immunoglobulin is not recommended. Future possibilities for prevention might include a CMV vaccine, and different vaccines are currently in development.
Antiviral Prophylaxis and Preemptive Therapy
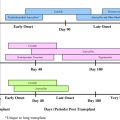
Antiviral prophylaxis is defined as the routine administration of an antiviral agent to all patients at risk. Preemptive therapy is initiated when CMV infection is detected, but before the development of CMV-associated symptoms. Both strategies have their benefits and drawbacks ( Table 1 ). If an effective antiviral is used for prophylaxis, it could be argued that monitoring would not be required. Additionally, prophylaxis may potentially prevent the indirect effects associated with CMV infection. Prophylaxis by definition results in some patients receiving the drug unnecessarily, however, exposing them to potential drug-related toxicities. The success of the preemptive treatment strategy is largely dependent on the early detection of CMV in blood. By allowing a limited amount of viral replication, preemptive therapy may stimulate immune responses and thereby promote CMV-specific immune reconstitution. Because both strategies are equally effective in preventing CMV disease, most transplant centers have moved toward preemptive strategies as pp65 antigenemia and DNA PCR-based assays have become readily available.
| Prevention Strategy | Patient Population | Timing Post-HCT | Initiation | First-line Choice: Induction | First-line Choice: Maintenance | Alternatives | Duration |
|---|---|---|---|---|---|---|---|
| Preemptive | Allogeneic HSCT recipients | <100 d | At first detection of CMV infection | GCV 5 mg/kg IV bid × 7–14 d and declining viral load | GCV 5 mg/kg IV qd | Foscarnet Valganciclovir Cidofovir | Indicator test negative and minimum 2–3 wk |
| Allogeneic HSCT or GVHD requiring steroid therapy or Early CMV infection | >100 d | pp65 Ag ≥5 cells/slide or ≥2 consecutively positive PCR/viremia | GCV 5 mg/kg IV bid × 7–14 d and declining viral load | GCV 5 mg/kg IV qd | Valganciclovir Foscarnet | Until indicator assay negative and minimum 2–3 wk therapy | |
| Autologous HSCT and CMV seropositive and at high risk a | <100 d | pp65 Ag ≥5 cells/slide (or at any level if CD34 ± selected graft) | GCV 5 mg/kg IV bid × 7 d and declining viral load | GCV 5 mg/kg lV qd | Foscarnet Valganciclovir Cidofovir | Until indicator assay negative and minimum 2 wk therapy | |
| Prophylaxis | Allogeneic HSCT recipients | <100 d | At engraftment | GCV 5 mg/kg IV bid × 5–7 d | GCV 5 mg/kg IV qd | Foscarnet Acyclovir b Valacyclovir b | Day 100 after HCT |
a Includes use of TBI in conditioning, recent fludarabine, or 2-chlorodeoxyadenosine, high-dose corticosteroids.
b Must be combined with active surveillance for CMV infection.
More recently, there has been great interest in using methods to determine CMV-specific immune reconstitution after HSCT as an additional means to determine the risk of CMV infection and disease. The usefulness of measuring T-cell responses as a guide for withholding therapy was evaluated in a small pilot study involving HSCT recipients more than 100 days after transplant. Although promising, this strategy requires validation in larger, randomized trials.
Antiviral Agents
High-dose acyclovir reduces the risk for CMV infection and possibly disease. Valacyclovir is the prodrug of acyclovir and is better absorbed, resulting in higher serum-concentration. High-dose valacyclovir is more effective than acyclovir in reducing CMV infection and the need for preemptive therapy with ganciclovir after HSCT, although there is no impact on survival. Routine monitoring for CMV infection is required if valacyclovir or acyclovir prophylaxis is used.
Ganciclovir is currently the first-line agent for CMV prophylaxis and preemptive treatment. Intravenous ganciclovir has been demonstrated to reduce the risk of CMV infection and disease compared with placebo, but does not improve overall survival. Neutropenia occurs in up to 30% of HSCT recipients during ganciclovir therapy increasing the risk of invasive bacterial and fungal infections. Therapeutic drug monitoring can be helpful to guide therapy and reduce the risk for toxicity, especially in the situation of pre-existing renal impairment.
Valganciclovir is an orally available prodrug of ganciclovir and administration achieves serum concentrations at least equivalent to intravenous ganciclovir. The results of several uncontrolled studies suggest that valganciclovir is comparable with intravenous ganciclovir in terms of efficacy and safety when used as preemptive therapy after allogeneic HSCT. Preliminary data from a randomized trial have been presented indicating little or no difference in efficacy or toxicity compared with intravenous ganciclovir. Until more data are available, however, caution should be exercised when choosing valganciclovir as preemptive therapy.
Foscarnet is as effective as ganciclovir for preemptive therapy after allogeneic transplantation. The commonly encountered toxicities of foscarnet make this drug a second-line agent, most appropriate when ganciclovir is contraindicated or not tolerated.
Cidofovir is a “broad-spectrum” antiviral with a long half-life allowing a once-per-week dosing schedule. The major toxicity with cidofovir, acute renal tubular necrosis, limits its use after HSCT.
Monitoring for CMV Infection and Initiation of Preemptive Therapy
qPCR assays for CMV DNA are increasingly used because of their performance characteristics allowing the development of institution-specific viral load thresholds for initiation of preemptive treatment, thereby avoiding unnecessary treatment of patients who are at low risk of progression to disease. It has been reported that the initial viral load and the viral load kinetics are important as risk factors for CMV disease. Currently, several different variations are used, making it difficult to establish validated universal viral load thresholds because of differences in assay performance and testing material (whole blood vs plasma).
If the preemptive therapy strategy is used, all patients who have undergone allogeneic HSCT should be monitored up to day 100 posttransplant on a weekly basis for CMV infection. Although CMV infection is rare in D-/R- patients, routine monitoring was effective in identifying CMV infection and preventing disease in a large cohort. The ideal duration and frequency of CMV monitoring later after HSCT have not been determined.
Various durations of preemptive antiviral treatment have been explored. Most centers now continue antiviral treatment until the designated viral marker is negative and the patient has received at least 2 weeks of antiviral therapy. If an assay less sensitive than DNA PCR, such as the pp65 antigenemia assay, is used, then preemptive therapy should be continued until two negative results are obtained. If a patient is still positive by PCR or pp65 antigenemia assay after 2 weeks of therapy, treatment should be extended until clearance is achieved. It has been shown that a low rate of viral load decrease is a risk factor for later-occurring CMV disease.
Special Populations
Patients with CMV disease occurring before planned allogeneic HSCT have a very high risk of mortality. After transplantation, a patient with documented pretransplant CMV disease should either be monitored for CMV very closely (ie, twice weekly), or be given prophylaxis with ganciclovir or foscarnet.
The optimal approach to CMV after CBT is not clear. One study described successful preemptive treatment with ganciclovir, whereas another combined high-dose valacyclovir prophylaxis with continued monitoring and preemptive therapy.
Antiviral resistance
Risk factors for drug resistance include prolonged (months) antiviral therapy, intermittent low-level viral replication in the presence of drug caused by profound immunosuppression or suboptimal drug levels, and lack of prior immunity to CMV. Drug resistance should be suspected in patients who have increasing quantitative viral loads for more than 2 weeks despite antiviral therapy. After start of antiviral therapy in treatment-naive patients, an increase in the viral load occurs in approximately one third of patients and is likely caused by the underlying immunosuppression (clinical resistance), not true drug resistance caused by mutations in the target genes for the antiviral agent used. If the viral load increases in patients who have received previous antiviral therapy, drug resistance should, however, be suspected.
Ganciclovir resistance most often is caused by mutations in the UL97 gene, but mutations in the UL54-encoded DNA polymerase can also occur. Several UL97 mutations that confer resistance have been described. Because different UL97 mutations confer varying degrees of ganciclovir resistance, however, some cases of genotypically defined ganciclovir-resistant CMV may still respond to therapy.
If ganciclovir resistance is documented or suspected, foscarnet is generally the second-line agent of choice. Unlike ganciclovir, foscarnet activity is not dependent on phosphorylation by the UL97 gene product. Resistance to foscarnet can occur and is caused by mutations in UL54. Because cidofovir is not phosphorylated by the CMV UL97 gene product, it is also active against ganciclovir-resistant UL97 mutants. Certain UL54 mutations, however, can confer cross-resistance between ganciclovir and cidofovir. Additional genotype testing of UL54 is indicated to evaluate for potential cross-resistance conferring mutations.
Drugs under evaluation, such as maribavir, may provide therapeutic options in the future. Maribavir inhibits the CMV UL97 kinase and is active against wild-type and ganciclovir-resistant CMV strains and has shown promising results in a small series of patients failing therapy with other antiviral agents either because of toxicity or resistance. Other drugs with possible anti-CMV activity include the arthritis drug leflunomide and the antimalaria compound artesunate.
Management of CMV disease
Several studies established the current standard of care for CMV pneumonia, which is treatment with ganciclovir (or foscarnet as an alternative agent) in combination with IVIG. These studies showed improved survival rates compared with historical controls. There does not seem to be a specific advantage of CMV-specific immunoglobulin (CMV-Ig) compared with pooled immunoglobulin. In specific clinical situations, however, such as volume overload, CMV-Ig may be preferred. Several studies have raised doubt regarding the beneficial effect of concomitant IVIG, but it is still considered as standard-of-care at most centers.
For GI disease, the standard therapy is most often intravenous ganciclovir for 3 to 4 weeks followed by several weeks of maintenance. Shorter courses of induction therapy (2 weeks) are not as effective. There is no role for concomitant IVIG in the treatment of GI disease. Recurrence may occur in approximately 30% of patients in the setting of continued immunosuppression and such patients may benefit from secondary prophylaxis until immunosuppression has been reduced. Foscarnet can be used as an alternative if neutropenia is present. Valganciclovir as maintenance treatment for GI disease has not been well studied.
CMV retinitis is typically treated with systemic ganciclovir, foscarnet, or cidofovir, with or without intraocular ganciclovir injections or implants. Fomivirsen is an antisense RNA molecule that targets mRNA encoded by CMV and is approved as second-line therapy for CMV retinitis in patients with AIDS.
Other manifestations of CMV disease, such as hepatitis and encephalitis, are uncommon and are typically managed with intravenous ganciclovir. The duration of therapy for these manifestations has not been well-established and should be tailored to the individual patient.
Adoptive immunotherapy
CMV-specific T cells can be generated by several different mechanisms to restore cellular immunity passively after transplantation. Several groups have reported a beneficial impact of adoptive immunotherapy on CMV viral loads in patients who had undergone HSCT. Despite these seemingly promising results, scientific questions remain unanswered (eg, the optimal cell type and dose for infusion) and technical hurdles persist (availability of clinical grade reagents) that preclude adoptive immunotherapy from becoming a routine clinical procedure at the current time.
Per Ljungman had support from the Karolinska Institute research funds, the European Leukemia Net, and the Swedish Children’s Cancer Fund. Michael Boeckh had support from the National Institute of Health ( NIH CA 18029 ).
A version of this article was previously published in the Infectious Disease Clinics of North America , 24:2.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree