Abstract
Several breast biopsy techniques are available for patients presenting with palpable and nonpalpable lesions. Current consensus guidelines recommend percutaneous biopsy as the initial tissue acquisition modality. Image guidance (ultrasound, mammogram, or magnetic resonance imaging) improves accuracy and precision in the biopsy of nonpalpable lesions. Here we review the biopsy techniques in most common use, as well as the indications for each approach.
Keywords
fine-needle aspiration (FNA), ultrasound-guided biopsy, stereotactic biopsy, magnetic resonance imaging–guided biopsy, open surgical biopsy
Currently there are multiple modalities available to obtain a tissue diagnosis for a patient who presents with a breast abnormality on physical examination or imaging. Due to advances in breast imaging and biopsy devices, the latter part of the 20th century saw a shift from open surgical biopsy to percutaneous biopsy as the initial tissue acquisition procedure. This paradigm shift was propelled by the multiple benefits and equivalent accuracy to open surgical biopsy offered by percutaneous biopsy. The International Breast Cancer Consensus Conference in 2009 and the American Society of Breast Surgeons have released consensus statements supporting the use of percutaneous biopsy as the initial method for tissue retrieval for breast disease diagnosis. A 2009 report by the Agency for Healthcare Research and Quality (AHRQ) comparing image-guided core needle biopsy to open surgical biopsy revealed an excellent safety profile for both modalities. Specifically, their comparative effectiveness review showed a less than 1% absolute incidence of adverse events for both biopsy techniques. However, it should be noted that the majority of patients who are referred for biopsy have benign lesions thus supporting the use of minimally invasive procedures over more invasive surgical procedures.
In the literature, percutaneous biopsies are also called minimally invasive breast biopsies. These biopsies include fine-needle aspiration (FNA) and core needle biopsies (CNB). Open surgical biopsies are sometimes referred to as excisional biopsies or incisional biopsies. Excisional biopsy indicates complete removal of the lesion, whereas incisional biopsy indicates removal of part of the lesion. For nonpalpable lesions, imaging modalities such as ultrasound (US), mammography, and magnetic resonance imaging (MRI) are useful adjuncts for identifying and localizing the lesion of interest.
The decision of when to perform a breast biopsy is dependent on the patient’s history, physical examination findings, and radiologic imaging. The main objective of the biopsy is to obtain a tissue diagnosis that can help dictate treatment and preoperative planning, if indicated. As a result, it is imperative to choose a biopsy technique optimizing the chances for an accurate diagnosis while minimizing costs, limiting patient discomfort, and reducing the need for a repeat procedure.
Fine-Needle Aspiration Biopsy
Fine-needle aspiration of breast masses is a safe and reliable diagnostic technique that can be performed in the office using local anesthesia. Compared with CNBs, FNA biopsies have less morbidity, lower cost, and faster turnaround time, with results often available immediately after aspiration. The recent trends away from FNA in favor of CNB can be attributed to the increasing importance of obtaining tumor biomarkers of estrogen receptor (ER), progesterone (PR), and human epidermal growth factor receptor-2 (HER2) status on needle biopsy samples for treatment planning of cancers. Nevertheless, there remains a role for FNA, primarily in the evaluation of palpable masses of low to intermediate suspicion for cancer and in resource-constrained settings where FNA may be more feasible than CNB.
Procedure
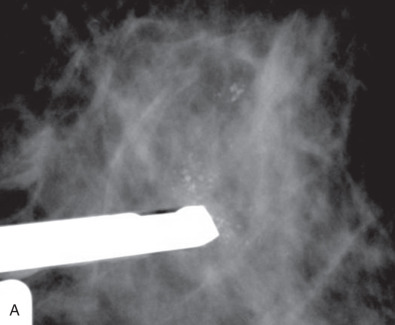
The skin overlying the palpable lesion is infiltrated with a local anesthetic. The breast lump is held relatively immobile, using one hand to gently but firmly stabilize the quadrant containing the mass. If the mass is not well defined, the procedure should be performed with ultrasound or mammographic guidance. FNA is facilitated using an “aspiration gun” to allow the operator to apply suction while maintaining the position of the needle tip in the mass ( Fig. 28.1 ). The procedure uses a 10- to 20-mL syringe and 22- or 25-gauge needle. The needle is inserted into the mass, and suction is applied to the syringe. Moving the needle into the lesion at various angles allows clumps of cells to be dislodged from the tumor, aspirated into the syringe, and submitted for cytologic examination. Local pressure is applied after the procedure. The procedure is well tolerated; hematoma is the most common complication but is rare.

Accuracy
The sensitivity of FNA ranges from 80% to 90% with a pooled sensitivity on meta-analysis of 92% (95% confidence interval 91%–93%). False-negative results have been shown to range from 6% to 11%. It is important to note that these rates do not differ markedly from results of CNB; in one study that directly compared FNA to CNB, the sensitivity of FNA was 93.8% compared with 90.1% for CNB. Furthermore, FNA has been shown to be more cost-effective than CNB without compromising diagnostic accuracy.
The diagnostic performance of the FNA technique relies more strongly on the skill and experience of the operator and cytologist than CNB. Thus the routine use of FNA requires local institutional expertise, including specialized training and close multidisciplinary communication among the cytopathologist, referring physician, and radiologist. False-positive results are unusual when the aspirated specimen is properly prepared and reviewed by a qualified cytopathologist. Thus lumpectomy may be safely performed on the basis of FNA confirmation of cancer. However, caution would dictate that mastectomy should not be undertaken without further tissue confirmation of malignancy due to the small risk of a falsely positive result.
False-negative results from FNA are much more common, and it must be emphasized that the absence of malignant cells in the aspirate does not rule out the presence of cancer. A recent meta-analysis reported that more than a quarter of all FNA samples may result in insufficient material for diagnosis. Thus any clinically or mammographically suspicious breast mass investigated with FNA where cytology is disconcordant with radiologic findings must be subjected to further diagnosis by means of CNB or surgical excision.
Cytopathology
The National Cancer Institute recommendation for the diagnosis of breast aspiration cytology has divided FNAB findings into five categories: C1 = unsatisfactory; C2 = no suspicious features; C3 = cells suspicious but probably benign; C4 = cells suspicious but probably malignant; and C5 = definitely malignant. Clinically, category C2 is defined as negative, and categories C3 through C5 are defined as positive, indicating additional workup or treatment ( Figs. 28.2 and 28.3 ).
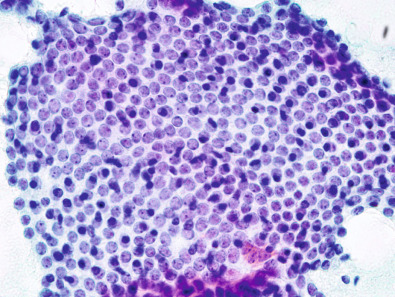
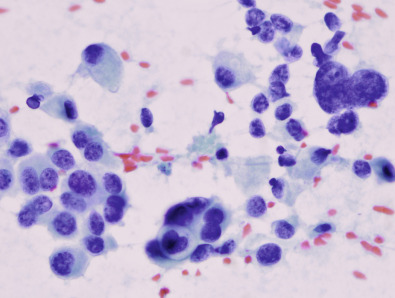
Biomarker evaluation for ER, PR, HER2, and other markers can often be performed on fine-needle aspirate by centrifuging the cellular material and performing immunohistochemical (IHC) staining on the resulting cell block. Several studies have shown that ER and PR can be successfully evaluated on FNA alone, and HER2 IHC testing as well as fluorescence in situ hybridization (FISH) have been performed. However, because of the loss of tissue architecture on a cellular aspirate, FNA cannot distinguish between in situ and invasive cancer. Because this determination is often critical to treatment planning, CNB may often be required to differentiate between these two diagnoses.
FNA remains the most cost-effective diagnostic procedure for the evaluation of breast masses in developing countries. As a procedure that is less invasive than CNB, there are clinical settings in which FNA can be considered a definitive procedure to confirm a diagnosis. However, FNA requires a more specialized expertise and coordinated multidisciplinary approach than CNB and thus may not be readily available at some centers. Radiologic concordance with cytologic findings is essential and this assessment should be performed as a routine component of FNA.
Direct Smear
Specimens for exfoliative cytologic analysis in the patient with suspected Paget disease of the breast may be obtained with direct smear of the weeping eczematoid lesion of the nipple. If the areola and surrounding skin are scaly and encrusted, a sterile glass slide can be used to scrape this area gently. The direct smear technique is simple and can be performed as an office procedure. The technique is rapid and inexpensive; however, histologic confirmation is required for definitive diagnosis. Comprehensive management of nipple cytology and secretions is reviewed in other chapters in this textbook.
Fluid Aspiration
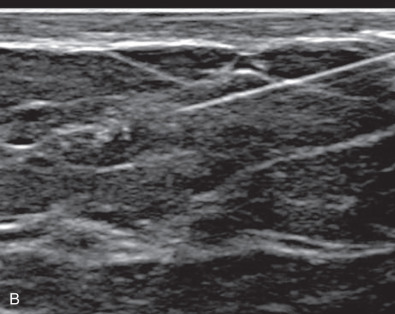
Fluid aspiration is indicated for those masses that on US evaluation are found to have a cystic component. Fluid from palpable breast cysts is simple to aspirate with a needle and syringe. If the cyst is not palpable, ultrasound can be used as a guide to direct the depth and location of the biopsy needle. The return of nonbloody fluid confirms the diagnosis of benign (nonproliferative) cystic disease, and, unless otherwise indicated, this fluid should not be submitted for cytologic examination. Bloody cystic fluid, on the other hand, is more likely to indicate malignancy and therefore should be examined cytologically, either by direct smear or after centrifugation of the aspirated contents. Cystic masses should not be palpable after aspiration because the walls of the cyst collapse and conform to the surrounding breast tissue. If fluid is not obtained or the mass persists, further workup is required. If the mass resolves and later recurs, clinical and mammographic evaluations are also indicated ( Fig. 28.4 ).

Core Needle Biopsy
Image-guided CNBs are currently the primary means by which a pathologic diagnosis is made for suspicious abnormalities identified by screening or diagnostic workups. Image guidance allows precise tissue sampling of the area of interest and ensures that the abnormality identified during diagnostic workup is correctly biopsied. Compared with surgical biopsies, image-guided biopsies have comparable sensitivity and specificity, are associated with fewer adverse events, have shorter recovery times, provide better cosmetic results, and are preferred by patients. Cost savings of image-guided biopsies are dependent on the specific equipment used but are considered to be significant compared with surgical excision. Finally, women who have cancer detected via image-guided biopsy are 15 times more likely to have their cancer treated with only a single surgical procedure. Biopsies may be performed via US, stereotactic, or MRI-guided approaches; strengths and weaknesses have been described with each approach ( Table 28.1 ). Digital breast tomosynthesis (DBT)-guided biopsies are also possible but are not currently performed with any frequency because of the recent implementation of DBT and limited availability of biopsy-capable devices.
| Biopsy Type | Indications | Advantages | Disadvantages |
|---|---|---|---|
| FNA | Palpable masses | Cost-effective compared with CNB and open surgical biopsy 80%–90% diagnostic accuracy Minimal patient discomfort Therapeutic for cyst aspiration Short turn-around time | Cannot distinguish between in situ and invasive diagnoses May require additional sampling by core needle or surgical biopsy for definitive histologic diagnosis Reliant on institutional cytologic expertise |
| CNB | Image-detected abnormalities | Less invasive than surgical biopsy Better cosmesis compared with surgical biopsy Women diagnosed with cancer on CNB have fewer surgical procedures Helpful in preoperative planning due to clip placement | Discordance in radiologic and pathologic findings warrants additional biopsies, which increase costs |
| Ultrasound-guided CNB | Preferred CNB approach Axillary lymph nodes | Flexibility of patient and operator positioning Real-time imaging and biopsy | Calcifications typically not visible on ultrasound |
| Stereotactic guided CNB | Calcifications and other lesions seen only on mammography | Only option available for most calcifications Specimen radiographs can confirm sample contains calcifications | Requires breast compression and ability to lie prone for duration of procedure Not feasible for some chest wall or subareolar lesions Breasts that compress to less than 1.5–2 cm may not be amenable to biopsy |
| MRI-guided CNB | Lesions only seen on MRI | Only option available for lesions seen only on MRI | Not feasible for some chest wall or subareolar lesions Requires contrast administration Most expensive CNB approach |
| Surgical biopsy | Lesions close to chest wall, nipple, or breast implant High-risk lesions on CNB (i.e., ADH, ALH) Discordant radiologic imaging and pathology results Inadequate tissue sampling by FNA or CNB | Most definitive of the three biopsy types | More likely to result in multiple surgical procedures if used as index biopsy More costly than CNB and FNA Often requires intravenous sedation Higher risk of postprocedure complications compared with FNA and CNB |
Several general principles apply to all forms of image-guided biopsy. First, before the start of the procedure, it is important to have a firm understanding of acceptable concordant and discordant pathology results. This aids in discussions with patients about potential outcomes and also ensures that all necessary noninvasive diagnostic steps are performed before the patient is subjected to a biopsy. Second, there are a large and steadily growing number of biopsy devices, clip deployment devices, and guidance systems. It is mandatory to fully understand the strengths and limitations of all biopsy equipment before use because failure to do so can result in increased procedure time, decreased accuracy, and need for additional open biopsies. Finally, deciding which image-guided modality to use should be based on the modality most likely to obtain a diagnostic tissue sample while also incorporating any special needs of the patient (i.e., inability to lay flat).
Complications related to image-guided biopsy procedures are usually uncommon and minor. The most common complaint from patients is soreness and bruising, especially the day after the procedure. Hematoma formation is common but usually small and self-limited. Antithrombotic usage is associated with an increased risk of nonclinically significant hematoma formation, thus the benefits of continuing antithrombotic therapy often outweigh the very low risk of severe bleeding. The risk of hematoma formation is associated with additional tissue sampling and smaller needle gauge. Patients are usually instructed to avoid any strenuous lifting or exercising after the procedure to avoid rebleeding. The likelihood of a vasovagal reaction has been reported to be as low as 1% and is more commonly seen when patients are positioned upright. Although image-guided biopsies are typically clean but not sterile, the risk of infection is extremely low. Among 40 studies including 25,688 procedures, the range of procedures with an infection was 0 to 2.91%. Finally, although injury to the regional structures including the development of a pneumothorax is possible, this risk should be negligible by maintaining a needle position parallel or near-parallel to the chest wall at all times. The reported risk of pneumothorax has been reported to be less than 1 in 2500 patients.
Ultrasound-Guided Biopsy
US is the preferred imaging-guided breast biopsy approach and is the only true real-time imaging approach. US is inexpensive, uses no radiation, requires no compression, does not require contrast, and takes less time than other image-guided biopsy approaches. Compared with FNA, the increased size of the tissue sample is associated with improved accuracy in some studies. Ultrasound is also the only image-guided modality capable of safely sampling axillary lymph nodes, although very deep nodes may be inaccessible.
Before starting a US-guided biopsy, the patient is scanned in a supine or anterior oblique position to identify the suspicious abnormality. A primary advantage of US-guided biopsies is the ability to position the patient and operator in the most comfortable orientation possible. The biopsy approach can be planned from any direction, and color Doppler can be used to avoid major blood vessels. Once the approach is decided, the site is cleaned and dressed in the usual fashion. The skin is anesthetized, and then under direct visualization anesthetic medication is administered along the proposed biopsy tract and around the abnormality to be biopsied. At any point during the procedure, if the patient complains of inadequate analgesia, additional medication can be administered. After a skin nick, typically an introducer needle with a coaxial sheath is advanced adjacent to the abnormality. The introducer needle is then replaced with the biopsy device.
