Infections in patients with cancer
Lior Nesher, MD  Kenneth V. I. Rolston, MD, FACP
Kenneth V. I. Rolston, MD, FACP
Overview
Patients with cancer have an increased risk of developing infections, owing both to their underlying disease and its treatment. This risk appears to be greatest in patients with hematologic malignancies and in hematopoietic cell transplant recipients. This is due primarily to the development of various immunologic defects such as neutropenia and impaired cellular and/or humoral immunity, each associated with a unique spectrum of infection. Newer therapeutic modalities for the treatment of some cancers are changing the spectrum of infections as are the increasing use of catheters and other medical devices. While bacterial infections are documented most often, opportunistic fungal and viral infections are being encountered with increasing frequency. The morbidity and mortality of infections in cancer patients is generally greater than in the general population. Thus, early diagnosis and the prompt administration of appropriate therapy are of paramount importance. Antimicrobial resistance among these pathogens has become a worldwide problem, which can only be partially tackled by the development of novel agents. Consequently, the importance of conducting frequent epidemiologic surveillance in order to detect local epidemiologic shifts and of infection prevention, infection control, and antimicrobial stewardship cannot be emphasized enough. The number of cancer survivors is steadily increasing. Many of these patients remain immunosuppressed for substantial periods of time. Keeping these survivors healthy and infection free will continue to be a challenge for years to come.
Infection remains a common problem in patients with cancer. The frequency and nature of infection depend on the type of underlying neoplastic disease.1 Multiple episodes of infection are not uncommon. Neutropenia, impaired cellular or humoral immunity, the use of catheters and other medical devices, splenectomy, surgery, radiation, nutritional status, and local factors such as obstruction increase the susceptibility of these patients to infection. Each risk factor is associated with a unique set of infections, although there is some overlap. Multiple factors often exist in the same patient. Table 1 lists defects in host defense mechanisms and the infections associated with those defects.
Table 1 Defects in host defense mechanisms and common infections associated with malignant diseases
| Disease | Prominent defect | Predominant infections |
| Acute leukemia, aplastic anemia | Prolonged neutropenia | Gram-positive cocci, Gram-negative bacilli, fungi (Candida, Aspergillus, Zygomycetes, Fusarium, Trichosporon) |
| Hairy cell leukemia | Neutropenia, impaired lymphocyte function | Gram-negative bacilli, Gram-positive cocci, mycobacteria (including nontuberculous) |
| Chronic lymphocytic leukemia, multiple myeloma | hypogammaglobulinemia (impaired humoral immunity) | Encapsulated organisms, Streptococcus pneumoniae, Haemophilus influenzae, Neisseria meningitides |
| Hodgkin disease | Impaired T-lymphocyte response | Pneumocystis jiroveci, Cryptococcus spp., mycobacteria, Toxoplasma, Listeria monocytogenes, Cryptosporidium, Candida, CMV |
| Hematopoietic stem cell transplant recipients | Neutropenia, impaired cellular and humoral immunity | Gram-positive cocci, Gram-negative bacilli, cytomegalovirus, Candida, Aspergillus, herpes viruses (HSV, VZV, CMV) |
| Breast cancer | Tissue necrosis, radiation damage, foreign bodies | Gram-positive cocci, Gram-negative bacilli, anaerobes (polymicrobial infections common) |
| Lung cancer | Local obstruction, tissue necrosis | Gram-positive cocci, Gram-negative bacilli, anaerobes (polymicrobial infections common) |
| Gynecologic malignancy | Local obstruction, tissue necrosis | Mixed aerobic and anaerobic enteric flora including Enterococcus spp. (polymicrobial infections common) |
Infections primarily associated with neutropenia
Types of febrile episodes
A specific causative pathogen is identified in about 20–25% of febrile episodes in neutropenic patients (microbiologically documented infections). An additional 20–25% of episodes have identifiable sites of infection (e.g., pneumonia, cellulitis, and enterocolitis) but have negative cultures (clinically documented infections). Approximately 40–45% have neither an obvious clinical focus of infection nor positive cultures, and are referred to as episodes of unexplained fever (Figure 1). The majority of these are presumed to be due to an occult infection and respond to antibiotic therapy. Fewer than 5% of febrile episodes are due to non-infectious causes such as transfusion reactions, tumor fever, or drug fever.2, 3
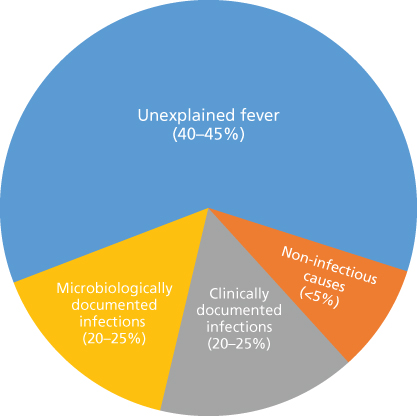
Figure 1 Types of febrile episodes in patients with neutropenia.
Sites of infection
The most common sites of infection encountered in neutropenic patients are the respiratory tract followed by the bloodstream (including central line-associated bloodstream infection-CLABSI), the urinary tract, skin and skin structure infections (SSSIs), and infections originating from the oro-pharynx and the gastrointestinal tract (Figure 2). Less frequent but clinically important sites include the central nervous system, bones, joints, and end organs such as the liver and spleen. Most microbiologically documented infections are caused by the patient’s own microflora with a minority being acquired from exogenous sources/environmental exposure.
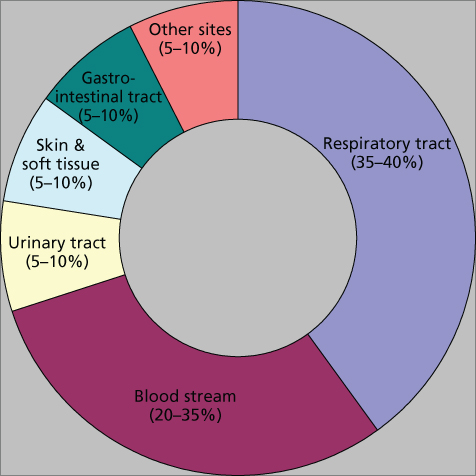
Figure 2 Common sites of infection in patients with neutropenia.
Infections primarily associated with impaired cellular and humoral immunity
Defects in cell-mediated immunity are common in patients with hematologic malignancies including malignant lymplomas, in allogeneic hematopoietic cell transplant (HCT) recipients, in recipients of high-dose corticosteroid therapy, and in patients treated with newer modalities (nucleoside analogs, monoclonal antibodies, and temozolomide) and result in an increase in infections caused by bacterial pathogens such as Legionella spp., Salmonella spp., Nocardia spp., Listeria monocytogenes, and Rhodococcus equi.4–6 Mycobacterial infections (Mycobacterium tuberculosis and nontuberculous mycobacteria) are also relatively common in such patients. Aspergillus spp., Pneumocystis jiroveci, and the endemic fungi (Cryptococcus neoformans, Histoplasma capsulatum, and Coccidioides immitis) cause most of the fungal infections in this setting.7, 8 Viral infections are predominantly caused by the herpes group of viruses with cytomegalovirus (CMV) being the most frequent. The community respiratory viruses are important causes of morbidity and mortality in allogeneic HCT recipients. Parasitic infections such as toxoplasmosis, strongyloidosis, and babesosis are also seen more frequently in patients with impaired cellular or humoral immunity.9–11 In patients with impaired humoral immunity, infections caused by encapsulated organisms such as Streptococcus pneumoniae and Haemophilus influenzae are common.
Infections in patients with solid tumors
Patients with solid tumors who are not significantly immunosuppressed also develop infections frequently. Risk factors include neutropenia that is generally short lived, disruption of normal anatomic barriers (skin and mucosal surfaces), obstruction caused by bulky or rapidly expanding tumors, radiation damage, surgical procedures, and the presence of various medical devices. The common sites of infection seen in patients with various solid tumors are summarized in Table 2. The site of infection depends on the location and size of the tumor or the site and nature of the medical device, radiation, or surgical procedure. Surgical site infections and catheter-related infections are caused most often by organisms colonizing the skin, although opportunistic pathogens such as Pseudomonas aeruginosa and other Gram-negative bacilli are beginning to emerge in this setting.12 Removal of the offending device and complete or partial relief of obstruction are important aspects of the management of infections in patients with solid tumors. If this is not feasible, prolonged suppressive therapy might be necessary.
Table 2 Common infections in patients with solid tumors
| Tumor location | Infection site or type |
| Breast | Wound infection; cellulitis or lymphangitis related to axillary node dissection; mastitis; breast abscess; bacteremia |
| Central nervous system (brain, meninges) | Wound infection; epidural/subdural infection; brain abscess; meningitis/ventriculitis; proximal and distal end-shunt-related infections; aspiration pneumonia; urinary tract infection; bacteremia |
| Genitourinary and prostate | Cystitis; urethritis; acute/chronic pyelonephritis ± bacteremia; catheter-related complicated urinary tract infection (nephrostomy/stents); wound infection; acute/chronic prostatitis; epididymitis, orchitis; pelvic abscess |
| Hepatobiliary-pancreatic | Wound infection; peritonitis; ascending cholangitis ± bacteremia; hepatic, pancreatic, or subdiaphragmatic abscess |
| Head and neck | Cellulitis; wound infection; deep facial space infection; mastoiditis/osteomyelitis; sinusitis; aspiration pneumonia; bacteremia; suppurative intracranial phlebitis; meningitis; brain abscess; retropharyngeal and paravertebral abscesses |
| Musculoskeletal (muscles, bones, joints) | Wound infections; pyomyositis; lymphangitis; bursitis; synovitis; septic arthritis; osteomyelitis; wound infection; prosthesis-related infections; bacteremia |
| Upper gastrointestinal | Esophagitis; tracheoesophageal fistula with pneumonitis/lung abscess; gastric perforation and abscess; feeding tube-related infections; mediastinitis/osteomyelitis |
| Lower gastrointestinal | Wound infection; intra-abdominal or pelvic abscess; peritonitis (perforation); enterocolitis; urinary tract infection; perianal/perirectal infection; sacral/coccygeal osteomyelitis |
Spectrum of infection
Bacterial infections
Currently, Gram-positive organisms cause ∼50% of bacterial infections in neutropenic patients (Table 3). Gram-negative bacilli account for ∼18%, and a substantial proportion, especially deep tissue infections, is polymicrobial.13–16 Bacteria commonly isolated from neutropenic patients are depicted in Table 4. Geographic and institutional variations do exist.17 Consequently, clinicians should consider local epidemiology and resistance patterns when initiating empiric antibiotic therapy.
Table 3 Bacterial infection in 2223 febrile episodes in neutropenic patients
| Infection typea | 2002–2003 | 2012–2013 | ||
| No. | % | No. | % | |
| Microbiologically documented | 262 | 26 | 321 | 26 |
| Gram-positive | 134 | 51 | 163 | 51 |
| Gram-negative | 51 | 20 | 55 | 17 |
| Polymicrobial | 71 | 27 | 92 | 29 |
| Anaerobic | 6 | 2 | 11 | 3 |
| Clinically documented | 210 | 21 | 298 | 24 |
| Unexplained fever | 521 | 53 | 611 | 50 |
a These data are derived from surveys conducted at the University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Table 4 Common infectious agents in patients with cancer
| Neutropenia |
| Bacteria |
| Gram-positive organisms |
| Coagulase-negative staphylococci |
| Staphylococcus aureus (including MRSA) |
| Enterococcus spp. (including VRE) |
| Viridans group streptococci |
| Gram-negative organisms |
| Escherichia coli |
| Klebsiella pneumoniae |
| Pseudomonas aeruginosa |
| Other Enterobacteriaceae |
| Stenotrophomonas maltophilia |
| Fungi |
| Candida spp. |
| Aspergillus spp. |
| Zygomycetes |
| Fusarium spp. |
| Cellular immune dysfunction |
| Bacteria |
| Listeria monocytogenes |
| Rhodococcus equi |
| Salmonella spp. |
| Mycobacteria |
| Nocardia spp. |
| Legionella spp. |
| Fungi |
| Aspergillus spp. |
| Cryptococcus spp. |
| Histoplasma capsulatum |
| Coccidioides immitis |
| Pneumocystis jiroveci |
| Protozoa |
| Toxoplasma gondii |
| Helminth |
| Strongyloides stercoralis |
| Viruses |
| Cytomegalovirus |
| Herpes simplex virus I and II |
| Varicella-zoster virus |
| Epstein–Barr virus |
| Humoral immune dysfunction |
| Streptococcus pneumoniae |
| Haemophilus influenzae |
Gram-positive bacteria
Coagulase-negative staphylococci (CoNS) are isolated most often and predominantly cause CLABSI.18 Staphylococcus lugdunensis is a more virulent species and needs more aggressive management.19, 20 Staphylococcus aureus is often associated with deep-seated infections (deep abscesses and endocarditis), and all patients with S. aureus bacteremia should be evaluated for such foci.21 Other sites of infection include SSSIs, pneumonia, bone and joint infections, and septic thrombophlebitis. Of concern is the increasing rate of methicillin resistance among S. aureus isolates, which is now >50% at many centers.22 Many of these isolates have developed tolerance or reduced susceptibility to vancomycin (referred to as the MIC creep), thereby reducing the efficacy of this agent.23–27 Alternative therapeutic agents include daptomycin, telavancin, dalbavancin, oritavancin, linezolid, tedizolid, and ceftaroline.28–30
Viridans group streptococci (VGS) are encountered often in patients with acute leukemia undergoing intensive chemotherapy and in allogeneic HCT recipients.31, 32 Risk factors include chemotherapy with agents that induce severe oral mucositis, prophylaxis with fluoroquinolones that can promote the selection of these organisms, and treatment of chemotherapy-induced gastritis with antacids or histamine type 2 (H2) antagonists.33, 34 Streptococcus mitis is the predominant species. Bacteremia is the most common manifestation. Some patients develop a rapidly progressive disseminated infection involving the bloodstream, lungs, central nervous system, and skin (Figure 3). This is associated with 25–35% mortality despite aggressive therapy.35 Of concern are reports that 20–60% of VGS are penicillin resistant at some institutions.31, 36 Beta-hemolytic streptococci also cause infections in neutropenic patients, but less often than VGS.37
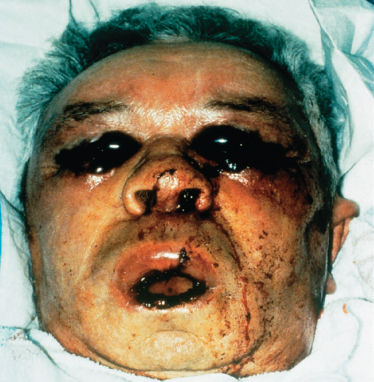
Figure 3 Invasive infection caused by α-hemolytic (viridans) streptococci. Note the hemorrhagic nature of the lesions in this patient with thrombocytopenia.
The enterococci colonize the lower intestinal tract.38, 39 Common sites of infection are the bloodstream, urinary tract, and intra-abdominal infections. Enterococcus faecalis is the predominant species accounting for 60–70% of isolates. Most E. faecalis strains are susceptible to penicillin, ampicillin, and vancomycin although these agents may lack bactericidal activity. Consequently, combinations of these agents with aminoglycosides are recommended for serious enterococcal infections. Enterococcus faecium isolates often express high-level resistance to the aminoglycosides, ampicillin, and vancomycin-resistant enterococcus (VRE). Risk factors for infection with VRE include intestinal colonization and the use of antimicrobial agents with significant activity against anaerobes (metronidazole, clindamycin, and imipenem).40 The administration of vancomycin (both oral and parenteral) is also a risk factor. Established therapeutic options for VRE include linezolid, daptomycin, oritavancin, and quinupristin/dalforpristin.41–43 Combination therapy may be necessary. Less common but important Gram-positive pathogens include Bacillus spp., Corynebacterium spp., Micrococcus spp., and Stomatococcus mucilaginosus.44, 45 Nocardiosis is caused by several Nocardia spp. (Nocardia asteroides complex, Nocardia brasiliensis, and Nocardia otitidiscaviarum). The most common sites of infection are the lungs (70%) and soft tissue sites (16%).46 Establishing a specific microbiologic diagnosis is of paramount importance as the differential diagnosis is wide. Trimethoprim/sulfamethoxazole remains the backbone of therapy although it is often combined with the carbapenems, tetracyclines, or aminoglycosides. L. monocytogenes is primarily acquired by the consumption of raw milk or products (cheese) made from raw milk. Bacteremia (75%) and meningoencephalitis (20%) are the most common manifestations.47
Immunoglobulins play an important role in the immune systems response to various infections by either opsonization or complement activation. Impaired humoral immunity leads to increased susceptibility to infections caused by S. pneumoniae and other encapsulated organisms.37 The widespread use of the conjugate pneumococcal vaccine has led to a reduction in the frequency of pneumococcal disease especially in pediatric populations.
Gram-negative bacteria
The intestinal tract serves as an important source of infection in neutropenic patients with the predominant pathogens being enteric Gram-negative bacilli. Escherichia coli, Klebsiella spp., and P. aeruginosa remain the three primary pathogens.13, 48 Other Enterobacteriaceae (Citrobacter spp., Enterobacter spp., Proteus spp., and Serratia spp.) are less common. Despite a decline in the frequency of Gram-negative infections due to antibacterial prophylaxis, the proportion caused by non-fermentative Gram-negative bacilli (NFGNB) such as P. aeruginosa, Stenotrophomonas maltophilia, and Acinetobacter spp. has increased.49 P. aeruginosa is the most frequently isolated and the most virulent NFGNB and causes between 15% and 20% of Gram-negative infections. It is also the most common Gram-negative organism isolated from polymicrobial infections.16 Infections caused by S. maltophilia are being documented more often in patients with hematologic malignancies and in HCT recipients.50, 51 The switch from trimethoprim/sulfamethoxazole (TMP/SMX), which is active against S. maltophilia, to the fluoroquinolones, which generally are not, as preferred agents for prophylaxis, may account for this increase. Other infrequent but important NFGNB include Achromobacter spp., Alcaligenes spp., and non-aeruginosa pseudomonads such as Pseudomonas putida and Pseudomonas fluorescens. Bacteremia is the most common site of infection followed by pneumonia and urinary tract infection. Fever is often the only manifestation of infection. Other manifestations such as ecthyma gangrenosum are uncommon (Figure 4). Polymicrobial infections and infections that are complicated by deep tissue involvement (pneumonia, neutropenic enterocolitis (NEC), and perirectal infections) are associated with greater morbidity and mortality.16, 52 The emergence of resistance to β-lactam agents and carbapenems is of great concern.53–55 Some organisms, especially P. aeruginosa and Acinetobacter spp., have become multi-drug resistant.56, 57 Very few options to treat such organisms (colistin, tigecycline, and combination regimens) currently exist, and very few novel agents are in the developmental pipeline.58, 59 Many institutions now conduct surveillance studies in high-risk patients looking for fecal colonization with VRE, P. aeruginosa, extended spectrum beta-lactamase (ESBL) producers, and carbapenem-resistant Enterobacteriaceae (CRE), because positive surveillance cultures often predict subsequent infection.60–63
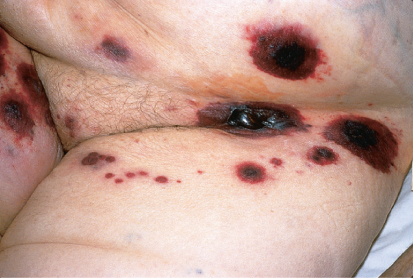
Figure 4 Multiple skin lesions (ecthyma gangrenosum) in a patient with Pseudomonas aeruginosa bacteremia.
Impaired cellular immunity is a risk factor for legionellosis. The most common Legionella species causing infection is Legionella pneumophila. Hospital water systems often harbor Legionella spp. and many cases of hospital-acquired legionellosis can be traced to such sources.64 Pneumonia is the most common manifestation. The detection of urinary antigens or recovery of the organisms on special media is required to make a specific diagnosis. The fluoroquinolones and macrolides are used most often for treatment and there appears to be no advantage to using them in combination. Non-typhoidal Salmonella and Campylobacter spp. infections are also seen with increased frequency in patients with impaired cellular immunity.65
Cell-mediated immunity plays an essential role in the control of mycobacterial infections. The association of tuberculosis and Hodgkin’s disease or hairy cell leukemia has been well established.66 Tuberculosis can precede the diagnosis of these malignancies, occur concomitantly, or develop during or after their treatment. Pulmonary infection that produces fever, cough, and weight loss is common. Diffuse pulmonary infiltrates with or without mediastinal enlargement are the most common radiographic findings. Nontuberculous mycobacterial infections are less common.67, 68 They produce pulmonary infections, lymphadenitis, SSSIs, catheter-related infections, and disseminated disease. The species isolated most often are Mycobacterium avium-intracellulare, Mycobacterium abscessus, Mycobacterium chelonae, Mycobacterium fortuitum, Mycobacterium kansasii, and Mycobacterium marinum. Prolonged, multiple drug therapy is usually administered for progressive infection.69
Anaerobes
Anaerobes are frequently involved in deep-seated, polymicrobial infections such as abdominal/pelvic abscesses, NEC, peri-rectal infections, complicated SSSIs, and pneumonia.52, 70 The organisms isolated most often include Peptococcus spp., Fusobacterium nucleatum, Bacteroides spp., and Prevotella spp.71 Clostridium difficile infection (CDI) is the leading infectious cause of diarrhea in cancer patients in whom there is increased severity of illness, higher mortality, and an increased risk of relapse and complications. Newer diagnostic tests are available including polymerase chain reaction (PCR)-based assays.72 Metronidazole is now considered inferior to vancomycin for the treatment of CDI.73 Agents such as rifaximin and nitazoxamide have been used with limited success.74–76 Fidaxomycin is an oral macrocyclic antibiotic that has been recently approved by the FDA for the treatment of CDI in adults, especially those individuals at greatest risk for relapse.77, 78 Fecal transplants have also been used successfully in this setting and appear to be promising.79 Preventive measures include strict enforcements of infection control practices, appropriate antimicrobial usage, and improved environment cleaning methods.
