Curative treatment of Severe Combined Immunodeficiency (SCID) by Hematopoietic Cell Transplantation (HCT) remains a challenge, in particular in infants presenting with serious, poorly controllable complications. In the absence of a matched family donor, HLA-haploidentical transplantation from parental donors represents a uniformly and readily available treatment option, offering a high chance to be successful. Concerning outcomes of HCT in SCID, other important parameters beside survival need to be taken into consideration, in particular the stability and robustness of the graft and its function, as well as potential late complications, related either to the disease or to the treatment.
Treatment of severe combined immunodeficiency (SCID) by hematopoietic cell transplantation (HCT) changed profoundly in the early eighties when Reisner and colleagues developed a procedure to efficiently reduce the number of T cells contained in marrow grafts responsible for graft-versus-host disease (GVHD), opening the possibility to prevent this complication after HLA-mismatched donor transplantation. The procedure used soybean agglutination in combination with sheep red blood cell rosette formation to fractionate bone marrow cells and provided precursor cell–enriched grafts with drastically reduced T-cell numbers. Reisner and colleagues and, subsequently, several other groups were able to demonstrate the potential of T-cell–depleted, HLA-haploidentical parental donor transplants to establish stable immunologic reconstitution in SCID patients with significantly reduced risk for acute and chronic GVHD. Newly developed donor T cells were found to be tolerant to cells of the HLA-haploidentical recipient. Other subsequently developed technologies for T-cell depletion of marrow grafts took advantage of lymphocyte-specific monoclonal antibodies, and more recently, antibodies directed at CD34 + are used to purify CD34 – expressing hematopoietic progenitor cells by positive selection from peripheral blood after their mobilization by granulocyte colony-stimulating factor (GCSF). In patients with SCID, for whom HCT represented the only available curative therapy, these advances led to profound changes in the prognosis of this otherwise lethal disorder, which became uniformly treatable regardless of the availability of a matched family donor. Over the last years, HCT from unrelated matched donors also has been markedly advanced, and both approaches now are well established in the treatment of the disorder. In this review, several pertinent findings in SCID patients undergoing HLA-haploidentical HCT are discussed, and several unresolved issues, such as the nonuniformity of B-cell reconstitution, graft resistance, the role of conditioning, and long-term immune reconstitution, are addressed.
Specific aspects of HCT in SCID
In contrast to other disorders, the profound immunodeficiency in SCID minimizes the risk of immunologic graft rejection, conceptually eliminating the need for immunosuppressive conditioning before transplantation. This approach of transplanting SCID patients without conditioning, providing a significant advantage because of the reduced toxicity of the procedure, has been widely explored in HLA-identical and HLA-nonidentical transplantation. Because the recipient’s hematopoietic system remains intact, there is no advantage for donor precursor cells to engraft and to replace this system, and indeed, evidence for sustained or substantial marrow engraftment by donor cells is usually lacking. Nevertheless, lymphocytes of donor origin develop, in contrast to other blood cells, which remain of recipient origin. The potential of HCT to induce stable immune reconstitution in the absence of marrow engraftment raises the obvious question regarding the underlying mechanisms of lymphocyte development in the absence of progenitor cell engraftment in the marrow.
Different outcomes after HLA-identical and haploidentical HCT without conditioning
The outcome of HCT in SCID when performed without conditioning differs substantially depending on whether nonmanipulated grafts from HLA-identical donors or manipulated, T-cell depleted grafts from HLA-nonidentical donors are used. Mature donor T cells contained in the graft have the capacity to undergo marked proliferation and expansion in the recipient, giving rise to an initial wave of T cells early after transplantation. These T cells are responsible for induction of GVHD but also are potentially beneficial and protective, in particular if GVHD is limited or absent, as is commonly the case in SCID patients after sibling transplantation. The scenario obviously is different after HLA-haploidentical transplantation, where this effect of expanding mature T cells is abolished. Development of T cells after T-cell depleted transplantation depends solely on de novo maturation in the thymus. This maturation process is slow. Newly differentiated T cells appear in the circulation only several months after transplantation. In contrast to early developing T cells, which carry predominantly a memory phenotype, newly differentiated T cells disclose a naive phenotype and are rich in a cell marker, the so called T-cell receptor excision circle (TREC), which indicates their recent thymus emigration. These cells are furthermore characterized by a diverse receptor repertoire, in contrast to the more skewed repertoire of early T cells arising by expansion. Simultaneously with the delayed development of naive T cells, the previously small thymus characteristic for SCID enlarges in size, as easily visualized by ultrasonography. Notably, after transplantation of unmanipulated grafts, a similar kinetics of slow development of naive T cells is observed. It is also interesting that T-cell development is similarly delayed in other congenital disorders after HLA-haploidentical, T-cell depleted transplantation, such as infantile osteopetrosis and Wiskott-Aldrich syndrome. Furthermore, in SCID patients receiving cytoreductive conditioning before transplantation for reasons that are discussed in more detail later in the article, the kinetics of slow T-cell reconstitution is not altered. The distinct pattern of T-cell reconstitution after HLA-identical and after HLA-haploidentical, T-cell–depleted transplantation is demonstrated in Fig. 1 .
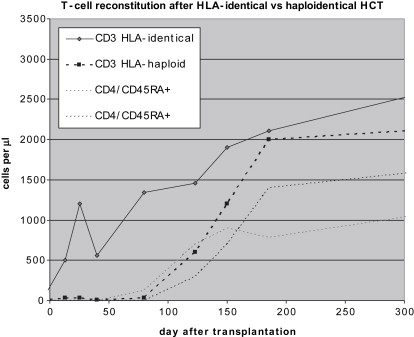
Patients remain profoundly immunodeficient for a prolonged period after HLA-haploidentical HCT because it takes up to 4 months until T cells become demonstrable, in contrast to patients after sibling transplantation, in whom early-appearing donor T cells can be effective to control and prevent infections.
Another marked difference after transplantation of grafts with and without T-cell depletion regards reconstitution of humoral immunity. Whereas transplantation of nonmanipulated grafts from HLA-identical donors commonly results in sustained B-cell reconstitution with detection of donor B cells in the circulation, patients after HLA-haploidentical, T-cell–depleted transplantation, when performed without cytoreductive conditioning, commonly fail to develop effective B-cell immunity. With rare exceptions, B cells of donor origin remain absent, as most evident in patients with B− SCID, who fail to develop circulating B cells. In most patients with B+ SCID, autologous B cells persist as expected but fail to become functional, as elegantly shown in a study by White and colleagues. Only exceptionally, such as in patients with IL7R deficiency, CD3 deficiency, and adenosine deaminase (ADA) deficiency, has the functional maturation of autologous B cells after effective T-cell reconstitution been observed. Because in the absence of conditioning, as noted earlier, evidence of substantial engraftment of donor precursor cells in the marrow is usually lacking after both HLA-identical and HLA-nonidentical transplantation, this discrepancy in humoral reconstitution requires an explanation. Graft manipulation and depletion of T cells usually also lead to drastic reduction of B-cell numbers in the grafts. As one possible explanation, it is conceivable that donor B cells arising after HLA-identical transplantation are derived from the pool of donor B cells contained in nonmanipulated grafts. The longevity of mature B cells and their potential to undergo extensive homeostatic proliferation analogous to T cells is well established in murine models and recently also in man, and it is conceivable that transfer of mature donor B cells into allogeneic hosts may be effective to establish stable long-term humoral immunity. Nevertheless, the heterogeneity of B-cell reconstitution remains incompletely understood and requires further studies.
Although most patients develop normal and sustained T-cell functions after HLA-haploidentical transplantation without conditioning, a proportion of patients fail to do so, showing either complete graft failures or subnormal T-cell reconstitution. Complete graft failure has been common in particular in ADA deficiency, in SCID patients characterized by the presence of poorly functional T cells, such as in Omenn syndrome, and in SCID patients with functional NK-cell systems. This experience again is in contrast to the outcome after nonmanipulated, HLA-identical sibling transplants, which almost uniformly, regardless of the underlying variant of SCID, will lead to engraftment and development of T-cell immunity. The less-uniform reconstitution after HLA-haploidentical transplantation may be because of several factors restricting T-cell development. One factor may be the absence of an engraftment-facilitating effect mediated by mature donor T cells. Also persistent viral disease, such as cytomegalovirus infections, may suppress lymphoid differentiation and cause poor reconstitution. Furthermore, in certain SCID variants, the environment of the thymus may limit homing of donor precursor cells to specific niches, as has been demonstrated in relevant murine SCID models. The most important factor for subnormal T-cell reconstitution probably is GVHD and its treatment, a complication that may also be induced by maternal T cells secondary to an intrauterine maternofetal transfusion and that may take a subclinical course. Furthermore, graft resistance may be mediated by NK cells, which function in subgroups of SCID patients. Importantly, graft failures can usually be overcome by using pretransplant cytoreductive conditioning. Because this approach also allows marrow engraftment of precursor cells and as a consequence donor B-cell development, conditioning has been used in a significant proportion of patients before initial transplantation.
Different outcomes after HLA-identical and haploidentical HCT without conditioning
The outcome of HCT in SCID when performed without conditioning differs substantially depending on whether nonmanipulated grafts from HLA-identical donors or manipulated, T-cell depleted grafts from HLA-nonidentical donors are used. Mature donor T cells contained in the graft have the capacity to undergo marked proliferation and expansion in the recipient, giving rise to an initial wave of T cells early after transplantation. These T cells are responsible for induction of GVHD but also are potentially beneficial and protective, in particular if GVHD is limited or absent, as is commonly the case in SCID patients after sibling transplantation. The scenario obviously is different after HLA-haploidentical transplantation, where this effect of expanding mature T cells is abolished. Development of T cells after T-cell depleted transplantation depends solely on de novo maturation in the thymus. This maturation process is slow. Newly differentiated T cells appear in the circulation only several months after transplantation. In contrast to early developing T cells, which carry predominantly a memory phenotype, newly differentiated T cells disclose a naive phenotype and are rich in a cell marker, the so called T-cell receptor excision circle (TREC), which indicates their recent thymus emigration. These cells are furthermore characterized by a diverse receptor repertoire, in contrast to the more skewed repertoire of early T cells arising by expansion. Simultaneously with the delayed development of naive T cells, the previously small thymus characteristic for SCID enlarges in size, as easily visualized by ultrasonography. Notably, after transplantation of unmanipulated grafts, a similar kinetics of slow development of naive T cells is observed. It is also interesting that T-cell development is similarly delayed in other congenital disorders after HLA-haploidentical, T-cell depleted transplantation, such as infantile osteopetrosis and Wiskott-Aldrich syndrome. Furthermore, in SCID patients receiving cytoreductive conditioning before transplantation for reasons that are discussed in more detail later in the article, the kinetics of slow T-cell reconstitution is not altered. The distinct pattern of T-cell reconstitution after HLA-identical and after HLA-haploidentical, T-cell–depleted transplantation is demonstrated in Fig. 1 .
Patients remain profoundly immunodeficient for a prolonged period after HLA-haploidentical HCT because it takes up to 4 months until T cells become demonstrable, in contrast to patients after sibling transplantation, in whom early-appearing donor T cells can be effective to control and prevent infections.
Another marked difference after transplantation of grafts with and without T-cell depletion regards reconstitution of humoral immunity. Whereas transplantation of nonmanipulated grafts from HLA-identical donors commonly results in sustained B-cell reconstitution with detection of donor B cells in the circulation, patients after HLA-haploidentical, T-cell–depleted transplantation, when performed without cytoreductive conditioning, commonly fail to develop effective B-cell immunity. With rare exceptions, B cells of donor origin remain absent, as most evident in patients with B− SCID, who fail to develop circulating B cells. In most patients with B+ SCID, autologous B cells persist as expected but fail to become functional, as elegantly shown in a study by White and colleagues. Only exceptionally, such as in patients with IL7R deficiency, CD3 deficiency, and adenosine deaminase (ADA) deficiency, has the functional maturation of autologous B cells after effective T-cell reconstitution been observed. Because in the absence of conditioning, as noted earlier, evidence of substantial engraftment of donor precursor cells in the marrow is usually lacking after both HLA-identical and HLA-nonidentical transplantation, this discrepancy in humoral reconstitution requires an explanation. Graft manipulation and depletion of T cells usually also lead to drastic reduction of B-cell numbers in the grafts. As one possible explanation, it is conceivable that donor B cells arising after HLA-identical transplantation are derived from the pool of donor B cells contained in nonmanipulated grafts. The longevity of mature B cells and their potential to undergo extensive homeostatic proliferation analogous to T cells is well established in murine models and recently also in man, and it is conceivable that transfer of mature donor B cells into allogeneic hosts may be effective to establish stable long-term humoral immunity. Nevertheless, the heterogeneity of B-cell reconstitution remains incompletely understood and requires further studies.
Although most patients develop normal and sustained T-cell functions after HLA-haploidentical transplantation without conditioning, a proportion of patients fail to do so, showing either complete graft failures or subnormal T-cell reconstitution. Complete graft failure has been common in particular in ADA deficiency, in SCID patients characterized by the presence of poorly functional T cells, such as in Omenn syndrome, and in SCID patients with functional NK-cell systems. This experience again is in contrast to the outcome after nonmanipulated, HLA-identical sibling transplants, which almost uniformly, regardless of the underlying variant of SCID, will lead to engraftment and development of T-cell immunity. The less-uniform reconstitution after HLA-haploidentical transplantation may be because of several factors restricting T-cell development. One factor may be the absence of an engraftment-facilitating effect mediated by mature donor T cells. Also persistent viral disease, such as cytomegalovirus infections, may suppress lymphoid differentiation and cause poor reconstitution. Furthermore, in certain SCID variants, the environment of the thymus may limit homing of donor precursor cells to specific niches, as has been demonstrated in relevant murine SCID models. The most important factor for subnormal T-cell reconstitution probably is GVHD and its treatment, a complication that may also be induced by maternal T cells secondary to an intrauterine maternofetal transfusion and that may take a subclinical course. Furthermore, graft resistance may be mediated by NK cells, which function in subgroups of SCID patients. Importantly, graft failures can usually be overcome by using pretransplant cytoreductive conditioning. Because this approach also allows marrow engraftment of precursor cells and as a consequence donor B-cell development, conditioning has been used in a significant proportion of patients before initial transplantation.
Survival and complications in SCID patients after HLA-haploidentical HCT
The exploration of HLA-haploidentical HCT in the treatment of SCID has resulted in a series of larger single- and multicenter studies, the latter in particular by groups collaborating in the European Group for Blood and Marrow Transplantation (EBMT) who established the Stem Cell Transplantation for Immunodeficiencies (SCETIDE) registry. In the following sections, several issues of HLA-haploidentical HCT are addressed based on these studies, including survival, immune reconstitution, complications, and late outcome.
A multicenter study published in 2003 analyzed the outcome after HCT in patients with primary immunodeficiencies treated in Europe between 1968 and 1999. The study included 475 patients with SCID. Donors in 294 of these cases were HLA-haploidentical parents. The majority (207 cases) had received pretransplant conditioning, consisting mostly of busulfan (8 mg/kg) and cyclophosphamide (200 mg/kg). The 3-year survival rate after HLA-haploidentical transplantation was 54%, which was significantly lower compared with a survival rate of 77% in patients after HLA-identical HCT ( P = .002) reported in the same study. Importantly, survival rates after haploidentical transplantation have improved significantly over time, from about 50% during the initial period up to 80% during the more recent period, as shown in Fig. 2 , where survival data of a more recent EBMT survey are demonstrated. It was also noted that the variant of SCID had an impact on survival after HLA-haploidentical HCT, because cases of B− SCID tended to have a poorer prognosis than those of B+ SCID, confirming similar observations in a previous analysis. In the former group, the use of conditioning before HCT was found to result in better survival, whereas in cases of B+ SCID, survival was not affected by conditioning. For patients with ADA deficiency (n = 26) and for patients with reticular dysgenesis (n = 8), 3-year survival rates were 30%.
In a separate study of 10 patients with reticular dysgenesis, cytoreductive conditioning before HLA-haploidentical HCT was found mandatory to obtain reconstitution of both lymphoid and myeloid functions in this disorder.
Independent predictors of poorer outcome, besides underlying variants of SCID, were the presence of pulmonary infection at transplantation, absence of a protected environment, and occurrence of acute GVHD (grade II or higher). The study also revealed that the incidence of GVHD had decreased over time, from 35% to 40% before 1996 to 22% thereafter ( P <.001), possibly related to the use of more stringent methods for T-cell depletion, a factor that partly accounted for the observed better survival with time. The main causes of death were infections (56%), GVHD (25%), and B-cell lymphoproliferative syndrome (5%).
In a large single-center study reported by Buckley and colleagues, 77 SCID patients were analyzed after HLA-haploidentical HCT. In this study, a survival rate of 77% was reported, with follow-up after transplantation ranging from 3 months to 16 years. All patients in this study underwent transplants without conditioning. Furthermore, the proportion of patients with B− SCID was very low, in contrast to the European study, in which this proportion was about 35%. An important prognostic factor for survival was noted to be age at transplantation; within the whole group of treated patients, infants who underwent transplant before the age of 3 to 5 months had a survival rate of 95% as compared with a survival rate of 76% in patients beyond that age.
In a previous multicenter EBMT study analyzing prognostic factors for long-term survival, normalization of T-cell immunity at 12 months was identified as a strong favorable indicator, whereas ineffective reconstitution, which may be because of poorly controlled GVHD or other complications, was found to be associated with a substantial mortality.
The basis for improved survival after HLA-haploidentical HCT during more recent periods includes several factors, such as earlier diagnosis resulting in fewer sick patients at the time of transplantation, more effective prevention and treatment of disease-related and transplantation-induced complications (notably infections and GVHD), and effective prevention of graft failure by using conditioning prior to transplantation in patients at risk for this complication.
Efficient prevention of GVHD has been related in particular to the use of highly purified, GCSF-mobilized CD34 + precursor cells from peripheral blood, which are obtainable in high numbers with minimal contamination by T cells. In addition to de novo GVHD caused by imperfect T-cell depletion of grafts, another cause for this complication has been a primary engraftment of transplacentally acquired maternal T cells, which is a common phenomenon in SCID. In the study by Buckley and colleagues discussed earlier, 27 of 28 cases with GVHD were noted to have circulating maternal T cells before transplantation. In the authors’ own experience of HLA-haploidentical HCT in a cohort of 137 SCID patients, 31 of 59 patients with maternal T-cell engraftment developed GVHD to variable degrees, whereas among the other 78 patients without maternal T cells, only 7 cases developed this complication (Friedrich W, unpublished data, 2009).
HCT without and with conditioning
Cytoreductive conditioning in SCID patients, for which most commonly a combination of busulfan and cyclophosphamide is used, has been found to offer several advantages, including, in particular, an improved prevention of graft failure and a high chance of sustained, complete immune reconstitution. In the authors’ experience of HLA-haploidentical HCT in SCID, they have observed better overall survival rates in patients who received preparative cytoreductive conditioning compared with those who did not, as demonstrated in Fig. 3 . Causes of death in the latter group were manifold but included a significant proportion of earlier patients showing complete graft failures. There are, however, obvious disadvantages with this approach, most importantly potentially acute and long-term toxic side effects. In particular, in infants suffering from persistent viral disease or other poorly controllable complications, the risks of conditioning may outweigh its potential benefits. As already mentioned, conditioning does not alter the kinetics of immune reconstitution. Also, the use of highly purified CD34 + cells at comparatively high numbers does not influence the slow pattern of T-cell reconstitution, whether conditioning is used or not. As outlined later in this article, based on studies in long-term surviving patients, some concern has been raised regarding the stability of thymic functions in nonconditioned patients. Nevertheless, the decision to use or not to use conditioning requires careful consideration in each individual case, taking into account potential advantages and disadvantages.