Either a vacuum-assisted device or a spring-loaded device may be used, typically with an 11- or 14-gauge size. Smaller needles are associated with a decrease in diagnostic accuracy. Samples are obtained under direct visualization and can be obtained from multiple sites within the same abnormality to reduce tissue under sampling. In addition, if multiple adjacent abnormalities need to be sampled, they can often be biopsied through the same skin incision. After sufficient samples have been obtained, typically at least 5, a biopsy marking clip is placed in the center of the abnormality. The patient is then bandaged, and two orthogonal mammogram pictures are obtained to document the position of the biopsy site.
There are few disadvantages and complications unique to US-guided biopsies. Compared with stereotactic and MRI-guided biopsies, US-guided biopsies require a great deal of technical expertise and thus successful sampling and complication rates will be more highly influenced by the experience of the operator. US-guided biopsies are theoretically at greater risk for complications such as pneumothorax or implant rupture because the biopsy device is typically held freehand by the operator and not in a fixed position parallel to the chest wall. Ultrasound is rarely able to identify mammographically detected microcalcifications unless there is an associated mass. Abnormalities initially identified via MRI may undergo a second look US to determine whether they are visible and amenable to US-guided biopsy, but many small lesions or areas of vague enhancement will not be seen on US and must undergo MRI-guided biopsy.
Stereotactic Core Needle Biopsy
Stereotactic-guided biopsies are primarily performed for mammographically detected microcalcifications. Less common indications for stereotactic CNB (SCNB) include asymmetries, architectural distortions, and masses that are seen only on mammography without a US correlate. There are two primary units used in a stereotactic biopsy: prone or upright. With a prone unit the patient lies on her abdomen and her breast falls through a hole in the table with the stereotactic unit positioned underneath. A prone unit is preferred because it provides the greatest flexibility for the operator to plan the biopsy approach, and with the breast is positioned in a pendant fashion, it allows sampling of more posterior lesions. With an upright unit the patient is seated, and an add-on device is attached to a mammography unit. An upright unit is typically reserved for facilities that do not have access to a prone table or for patients who are unable to lie flat, typically because of back pain or respiratory difficulties.
Whether the patient is positioned prone or upright, the shortest distance to the abnormality is typically chosen as the direction of approach. Of note, a caudal-cranial approach is not possible with an upright unit because the patient’s bent legs will interfere with the biopsy device. A small windowed paddle is used to compression the breast in the area of interest. Images are taken and adjustments in the positioning of the paddle are made until the abnormality of interest is identified ( Fig. 28.5 ). A pair of stereotactic images is then obtained at +15 and –15 degrees to triangulate the lesion. Using the stereotactic software, the computer calculates X and Y coordinates as well as the Z depth. The breast is locally anesthetized, and the biopsy device is lined up with the calculated coordinates and fired into final position. Prefire or postfire images may be obtained and small adjustments to the needle can be made secondary to displacement from the administration of medication or patient motion. Vacuum-assisted samples are then obtained circumferentially or directionally as needed. Typically an 11- or 14-gauge biopsy device is used, and 6 to 12 samples are obtained. If calcifications were biopsied, the specimens can be radiographed to ensure that there was adequate tissue sampling; however, the goal of the biopsy is to sample the calcifications and not remove all of them. If needed, additional samples can be taken at the same site or adjustments to the position of the biopsy device can be made accordingly. A clip is then placed and imaged while the patient is still in place to confirm clip deployment. The patient is then bandaged, and two orthogonal mammographic images are obtained to document the position of the clip. This is especially important in the case of biopsied calcifications, which may be completely removed during the procedure.