Fungal infections
Prolonged neutropenia (>7 to 10 days) is a key risk factor for the development of invasive fungal infections (IFIs). The most common causes of IFIs in neutropenic patients are Candida spp. and Aspergillus spp.80 The epidemiology of IFIs has changed substantially over the past 10–15 years.81, 82 Before the availability of agents such as fluconazole, invasive candidiasis was quite common with Candida albicans being the predominant species. The routine use of azole prophylaxis has led to a decrease in the frequency of candidiasis with manifestations such as oro-pharyngeal candidiasis (thrush), esophagitis, and chronic systemic (hepatosplenic) candidiasis becoming almost of historical interest. Candidemia, often catheter-related, is now the most common manifestation, with the alimentary tract being the predominant portal of entry. Recent studies have demonstrated a major shift from C. albicans to non-albicans Candida spp. (Candida glabrata, Candida tropicalis, Candida krusei, and Candida parapsilosis).82–84
There are no characteristic physical signs and symptoms of disseminated candidiasis. Often, the only indication is persistent fever and a gradual worsening of the patient’s clinical condition. Some patients have ocular infection causing blurred vision, pain, scotomata, or loss of visual acuity. Nearly 10% of patients develop characteristic erythematous macronodular skin lesions (Figure 5). A chronic form of Candida infection known as chronic disseminated candidiasis has been described, usually after neutrophil recovery. Patients remain febrile and debilitated with substantial weight loss. Symptoms including right upper quadrant or shoulder pain may appear. Alkaline phosphatase levels are usually elevated. Hepatosplenomegaly may be detected. Imaging of the liver and spleen reveals multiple lesions. Approximately 90% of the patients respond to appropriate antifungal therapy.62 This type of infection has virtually disappeared from institutions where azoles or echinocandins are used for antifungal prophylaxis. Treatment should be guided by in vitro antifungal susceptibility data.85 Breakthrough C. parapsilosis fungemia may develop during echinocandin therapy, as may C. glabrata infection during azole therapy. Echinocandin resistance among C. glabrata isolates appears to be increasing.86 C. krusei isolates are resistant to fluconazole. The mortality associated with systemic candida infections is ∼40%. Guidelines for the management of candidiasis have been published by various societies.87–90
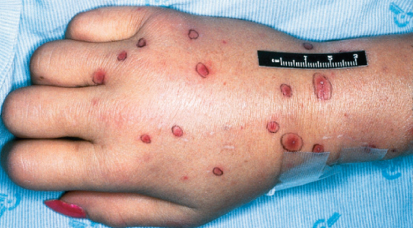
Figure 5 Characteristic macronodular cutaneous lesions in a patient with acute leukemia and disseminated Candida krusei infection.
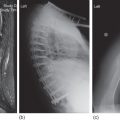
Aspergillosis is also frequent in patients with persistent neutropenia. Other risk factors include prolonged high-dose corticosteroid therapy, graft-versus-host disease (GVHD), repeated cycles of neutropenia, respiratory viral infections, and advanced age.91 The most common pathogen is Aspergillus fumigatus.92 Infection is usually acquired by inhalation of spores. Outbreaks of aspergillosis associated with construction within or adjacent to the hospital have occurred. More than 70% of infections involve the lungs (IPA, invasive pulmonary aspergillosis), and approximately 35% of patients have hematogenous dissemination to other organs.93 Often, the only evidence of infection is prolonged fever with pulmonary infiltrates that fail to respond to antibacterial therapy. High-resolution CT scanning of the lung is helpful in the early diagnosis of aspergillosis.94, 95 Characteristic findings in early stage disease are multiple nodules with a halo of surrounding ground-glass attenuation that represents hemorrhage surrounding a region of pulmonary infarction (Figure 6).94–96 As healing occurs, the infarcted tissue becomes necrotic and retracts from the viable tissue leaving an air crescent. Aspergillus sino-orbital infection is being diagnosed with increasing frequency in patients with acute leukemia and in HCT recipients, accounting for at least 15% of cases of aspergillosis (Figure 7). Infections may erode through the base of the skull and invade the brain or cause destruction of the paranasal and facial structures and the eye. A localized form of aspergillosis has been described in association with intravascular catheters.97 Aspergillus spores may be deposited at the time of insertion or may be impregnated in materials used for catheter dressings. These infections are potentially serious because they can disseminate.97, 98 Skin lesions, manifested as sharply defined black eschars, occur in about 5% of patients with disseminated infection. Voriconazole is the preferred agent for the treatment of invasive or disseminated aspergillosis. Posaconazole and lipid preparations of amphotericin B are used more often for salvage therapy or when voriconazole intolerance occurs. Combination therapy with a triazole and an echinocandin may be useful for salvage therapy of invasive aspergillosis.85, 90, 99, 100 A recent randomized trial has shown higher survival in patients with invasive aspergillosis treated with voriconazole and anidulafungin than those treated with voriconazole monotherapy.101
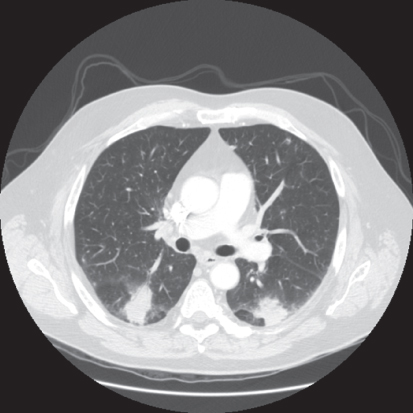
Figure 6 Rounded pulmonary lesions with surrounding halo, compatible with invasive pulmonary aspergillosis.
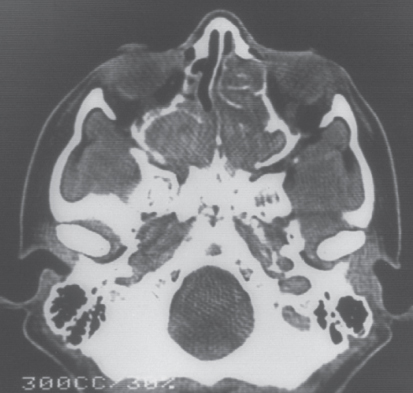
Figure 7 Pansinusitis caused by Aspergillus spp. in an allogeneic bone marrow transplant recipient with persistent fever.
Cryptococcosis is caused by two sibling species, C. neoformans and Cryptococcus gatti. The primary site of infection is the lung, which follows the inhalation of spores. Dissemination usually involves the central nervous system (meningoencephalitis and cryptococcoma). Fever and meningeal symptoms are common. Cerebrospinal fluid (CSF) abnormalities include raised opening pressure, lymphocytosis, elevated proteins, and low glucose levels. Cryptococcal antigen is also detected in the CSF and serum in most cases. Induction therapy with amphotericin B or its lipid formulations plus 5-fluorocytosine followed by maintenance therapy with fluconazole is the current standard of care.102 Histoplasmosis and infections caused by other endemic fungi are less common.8 They usually cause pulmonary, CNS, or disseminated infection and should be considered in the differential diagnosis in endemic areas. Pneumonia caused by P. jiroveci has traditionally been associated with impaired cellular immunity, which results in the reactivation of dormant infection.103, 104 The clinical presentation is usually subacute. Clinical features include fever, a non-productive cough, and progressive dyspnea. The most common CT findings are diffuse bilateral ground glass pulmonary infiltrates with apical predominance and peripheral sparing.105 The diagnosis is often made by demonstrating the organisms on respiratory specimens including biopsy tissue using stains such as methenamine silver or toluidine blue (Figure 8). Staining methods have been supplanted by sensitive molecular techniques including semi-quantitative or quantitative PCR.106, 107 High-dose TMP/SMX remains the agent of choice for prophylaxis and treatment. Alternative agents include pentamidine, atovaquone, clindamycin plus primaquine, and dapsone plus trimethoprim.108
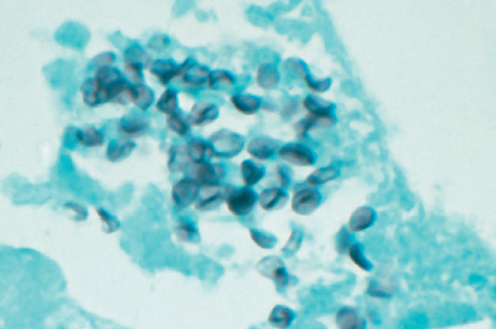
Figure 8 Gomori methenamine silver (GMS) stain from a bronchoalveolar lavage specimen demonstrating multiple organisms in a patient with Pneumocystis carinii pneumonia.
Other opportunistic fungi
Mucormycosis (zygomycosis) is still relatively uncommon with the most common pathogens being Rhizopus spp., Mucor spp., Lichtheimia (formerly Absidia) corymbifera, Rhizomucor spp., and Cunninghamella bertholettiae. Invasive pulmonary infection is the most common form of infection. Some patients develop sinopulmonary, rhinocerebral, gastrointestinal, cutaneous, or disseminated infection.109, 110 The clinical presentation of mucormycosis is often indistinguishable from aspergillosis. Mucormycosis should be considered in patients who are immunosuppressed and who develop sinusitis or IFI after prolonged exposure to voriconazole, which is not active against these organisms.111–113 Early diagnosis and administration of antifungal therapy is critical along with surgical debridment of infected tissue. Control of underlying diseases and reversal of risk factors when feasible is also of paramount importance.85, 114 Amphotericin B products and posaconazole are the only antifungal agents with reliable activity against Mucorales.115 Isavuconazole has also just been approved for the treatment of mucormycosis. Lipid preparations of amphotericin B are preferred for initial therapy, and there is some evidence to suggest that combining these preparations with an echinocandin might be beneficial.116 Therapy is often switched to posaconazole when feasible. Trichosporon beigelii can cause disseminated infection particularly in patients with severe neutropenia. A variety of skin lesions have been described and occur in approximately 30% of patients. Portals of entry include the gastrointestinal tract, respiratory tract, and intravenous catheter sites. Fusarium spp. have emerged as significant pathogens in patients with neutropenia.117 Localized infections of the lung, sinuses, and skin occur, but most patients have disseminated infection. Cutaneous and subcutaneous skin lesions are frequent.118 Similarly to Aspergillus spp., these organisms invade blood vessels, causing thrombosis and infarction. Usually Fusarium spp. can be isolated readily from blood culture or tissue specimens. It may be difficult to distinguish Fusarium from some other fungi on histopathologic examination. Recovery from this infection depends on resolution of neutropenia, and currently available antifungal agents are at best only marginally effective.119, 120
Various other fungi cause significant infection in neutropenic patients. These organisms include Blastoschizomyces capitatus, Scedosporium spp., Geotrichum candidum, and Malassezia furfur.121 The majority of patients who are infected have hematologic neoplasms, especially acute leukemia.122
Viral infections
Viral infections are uncommon in patients with neutropenia unless they are HCT recipients. Herpes viruses are identified most frequently, especially herpes simplex viruses (HSV), varicella-zoster virus (VZV), and CMV. Most adults are HSV seropositive, and reactivation can occur in ∼60–80% of patients undergoing HCT or intensive chemotherapy for hematologic malignancies. Reactivation generally occurs while patients are still severely neutropenic with oral mucositis/ulceration being the most common manifestation. Esophagitis indistinguishable from Candida esophagitis occurs occasionally. Encephalitis and dissemination are uncommon. HSV prophylaxis is recommended in patients undergoing HCT or remission induction therapy for leukemia.123 Reactivation of latent VZV also occurs and prophylaxis to prevent recurrence of VZV infection in seropositive patients is recommended for the first year following allogeneic HCT.124, 125 Community respiratory viruses, including RSV, influenza A and B viruses, parainfluenza viruses, human metapneumovirus, human coronaviruses, and human rhinoviruses, are common among HCT recipients and patients with acute leukemia in whom upper respiratory tract infection can progress to pneumonitis, which is associated with substantial morbidity and mortality.126 Testing for respiratory viruses is recommended in high-risk patients. Specimens include nasopharyngeal swabs, washes, or aspirates, tracheal aspirates, and bronchoalveolar lavage specimens. Optimum treatment for most of these viral infections, except for influenza viruses, remains to be determined. Ribavirin therapy for upper respiratory infection with RSV deters progression to pneumonia and may improve overall outcome in HCT recipients.127
Reactivation of EBV may occur after HCT or following chemotherapy with purine analogs such as fludarabine. EBV infection may be responsible for Richter transformation or development of Hodgkin disease in patients with chronic lymphocytic leukemia. In recipients of HCT or solid-organ transplants, uncontrolled proliferation of EBV infected B cells may occur, producing post-transplant lymphoproliferative disorders (PTLDs). In younger patients, a mononucleosis-like syndrome is a common presentation. Fever, sore throat, and lymphadenopathy are typical findings. Dissemination can occur from localized nodular lesions and can be fulminant and rapidly fatal. It is important to monitor high-risk patients (umbilical cord blood transplants, haploidentical transplants, and T-cell-depleted transplants) for EBV DNA viral load using PCR assays. Rituximab is recommended for preemptive therapy or the treatment of PTLD.128, 129
HHV-6 is being recognized as an important pathogen in HCT recipients.130, 131 Serologic reactivation accompanied by specific manifestations, including fever, rash, pneumonitis, hepatitis, myelosuppression, and neurologic dysfunction, have been described in recipients of bone marrow, kidney, and liver transplants. HHV-6 viremia is not necessarily associated with increased mortality, and routine screening is not necessary.132, 133 Ganciclovir and foscarnet inhibit viral replication, and therapy with these agents may be useful in patients with severe infections. Immunotherapeutic prevention and treatment strategies are being developed.134, 135
CMV infection occurs frequently in immunocompromised patients, especially those with impaired cellular immunity. It is a common complication in patients undergoing allogeneic HCT and has a negative impact on overall survival after transplantation.136 CMV seropositivity in HCT recipients is a major risk factor. Without prophylaxis, 50–80% of seropositive patients undergoing HCT reactivate latent infection. CMV seropositivity, in both the HCT recipient and the donor, has an impact on the subsequent development of CMV end organ disease.137 Other risk factors for CMV infection include total body irradiation, umbilical cord blood transplantation, the use of T-cell-depleted stem cells, treatment with purine analogs (fludarabine and cladribine), and monoclonal antibodies against CD20 (rituximab), CD52 (alemtuzumab), GVHD, and advanced age.138
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree