Janet R. Gilsdorf
Infections in Asplenic Patients
After its discovery, which occurred sometime during the dawn of human dissection, the true role of the spleen in health and disease remained a mystery for many generations. At the time of Galen, the spleen was thought to be important in removing “black choler” from the body; black choler (or black bile), according to Hippocrates, was one of the four humors thought to regulate bodily functions. The spleen and its various secretions were also implicated in a number of emotional imbalances. Excess black choler due to splenic failure was thought to be the cause of melancholy, whose name is derived from the words for “black” and “bile.” Further, a person described as “splenetic” was hot-tempered or hasty in judgment and “venting one’s spleen” suggests a burst of pent-up anger. Black choler, a cold humor, was believed to counteract the effects of the two hot humors (blood and phlegm). Although the fourth humor, yellow bile, was known to be excreted by the gallbladder into the intestine, the mode of excretion of black bile was not known to the ancients.1
More recently, the spleen has been recognized for its important role in protecting vertebrates from infection. It represents approximately 25% of the total lymphoid mass of the human body and contains half the body’s monocytes and immunoglobulin-producing B lymphocytes.2 Although not vital to human survival, the spleen plays an important role in the host’s ability to combat both invasive bacteria, particularly those possessing polysaccharide capsules, and intraerythrocytic parasites.
Anatomy of the Spleen
The embryonic precursor of the spleen is the mesenchyme of the dorsal mesogastrium, and the splenic red and white pulp are developed by the sixth month of fetal life. Located normally posteriorly in the left side of the peritoneal cavity, the spleen sits below the diaphragm and its hilum, which contains the splenic artery and vein, is in close proximity to the tail of the pancreas. The normal organ is roughly the size of the patient’s fist and weighs between 80 and 200 g in adult males and 70 and 180 g in adult females, but it can quickly enlarge in response to infection or inflammation. Aerated blood enters the spleen from the aorta via the splenic artery and short gastric arteries and, like the thymus, the spleen possesses only efferent lymphatic vessels. The normal spleen, which receives approximately 6% of the cardiac output,3,4 is surrounded by a fibromuscular capsule.
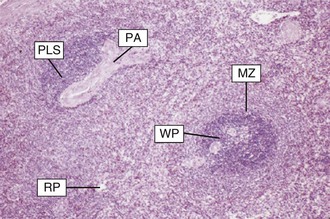
The spleen consists of three anatomically distinct zones: red pulp, white pulp (seen as white nodules on the cut surface), and marginal zone (Fig. 316-1). Red pulp, the largest component of the spleen, is composed of a complex network of endothelium-lined venous sinuses and the Billroth cords, which contain fibrils, connective tissue, and large numbers of macrophages. White pulp, made up of islands within the red pulp that are composed of reticular structures that surround penicillary arterioles, contains primarily T lymphocytes within the periarteriolar lymphoid sheath, as well as fewer B lymphocytes and natural-killer lymphocytes. Lymphoid follicles arise within this sheath and, on immunologic stimulation, the activated follicles form germinal centers similar to those seen in reactive lymph nodes. Located at the intersection of the red pulp and white pulp, the marginal zones are composed primarily of B cells (including memory cells) but also contain both T cells and antigen-presenting cells such as Toll-like receptor-bearing macrophages or dendritic cells. Thus, white pulp and the marginal zones, where antigen-presenting cells, bacterial antigens, and T and B lymphocytes are in close proximity, are sites of considerable importance in the coordinated immune response to circulating antigens.5
Function of the Spleen
The contribution of the spleen to controlling overwhelming infection was first recognized in 1952 by King and Schumacker,6 who reported life-threatening bacterial infections in splenectomized infants. Since then, the mechanisms by which the spleen combats bacterial infection, such as filtration, phagocytosis, and opsonization of bacteria, as well as regulation of inflammatory responses, have been carefully elucidated. The percentage of CD8+ T cells is higher in the spleen than in the peripheral blood, leading to an inverse splenic CD4/CD8 ratio. Further, both CD4 and CD8 cell populations in the spleen show a higher number of activated cells and the splenic CD8+ T cells show a more differentiated cytotoxic CD27−CD45RA+ memory phenotype.7 The multiple types of immune cells in close approximation within the spleen engage in complex interactions; are subject to the varied effects of autocrine and paracrine signaling; and migrate into, within, and out of the spleen—all contributing to the extraordinary repertoire of innate and adaptive immune responses important in protecting the host against bacterial and parasitic infections.
Regulation of Inflammation
The impact of the spleen on inflammation is evident by the increased production of pro-inflammatory cytokines during sepsis, which is, in part, mediated by nicotinic acetylcholine receptors in the spleen.8 In splenectomized mice, nicotine administration, which mimics vagus nerve stimulation, results in increased tumor necrosis factor (TNF) production and lethality from polymicrobial sepsis. In addition, TNF has been shown to be upregulated following splenectomy9 and, thus, removal of the TNF and cholinergic anti-inflammatory regulatory pathways may contribute to the inflammatory storm seen during postsplenectomy sepsis. Further, cytokines produced by splenic macrophages appear to modulate the febrile response to bacterial components such as lipopolysaccharide (LPS).10
Filtration and Clearance
The red pulp serves as a filter that provides grooming (antibody removal), pitting (intracellular material removal), and culling (cell destruction) functions to clear the blood of debris. As peripheral blood percolates through the splenic sinusoids, damaged cellular elements, senescent erythrocytes, cell-associated antibodies, circulating unopsonized bacteria, erythrocytes harboring malarial or babesial parasites, and foreign particles are removed, and platelets, erythrocytes, and iron are sequestered. The major defect associated with infectious risk of patients with asplenia or hyposplenia is impaired clearance of poorly opsonized particulate antigens such as bacteria.11
Adaptive Immunity
After exposure of the host to microbial antigens (e.g., through vaccination or bacteremia), T cell–dependent B-cell activation in the spleen begins through antigen interactions with T-helper lymphocytes, stimulating B lymphocytes to rapidly differentiate into either antigen-presenting cells or IgM-producing plasma cells. Following activation in the marginal zone, B and T cells, along with dendritic cells, are attracted to the periarteriolar lymphoid sheath of the splenic white pulp by cell-specific chemokines, where they facilitate T cell–dependent B-cell responses. Activated B cells undergo further maturation and clonal expansion in the splenic germinal centers, where, through contact with activated T cells, they undergo isotype switching into plasma cells capable of producing high-affinity antibodies or switched memory B cells.12 Antigen-specific plasma cells that have differentiated in the white pulp lodge in the red pulp in close proximity to macrophages, where antigenic sampling occurs. This process is mediated by upregulation of CXC-chemokine receptor 4 (CXCR4), which binds CXC-chemokine ligand 12 (CXCL12) expressed in the red pulp.13 Generation of IgM and IgG2 antibodies against polysaccharide antigens, which represent largely T cell–independent responses, occurs in the marginal zone of the spleen and is profoundly compromised by splenectomy.14
Innate Immunity
The marginal zone of the spleen is responsible for trapping and processing circulating antigens in the course of peripheral blood circulating through its rich beds of macrophages. Marginal zone macrophages are proficient in processing carbohydrate antigens through lectin receptors on their surfaces and through their scavenger activities. Unlike other B-cell lineages, marginal zone B cells, characterized as IgM+IgD+CD27 memory cells, develop during ontogeny and mutate their immunoglobulin receptors during the first years of life without prior engagement in an immune response. On stimulation with thymus-independent polysaccharide antigens expressed by encapsulated bacteria, the prediversified IgM memory B cells of the marginal zone do not differentiate into switched memory cells, but rather represent an immediate innate immune defense, as opposed to a memory-dependent adaptive defense, against invading pathogens.15,16 Thus, in asplenic hosts, this polysaccharide-specific immune function is lost, placing the patients at increased risk of infection with polysaccharide encapsulated bacteria.
Hematopoiesis/Hemostasis
During the second trimester of fetal life, hematopoiesis actively occurs in the spleen and then wanes in the third trimester, although hematopoietic stem cells remain in the spleen through adult life. In the face of bone marrow failure, such as myelofibrosis, extramedullary hematopoiesis may occur in the spleen with the appearance of erythroid and megakaryocytic, and, to a lesser extent, myeloid precursors. Further, the spleen contributes to hemostasis through the production of factor VIII and von Willebrand factor.17
Types of Asplenia
Congenital Asplenia
Congenital asplenia is rarely seen as an isolated clinical entity. It is, however, more commonly associated with many types of cyanotic congenital heart disease with heterotaxy, previously known as Ivemark syndrome, and is characterized by bilateral right-sidedness in which the liver is central, both lungs have three lobes, and the spleen is reduced in size or absent.18 The degree of splenic function is variable in these children, dependent on the amount of splenic tissue present. In contrast, bilateral left-sidedness, characterized by polysplenia, dextrocardia, and other congenital cardiac defects, is not associated with splenic dysfunction.
Acquired Asplenia
The most dramatic and predictable loss of splenic function occurs following surgical splenectomy or splenic infarction secondary to sickled erythrocytes, which progressively develops over the first few years of life,19 or to other embolic or thrombotic events in the splenic vasculature. Surgical splenectomy may be indicated in patients with severe splenic trauma, patients with intractable immune-mediated thrombocytopenia or anemia, patients with spherocytosis, or to relieve the effects of hypersplenism in patients with splenomegaly. Accessory spleens, present in up to 15% of patients at autopsy, may contribute to persistent immune cytopenias following splenectomy. Partial splenectomy, with retention of splenic immune function, may be an option in some patients with splenic trauma,20 such as those who are hemodynamically stable and require fewer than two units of erythrocytes for resuscitation. The ability of either accessory spleens or residual splenic tissue to protect against severe infections in splenectomized patients is unclear, although overwhelming infections have been reported in both patients with accessory spleens and those with various amounts of residual splenic tissue.21 In patients with hemoglobinopathies, the greatest splenic dysfunction, which ultimately leads to splenic atrophy and asplenia, occurs in those with homozygous sickle cell anemia, followed sequentially by hemoglobin S/β-thalassemia major disease, hemoglobin SC disease, and hemoglobin S/β-thalassemia intermedia.
Acquired Hyposplenia
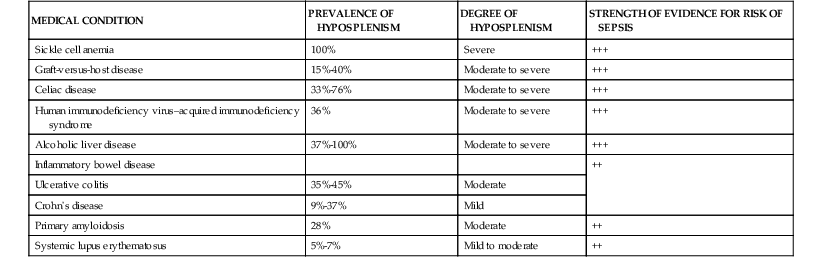
Functional hyposplenism related to impaired function of the splenic macrophage-associated Fc receptors may be associated with a variety of immunologic, rheumatologic, and inflammatory disorders (Table 316-1).22,23 Those at particular risk include patients with graft-versus-host disease following bone marrow/stem cell transplantation, patients with human immunodeficiency virus (HIV) infection, and those with celiac disease. In the late stages of HIV–acquired immunodeficiency syndrome (AIDS), atrophy of lymphoid follicles and T-cell depletion contribute to hyposplenism. Approximately a third of patients with celiac disease demonstrate impaired splenic function, and increased susceptibility to severe pneumococcal infection is seen among those with premalignant or malignant complications, concomitant autoimmune disorders, or thromboembolism, and among the elderly.24 Further, splenic function is also compromised by a variety of clinical therapies.25 For example, corticosteroid therapy impairs the affinity of Fc receptors on splenic macrophages for opsonized IgG to a greater extent than hepatic, pulmonary, and bone marrow macrophages. Further, intravenous immume globulin decreases the phagocytic function of the spleen temporarily by binding to Fc receptors and impeding its recognition of opsonized particles. Although ionizing radiation does not affect phagocytic cells, it has a particularly profound, albeit transient, dose-dependent effect on T and B cells.
Assessment for Splenic Function
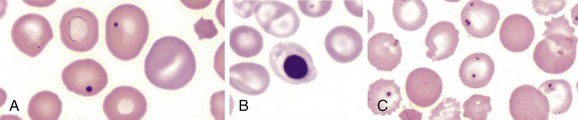
Splenic function is most simply assessed by examination of a peripheral smear for presence of the particulates normally filtered by the spleen. Such findings include red cells containing Howell-Jolly bodies (basophilic DNA remnants of red cell precursors), Pappenheimer bodies (abnormal iron granules), or presence of pitted or pocked red cells by phase-contrast microscopy (Fig. 316-2). The percentages of both pocked and pitted cells have been used to predict splenic function—normally individuals possess less than 2% of these cells, splenectomized patients possess up to 60%, and hyposplenic patients have intermediate values.26,27 Percentages of red cells possessing Howell-Jolly bodies, which are present when the spleen is largely nonfunctional, is somewhat less sensitive than percentages of pocked or pitted cells.26 With further validation, the number of IgM memory B cells, which correlates with functional splenic volume, may be a valuable parameter to assess splenic function.15,28
A variety of imaging studies may be useful in defining the presence, size, and, occasionally, function of the spleen. Abdominal ultrasonography and computed tomography (CT) scans can describe the size and position of the spleen, whereas radionuclide scintigraphy, which assesses phagocytic uptake of technetium-99m sulfur colloid, assesses anatomic features and correlates with phagocytic and immunologic function.28,29 In a study using radionuclide scintigraphy in patients older than age 18 years, all patients homozygous for sickle cell disease were asplenic and heterozygous patients showed increased anatomic splenic volumes but decreased functional splenic volumes. In patients with splenosis (i.e., ectopic splenic tissue implanted in the abdomen following traumatic splenic rupture) or patients with accessory spleens that were retained following splenectomy, the immunologic defects related to splenic function are difficult to access, but overwhelming sepsis has been described in patients with documented residual splenic tissue.21
The immunologic defects associated with asplenia or hyposplenia reflect impairment of the many normal immunologic functions of the spleen, including compromised clearance of circulating bacteria, decreased opsonization of bacteria, impaired phagocytosis, and dysregulation of inflammatory responses (Table 316-2).
TABLE 316-2
Immunologic Defects after Splenectomy
| DEFECT | MECHANISM |
| Decreased clearance of unopsonized bacteria | Depleted red pulp macrophages |
| Decreased clearance of cell-associated antibodies | Depleted red pulp macrophages |
| Decreased clearance of erythrocytes harboring malarial or babesial parasites | Depleted red pulp macrophages |
| Decreased processing of carbohydrate antigens | Depleted marginal zone macrophages |
| Decreased B-cell activation | Depleted marginal zone T cells |
| Decreased IgG2 and IgM antibodies | Depleted B cells in the germinal centers |
| Decreased CDC-chemokine ligand 12 (CXCL12) | Depleted CSC-chemokine receptor 4 (CDCR4) |
| Increased tumor necrosis factor (TNF) | Depleted splenic nicotinic acetylcholine receptors |
| Decreased recognition of pathogen-associated molecular patterns | Decreased Toll-like receptor networks in marginal zone macrophages |
| Decreased macrophage scavenger function | Decreased macrophage receptor with collagenous structure (MARCO) in marginal zone macrophages |
Epidemiology and Risk Factors of Sepsis in Asplenic Patients
Although sepsis related to reduced splenic function is relatively rare, when it occurs it often progresses rapidly to overwhelming, severe sepsis and may result in a fatal outcome. Risk factors for severe infection in asplenic patients include those related to age (both very young and old age); indication for, and time since, splenectomy; underlying diseases or medical conditions; and vaccine status (Tables 316-3 to 316-5). In a large Danish National Patient Registry30 the overall rate of any infection requiring hospital care among splenectomized patients was 7.7 per 100 person-years compared with 2 per 100 person-years in the general population. The relative frequency of sepsis associated with hyposplenism or splenectomy among various groups of patients, however, is difficult to accurately assess, because the many studies include patients of different ages, vaccination status, indications for splenectomy, and underlying diseases leading to hyposplenism. Nevertheless, the risk of sepsis is clearly dependent on the age of the patient (see Table 316-4). Overall, children younger than 16 years of age have the same risk of sepsis associated with splenectomy or asplenia as young adults, although infants are at higher risk. Among patients with hemoglobinopathies the risk is greater for children than adults. In adults, the risk for severe infection after splenectomy increases among patients older than 50 years of age, with the highest risk among those older than 70 years of age.31 The risk of sepsis is also dependent on the underlying disease associated with hyposplenism or the indication for splenectomy (see Table 316-5).2,30,32 Post-traumatic splenectomy is associated with a lower rate of sepsis than splenectomy due to hematologic abnormalities,31 possibly due to regeneration of splenic tissue after post-traumatic splenectomy. Such regenerated splenic tissue, however, is histologically and functionally different from normal spleen tissue and its ability to protect against sepsis may be limited.33
TABLE 316-3
Risk Factors for Hyposplenia/Postsplenectomy Sepsis
| RISK FACTOR | MECHANISM |
| Young age | Immune immaturity and naiveté |
| Old age | Immune senescence, comorbidities |
| Time since splenectomy | Risk at 0-90 days > 91-365 days > more than 1 yr |
| Indication for splenectomy: Splenectomy 2° to immune cytopenias > 2° to trauma > 2° to incidental | Unknown immune dysfunction associated with immune cytopenias |
| Hemoglobinopathies | Splenic infarction |
| Lack of appropriate vaccines | Impaired acquired immunity |
| Immunosuppressive drugs | Immunosuppression |
| Human immunodeficiency virus–acquired immunodeficiency virus | Immunosuppression |
| Amyloidosis/sarcoidosis | Splenic infiltration |
| Autoimmune diseases | Innate immunosuppression |
| Splenic radiation | Impaired splenic function |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree