Fig. 26.1
Schematic mechanism of two immunohistochemistry methods. Top: secondary antibodies and enzymes link to polymer molecule. Bottom: biotinylated secondary antibody and labeled streptavidine
Immunohistochemistry has wide application including research uses, diagnostic purposes, and prognostic and therapeutic aims. IHC is a nice technique for tracking of proteins and haptens, so it is used to define expression of specific genes at the level of proteins. It is also very useful in diagnostic pathology including definition of cellular lineage (epithelial, vascular, lymphoid, etc.) or subtyping of some specific lesions and malignancies such as malignant lymphomas. Prognostic and therapeutic applications have gradually become widely popular such as the definition of hormone receptor status of breast cancer (ER, PR, and AR) and oncogene products (e.g., Her2, EGFR, c-kit, etc.) which could be a part of guidelines for targeted therapy of the tumors.
26.2 Immunohistochemistry of Skin Tumors
26.2.1 Markers of Normal Skin
Skin tissue is composed of epidermal and adnexal components as well as mesenchymal dermal components. All epithelial cells in the epidermis, folliculosebaceous unit, and sweat glands reveal pan-keratin markers such as AE1/AE3 (Fig. 26.2a). Keratinized squamous cells and proliferative keratinocytes express cytokeratin (CK) 6/16, nonkeratinized squamous cells reacts with CK4/13, and basal keratinocytes exhibit reactivity for CK5/14/15 (Fig. 26.2b). Squamous cells in palm and sole are reactive for CK1/9/10 [1, 2]. Eccrine and apocrine glands comprise sweat structures of the skin. Normal eccrine glands show reactivity with CD7, CD20 (Fig. 26.2c, d), CEA, and S100, while apocrine glands exhibit immunostaining for CEA and GCDFP15 [3, 4]. Sebaceous glands exhibit reactivity for CK10 as well as EMA rimming cytoplasmic lipid vesicles (Fig. 26.2e) [5]. Normal melanocytes express S100, HMB45, and MART-1/melan-A but do not react with tyrosinase [6]. Langerhans cells are stained with CD1a (Fig. 26.2f), S100, langerin, and CD31 [7]. Displaying neurotactile differentiation, Merkel cells of normal skin are reactive for CK20, MOC-31, neurofilament, and CD56 [8–10]. Markers of the normal epidermal components are depicted in Fig. 26.3. The immunoprofile of normal skin components and respective cancers is summarized in Table 26.1.
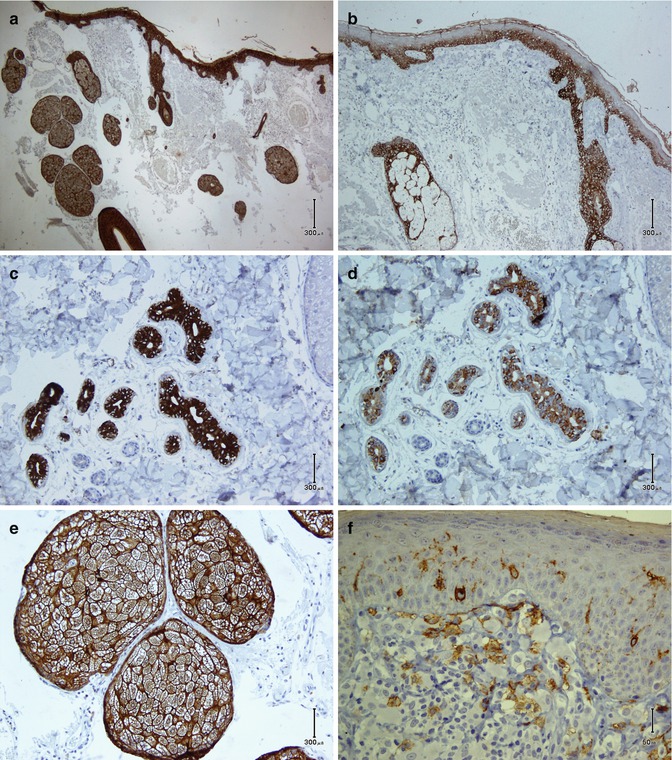
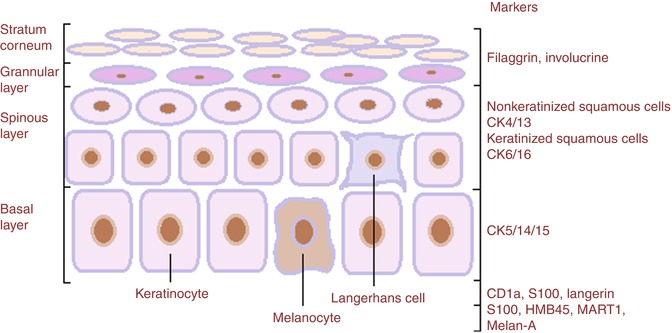

Fig. 26.2
Normal skin. (a) Pan-keratin of AE1/AE3 stains the epidermis, folliculosebaceous unit epithelium, and sweat glands. Basal keratinocytes are highlighted by CK5 (b). Sweat glands are immunostained by CK7 (c) and CK20 (d). EMA (e) reacts with sebaceous glands rimming cytoplasmic vacuoles, and CD1a highlights dendritic Langerhans cells in the epidermis (f)

Fig. 26.3
Immunohistochemistry antibodies in schematic normal epidermal components
Table 26.1
Immunoprofile of normal epidermis, folliculosebaceous, and sweat gland structures in comparison with respective tumors
Cell | Antibodies | Tumor | Markers |
|---|---|---|---|
Keratinocyte | CK6/16 | Squamous cell carcinoma | EMA, p63 |
Basal keratinocyte | CK5/14/15 | Basal cell carcinoma | BerEp4 |
Eccrine cell | CK7, CK20, CK5/14, CK1/10, CEA, S100 | Eccrine carcinoma | EMA, CEA, CD15, p63, S100 |
Apocrine cell | CEA, GCDFP15 | Apocrine carcinoma | EMA, CEA, CD15, p63, CA72.4, GCDFP15 |
Trichogenic cell | CK14/15/19 | Trichilemmal carcinoma | CEA, S100 |
Proliferating trichilemmal carcinoma | EMA, CD34 | ||
Sebaceocyte | CK5/14/15, CK8/18 | Sebaceous carcinoma | EMA |
26.2.2 Epithelial Tumors
Squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) are derived from the spinous layer and basal layer of the epidermis, respectively. Well-differentiated SCC expresses high molecular cytokeratin, while those with poor differentiation express low molecular cytokeratin. Cytokeratin, p63, and vimentin are present in the sarcomatoid variant of SCC [11]. EMA, one of the human milk fat globule proteins not expressed in normal keratinocytes, is expressed on malignant squamous cells. Basal cell carcinoma expresses BerEp4 (Fig. 26.4) but does not demonstrate reactivity with EMA and p63, distinguishing it from SCC [12].
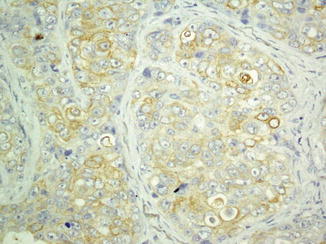

Fig. 26.4
Immunoreaction of basal cell carcinoma with BerEP4
26.2.3 Sweat Gland Tumors
Malignant eccrine tumors are distinct from benign eccrine tumors by displaying reactivity with EMA. Eccrine tumors display CEA, CD15, and p63 which are also common with apocrine tumors. Differentiating markers of apocrine tumors are TAG-72 (CA72.4) and GCDFP15 (Fig. 26.5) which are not expressed on eccrine tumors [4]. S100 is demonstrated in 50 % of eccrine tumors, but not in apocrine tumors. A remaining challenge is distinguishing primary eccrine carcinoma from metastatic carcinoma by immunoprofile of CK5/6 and p63 which are positive in eccrine carcinoma, but not in metastatic carcinoma [13]. Paget disease is an intraepidermal extension of neoplastic cells into the epidermis which shares similar histopathologic features with malignant melanoma and Bowen disease. Immunohistochemistry study can be a helpful method in differentiating these tumors as denoted in Table 26.2 [14]. CK20 and GCDFP-15 are useful markers in distinguishing primary and secondary perianal Paget diseases, respectively [15].
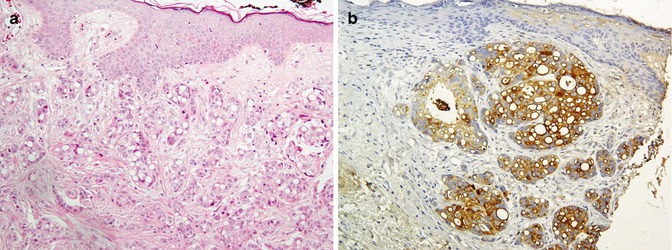

Fig. 26.5
Primary skin apocrine carcinoma (a) immunostained by GCDFP15 (b)
Table 26.2
Immunophenotype of mammary and extramammary Paget disease (PD), Bowen disease, and malignant melanoma
Makers | Mammary PD | Extramammary PD (apocrine carcinoma in situ) | Bowen disease (SCC in situ) | Melanoma (in situ) |
|---|---|---|---|---|
CK7 | + | + | − | − |
CEA | + | + | − | − |
CAM5.2 | + | + | − | − |
GCDFP15 | + | + | − | − |
MUC1 | + | + | − | − |
MUC5AC | − | + | − | − |
CA15-3 | + | − | − | − |
CA72.4 | − | + | − | − |
KA-93 | − | + | − | − |
CK5/6 | − | − | + | − |
S100/HMB45/MART | − | − | − | + |
26.2.4 Trichogenic Tumors
Tumors with trichilemmal differentiation display reaction with CK14/15/19, BerEP4, and p63 but do not react with EMA (except proliferating trichilemmal tumor), CEA, S100, CD15, CA72.4, HMB45, and GCDFP15 [3]. Trichilemmal carcinoma displays reactivity with CEA and S100, and proliferating trichilemmal carcinoma (malignant proliferating tumor) shows reactivity with EMA and CD34 [17]. Desmoplastic trichoepithelioma shares histopathologic similarities with infiltrating BCC and microcystic adnexal carcinoma. The immunoprofile of these tumors are demonstrated in Table 26.3.
Table 26.3
Immunoprofile of desmoplastic trichoepithelioma (DTE), infiltrating basal cell carcinoma (IBCC), and microcystic adnexal carcinoma (MAC)
Tumor | DTE | IBCC | MAC |
|---|---|---|---|
Panel antibodies | EMA, CK5/6, CD10 (stroma), CK15, CK20, p63, Bcl-2, BerEP4 | CK5/6, CD10 (epithelial), p63, Bcl-2, BerEP4, stromelysin-3, p53 | EMA, CK7, Ck5/6, CK15, p63, SMA |
26.2.5 Sebaceous Tumors
Sebaceous tumors exhibit reactivity with CK5/14/15, CK8/18, EMA, CD15, anti-adipophilin (ADP) and androgen receptor. CK15 is positive in sebaceoma but does not exhibit reactivity with sebaceous carcinoma [21]. Sebaceous tumors do not express CEA, S100, CA72.4, and GCDFP-15 in comparison with sweat gland tumors, which are positive for these markers [4, 22]. Sebaceous carcinoma is differentiated from BCC by showing reactivity for EMA (Fig. 26.6) and negative reaction to BerEP4, vice versa of BCC [23]. Proliferating markers are good markers to differentiate sebaceous adenoma from sebaceous carcinoma (Table 26.4).
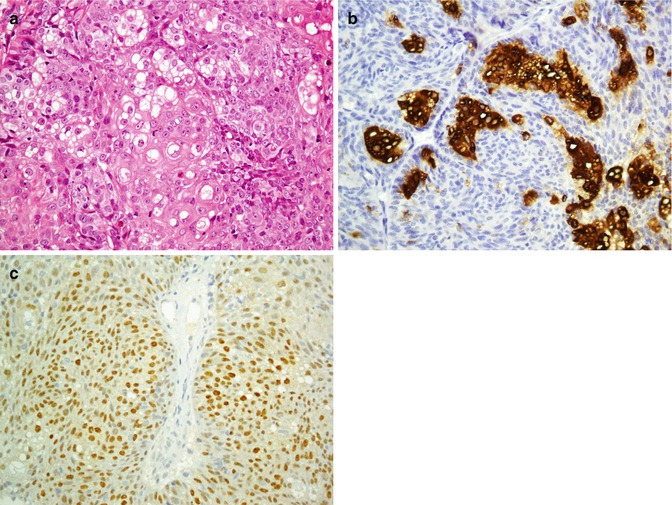

Fig. 26.6
Sebaceous carcinoma (a). Sebocytes are stained with EMA (b). Nuclear reactivity of tumor cells for androgen receptor (c)
Table 26.4
Immunoprofile of sebaceous adenoma (SA) and sebaceous carcinoma (SC)
Tumor | Ki-67 (%) | p53 (%) | Bcl2 (%) | p21 (%) |
|---|---|---|---|---|
Sebaceous adenoma | 10 | 11 | 56 | 34 |
Sebaceous carcinoma | 30 | 50 | 7 | 16 |
26.2.6 Melanocytic Tumors
Being a sensitive but a nonspecific marker of melanoma, S100 is a calcium-binding protein given its name because of solubility in 100 % saturated ammonium sulfate solution. Other S100-positive tumors include undifferentiated carcinoma, nerve sheath and glial tumors, adipose tumors, and histiocytic and Langerhans cell proliferations [26, 27]. Considering as highly specific marker of melanocytes, the gp100 group includes HMB-45 and MART-1/melan-A with 60 and 80 % sensitivity, respectively. Melanoma antigen recognized by T-cells-1 (MART-1) is a protein which serves as a potential target for cytotoxic T lymphocytes recognized by two monoclonal antibodies (mAbs), A103 and melan-A [28]. Desmoplastic/spindle cell variant of melanomas does not show reactivity with HMB45 and MART/melan-A. Instead, these melanomas are more reactive with S100, p75-NGF-R, and tyrosinase [29]. Small cell melanoma is another variant of the melanoma which could be distinguished from other small cell undifferentiated tumors of the skin and subcutaneous tissue by Abs panel (Fig. 26.7). The immunoprofiles of these tumors are summarized in Table 26.5.
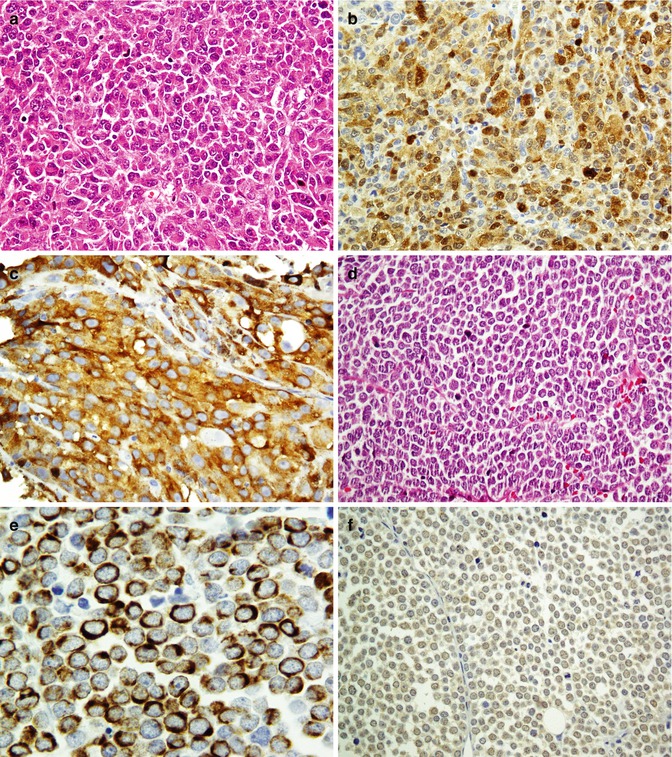

Fig. 26.7
Small round cell tumor in the skin. Malignant melanoma (a) reacts with S100 (b) and melan-A (c) antibodies. Merkel cell carcinoma (d) immunostained by CK20 as paranuclear dots (e) and shows weak reaction with CD99 (f)
Table 26.5
Immunopanel of small cell melanoma (SCM), Merkel cell carcinoma (MCC), small cell squamous carcinoma (SSCC), small cell eccrine carcinoma (SEC), peripheral neuroectodermal tumor/extraskeletal Ewing sarcoma (PNET/ES), lymphoma, rhabdomyosarcoma (RMS), and metastatic pulmonary small cell carcinoma (MPSC)
Panel antibodies | SCM | MCC | SSCC | SEC | PNET/ES | Lymphoma | RMS | MPSC |
|---|---|---|---|---|---|---|---|---|
S100/HMB45/MART | + | − | − | − | − | − | − | − |
CK20/CD56/SYN/CGN | − | + | − | − | − | − | − | − |
CK/EMA | − | − | + | + | − | − | − | + |
CD15/MOC31/TAG-72 | − | − | − | + | − | − | − | − |
CD99/CD56/SYN/CGN | − | + | − | − | + | − | − | − |
LCA/CD3/CD20 | − | − | − | − | − | + | − | − |
DES/MSA/MYG | − | − | − | − | − | − | + | − |
CEA/TTF-1 | − | − | − | − | − | − | − | + |
26.2.7 Prognostic Markers of Melanoma
Detection of BRAF p.V600E mutation by immunohistochemistry in melanomas could be used as a first step to identify patients with melanoma as candidates for BRAF inhibitors. Displaying by immunohistochemistry, melanoma progression is correlated with MERTK expression: highest in metastatic melanomas, followed by primary melanomas and nevi [32, 33]. Other prognostic markers correlated with melanoma progression and prognosis include MIB-1 (Ki-67), Bcl2, p53, p16, cyclin-D1, cyclin-D3, osteopontin, NM23, E-cadherin, beta-catenin, Wnt5a/frizzled, Cdc42, and CXCR4 [34–40].
26.2.8 Specific Mesenchymal Tumors of the Skin
Mesenchymal tumors are discussed in soft tissue tumors, but some tumors which are more seen in skin are discussed here. Kaposi sarcoma which originates from endothelial cells is an intermediate malignant potential vascular tumor of the skin positive for a highly sensitive and specific Ab called HHV8 latent nuclear antigen-1 [41]. Dermatofibrosarcoma protuberance is an intermediate tumor of fibrohistiocytic cell origin which is diffusely positive for CD34 (Fig. 26.8) and negative for factor XIIIa separate from dermatofibroma which is in reverse of DFSP (CD34−, factor XIIIa+) [42]. Considering it as a superficial variant of malignant fibrous histiocytoma, atypical fibroxanthoma is a fibrohistiocytic tumor exhibiting reactivity with vimentin, CD10, and CD99 (Fig. 26.9) [43]. Among tumors with smooth muscle differentiation, leiomyoma and leiomyosarcoma are reactive for SMA, desmin, and caldesmon similar to extracutaneous equivalents [44, 45]. Neurothekeoma (NTKs) is a distinctive neoplasm of the skin showing schwannian and neuroectodermal differentiation which typically labels with S100 (conventional variant), CD99, and NKI-C3 (cellular variant) [46].
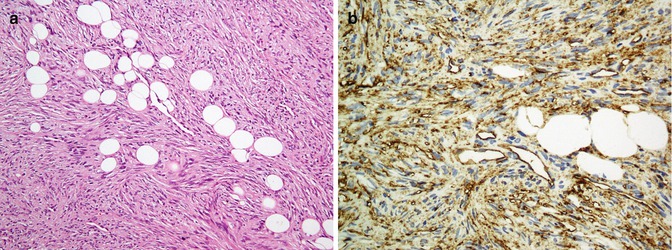
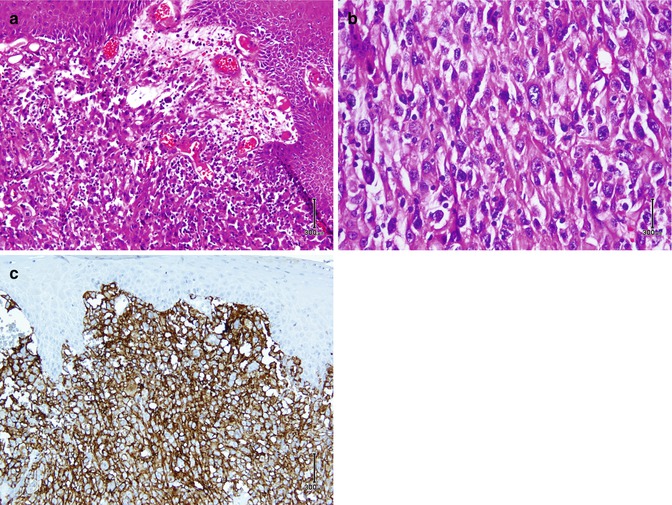

Fig. 26.8
Dermatofibrosarcoma protuberans. Spindle fibrohistiocytic cells, entrapping subcutaneous fat tissue (a) highlighted by CD34 (b)

Fig. 26.9
Atypical fibroxanthoma. Atypical pleomorphic cells with vesicular nuclei in the dermis (a, b) are immunostained by CD10 (c)
26.3 Immunohistochemistry of Head and Neck Tumors
26.3.1 Tumors of the Nasal Cavity and Paranasal Sinuses
Tumors of the nose and paranasal sinuses can be categorized in two groups of small cell carcinomas and undifferentiated carcinomas. Small cell carcinomas of the nasal cavity and paranasal sinuses include olfactory neuroblastoma (ONB), melanoma, lymphoma, rhabdomyosarcoma, small cell neuroendocrine carcinoma, and ES/PNET (Table 26.6). Undifferentiated carcinomas include sinonasal undifferentiated carcinoma, undifferentiated nasopharyngeal carcinoma (Fig. 26.10), and undifferentiated neuroendocrine carcinoma (Fig. 26.11) [47, 48]. All poorly differentiated and undifferentiated carcinomas express cytokeratin [49]. Undifferentiated nasopharyngeal carcinoma reacts with EBV, and undifferentiated neuroendocrine carcinoma is positive for neuroendocrine markers and S100 [50]. NUT midline carcinoma (NMC) is an aggressive tumor with translocation of the NUT (nuclear protein in testis) gene resulting in the formation of BRD4–NUT fusion gene. Recently, new mAbs against the NUT Ag have been designed which will improve the diagnosis of NMC [51]. Immunohistochemistry of poorly differentiated and undifferentiated carcinomas are denoted in the Table 26.7.
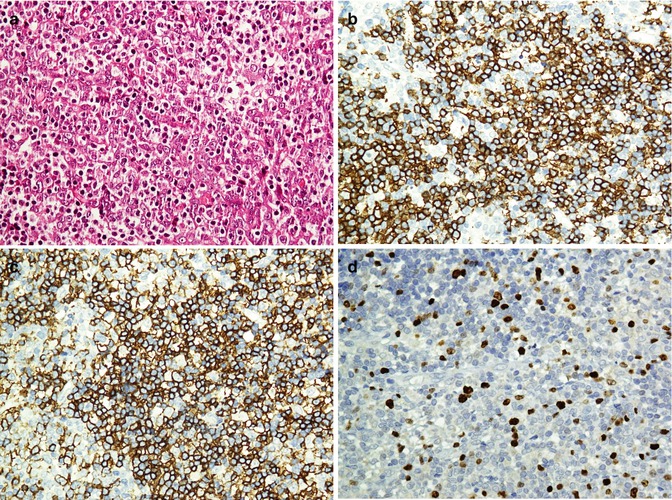
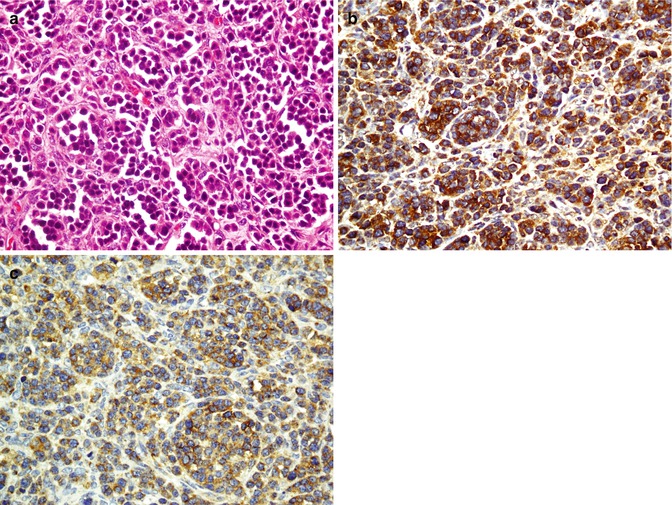
Table 26.6
Immunohistochemistry of small cell carcinomas of nasal cavity: olfactory neuroblastoma (ONB), rhabdomyosarcoma (RMS), Ewing sarcoma/peripheral neuroectodemal tumor (ES/PNET), and small cell neuroendocrine carcinoma (SNEC)
Tumor | ONB | Melanoma | Lymphoma | RMS | SCC | ES/PNET | SNEC |
|---|---|---|---|---|---|---|---|
Immunoreactive markers | SYN | HMB45, S100, vimentin | LCA, vimentin | Desmin, Myogenin, vimentin | AE1/AE3, EMA, SYN | CD99, SYN | Cytokeratin, neuroendocrine markers |

Fig. 26.10
Undifferentiated nasopharyngeal carcinoma shows infiltration of large undifferentiated cells with intermixed small lymphocytes (a). Cytokeratin antibody highlights malignant cells (b), and intermixed lymphocytes react with LCA (c). Ki-67 antibody reacts with about 20 % of malignant cells (d)

Fig. 26.11
Neuroendocrine carcinoma (a). Tumor cells are immunostained with synaptophysin (b) and NSE (c)
Table 26.7
Immunohistochemistry of poorly differentiated and undifferentiated carcinomas of nasal cavity: sinonasal undifferentiated carcinoma (SNUC), undifferentiated neuroendocrine carcinoma (UNEC), and undifferentiated nasopharyngeal carcinoma (UNPC)
Markers | SNUC | UNPC | UNEC (Fig. 26.11) |
|---|---|---|---|
Cytokeratin | + | + | + |
EBV | − | + | − |
Neuroendocrine | − | − | + |
CD99 | − | − | +/− |
S100 | − | − | + |
26.3.1.1 Theranostic Application
In olfactory neuroblastoma, immunoreactivity with bcl-2 may predict response to neoadjuvant chemotherapy and seems to be associated with worse survival [52].
26.3.2 Tumors of the Larynx, Nasopharynx, and Oropharynx
Squamous cell carcinoma (SCC) is the most common malignancy in the head and neck. Typically, head and neck SCCs are positive for cytokeratin cocktails, AE1/AE3, and pan-cytokeratin. Human papilloma virus (HPV) is detected in some SCCs of the oropharynx and known as a risk factor of head and neck SCCs [60, 61]. Being as a variant of SCC, basaloid squamous cell carcinoma (BSCC) is another tumor with predominance of basaloid components. Basaloid squamous cell carcinomas express p63 which is relatively specific but also found in other squamous tumors (Fig. 26.12). Neuroendocrine markers are negative in BSCC [62]. Spindle squamous cell carcinoma (SSCC) is a cytokeratin-negative SCC in which spindle cell component is uniformly and strongly positive for vimentin [63]. Undifferentiated nasopharyngeal carcinoma shows reactivity to EBV immunostaining as well as some SCCs and BSCCs [64, 65].
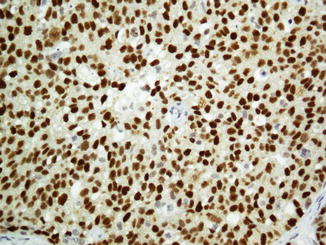

Fig. 26.12
P63 immunoreaction in basaloid squamous cell carcinoma
26.3.2.1 Prognostic Marker
As a transcription repressor of E-cadherin, Snail-1 is expressed in more than half of the cases of SSCC but not in SCC. In addition, it can be a novel marker for the prediction of metastasis [66].
26.3.3 Tumors of the Salivary Glands
Salivary glands are tubuloacinar exocrine glands having two-layered epithelium which comprise of luminal (acinar and ductal cells) and abluminal (myoepithelial and basal cells). Luminal cells are positive for low molecular cytokeratin, whereas myoepithelial and basal cells react with high molecular cytokeratin and myoepithelial markers. The majority of salivary gland carcinomas can be diagnosed by routine hematoxylin and eosin (H&E)-stained slides, and immunohistochemical (IHC) staining has only a limited role in the diagnosis of salivary gland tumors [47, 67]. Figure 26.13 summarized the various components of the normal salivary glands with an emphasis on the immunohistochemistry Abs.
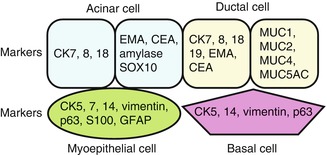

Fig. 26.13
Normal salivary gland components with immunohistochemistric antibodies
26.3.4 Immunohistochemistry of Salivary Gland Tumors
The most common malignant tumors of salivary glands consist of acinic cell carcinoma, adenoid cystic carcinoma (Fig. 26.14), basal cell adenocarcinoma, epithelial-myoepithelial carcinoma, mucoepidermoid carcinoma (Fig. 26.15), myoepithelial carcinoma, polymorphous low-grade adenocarcinoma, and salivary duct carcinoma. All tumors are cytokeratin positive; however, different immunoprofile patterns exist [68]. C-kit (CD117) is positive in acinic cell carcinoma and adenoid cystic carcinoma [69, 70]. Acinic cell tumor and mucoepidermoid carcinoma demonstrate reactivity with membrane-bound mucin (MUC) [71, 72]. Myoepithelial carcinomas are positive for both epithelial and myoepithelial markers but do not exhibit reaction with EMA and CEA [73]. Malignant monophasic salivary gland tumors include acinic cell carcinoma, myoepithelial carcinoma, mucoepidermoid carcinoma, and polymorphous low-grade adenocarcinoma. Immunophenotype profiles of monophasic and biphasic tumors are denoted in Tables 26.8 and 26.9. Application of CK7 and CK20 is a useful panel in distinguishing primary salivary gland carcinoma (CK7+, CK20−) from metastatic carcinoma (CK7−, CK20+) [74].
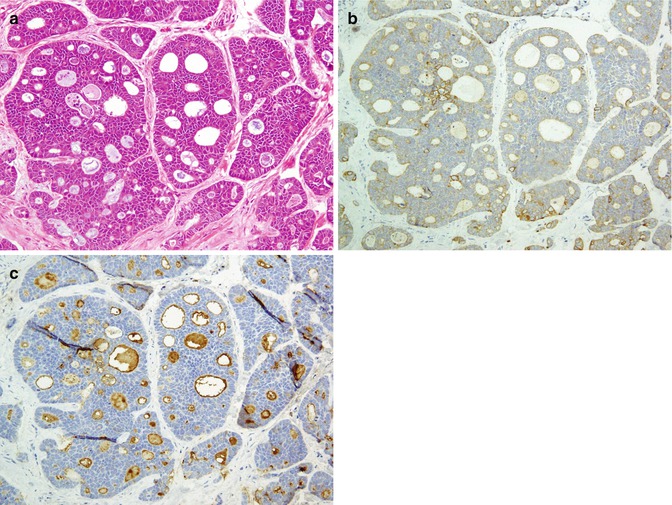
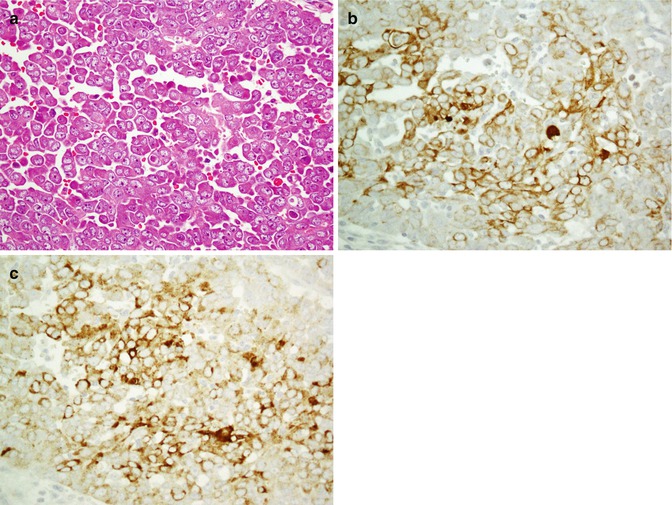

Fig. 26.14
Adenoid cystic carcinoma with typical cribriform pattern (a) shows immunoreaction with EMA (b) and CEA (c)

Fig. 26.15
Poorly differentiated mucoepidermoid carcinoma with polygonal atypical epidermoid cells (a) exhibits immunostaining with CK7 (b) and EMA (c)
Table 26.8
Immunophenotype of monophasic malignant salivary gland tumors: acinic cell carcinoma (ACC), myoepithelial carcinoma (MC), mucoepidermoid carcinoma (MEC), and polymorphous low-grade adenocarcinoma (PLGC)
Tumor | AC | MC | MEC | PLGC |
|---|---|---|---|---|
Epithelial Markers | CAM5.2, CK7/8/18, EMA, CEA, MUC3 | AE1/AE3, CAM5.2, CK14, 34βE12 | CAM5.2, CK7/8/14/18/19, EMA, CEA, MUC1/4/5 AC, 5B | CAM5.2, CK7, 14, EMA |
Myoepithelial/ basal markers | N | p63, calponion, SMA, myosin | p63 (epidermoid component) | p63 |
Other markers | C-kit, S100 | Vimentin, S100, GFAP | – | S100 |
Table 26.9
Immunophenotype of biphasic malignant salivary gland tumors: adenoid cystic carcinoma (ACC), basal cell adenocarcinoma (BCA), epithelial-myoepithelial carcinoma (EMC), and salivary duct carcinoma (SDC)
Tumor | ACC | BCA | EMC | SDC |
|---|---|---|---|---|
Epithelial markers | CAM5.2, CK7/14/19, EMA, CEA | AE1/AE3, CAM5.2, CK7, EMA, CEA | AE1/AE3, CAM5.2, CK14 | AE1/AE3, EMA, CEA |
Myoepithelial/basal markers | p63, calponin | p63, calponin, SMA | p63, calponin, SMA | p63 |
Other markers | C-kit, S100 | C-kit, S100 | S100 | AR, GATA3, HER2/neu |
26.3.4.1 Prognostic Marker
In mucoepidermoid carcinoma, MUC1 expression is correlated with tumor progression and worsened prognosis, whereas MUC4 expression is related to a better prognosis [72].
26.3.5 Tumors of Thyroid and Parathyroid Glands
The functional unit of thyroid is the follicle which is composed of follicular cells and C cells. Follicular cells exhibit reactivity with thyroglobulin, TTF1, PAX8, AE1/AE3, EMA, and CK7 and CK8/18/19, whereas C cells are positive for calcitonin, TTF1, CK7, synaptophysin, and chromogranin. Being as a nuclear transcription factor, TTF1 is expressed on follicular and C cells. A follicular cell-specific marker is thyroglobulin which does not react with C cells (Fig. 26.16). As a member of the paired box (PAX) gene family, PAX8 is a sensitive marker of thyroid tumors similar to TTF1. Among intermediate filaments, CK19 is more expressed in papillary carcinoma than other tumors. Parathyroid hormone (PTH) and parafibromin are markers of parathyroid tumors. Parafibromin is uniformly expressed in parathyroid adenomas, whereas its expression is often reduced in parathyroid carcinomas. Table 26.10 shows an immunopanel of thyroid and parathyroid tumors. Figure 26.17 depicts thyroid medullary carcinoma.
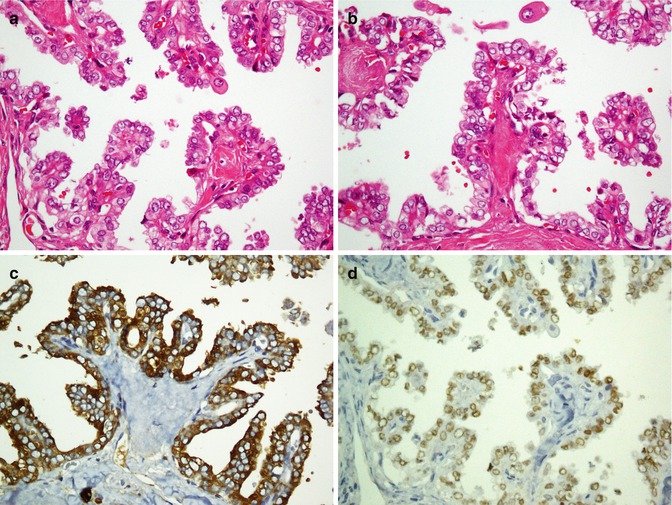
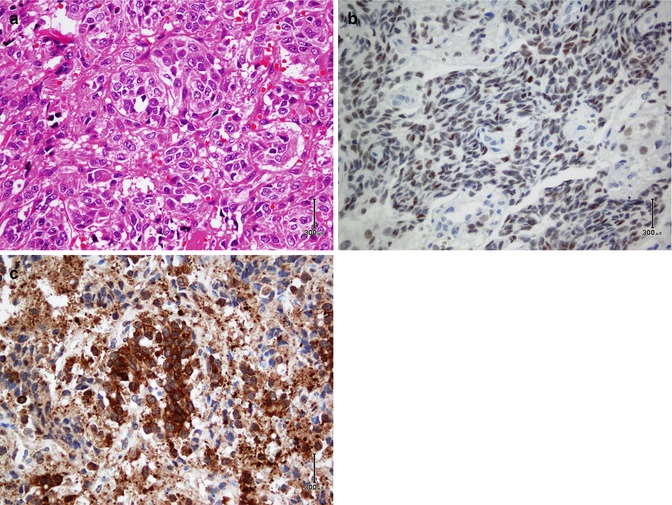

Fig. 26.16
Thyroid papillary carcinoma. Papillary projections with intranuclear inclusions (a) and Orphan Annie nuclei (b) are highlighted by thyroglobulin in the cytoplasm (c) and TTF1 in the nuclei (d)
Table 26.10
Immunopanel of thyroid and parathyroid tumors
First-choice antibody panel | Second-choice antibody panel | Consistent with |
|---|---|---|
CK+, TTF1+, TGB+ | PAX8+, CK19+ | Papillary carcinoma (Fig. 26.16) |
PAX8±, VIM+ | Follicular carcinoma | |
CK+, TTF1+, TGB− | Calcitonin+, SYN+, CGN+ | Medullary carcinoma (Fig. 26.17) |
CK±, TTF1+, TGB− | p53+, VIM+, PAX8± | Anaplastic carcinoma |
CK+, TTF1−, TGB− | PTH+, CGN+, parafibromin± | Parathyroid tumor |

Fig. 26.17
Thyroid medullary carcinoma. Solid nests with medium-sized atypical cells (a) exhibit immunoreaction with calcitonin (b) and chromogranin (c)
26.4 Immunohistochemistry of Lung Tumors
26.4.1 Adenocarcinoma
The most frequent IHC pattern observed in lung tumors is positivity for CK7, TTF1, and Napsin A, along with negative staining for CK20, CDX2, and MUC2. It is highly advocated to consider the fact that there are recently increasing reports of primary pulmonary adenocarcinomas with intestinal differentiation which are CK7 and TTF1 negative but CK20 positive which can be highly misinterpreted as metastatic colorectal adenocarcinomas. Therefore, the importance of physical examination and imaging studies is highlighted. It should be noted that neuroendocrine markers including chromogranin, synaptophysin, NSE, and Leu7 (CD57) can be positive in lung non-neuroendocrine carcinomas such as adenocarcinomas and SCC. Recent studies have shown EGFR, Her2, and BRAF mutations in lung cancers which can increase the chance for targeted therapies in these cancers [103–106].
26.4.2 Mesothelioma
Neoplasms of the pleura are very rare, and most tumors in this area are usually metastatic lesions. One of the most important applications of IHC is to assist pathologists in differentiating mesotheliomas from lung adenocarcinomas [107–109]. Table 26.11 shows the most frequent markers stained by IHC staining in mesothelioma compared with pulmonary adenocarcinoma (Fig. 26.18).
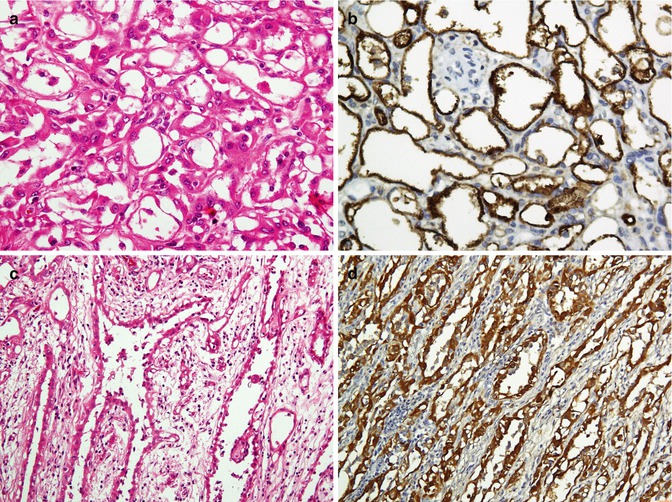
Table 26.11
Immunohistochemistric differentiation of pulmonary adenocarcinoma (PAC) and malignant mesothelioma
Marker | Pulmonary AC | Mesothelioma | Comment |
|---|---|---|---|
Calretinin | R | Usually + | The most specific and reproducible positive marker in mesothelioma |
CDX2 | R | − | About 13 % positive, in pulmonary mucinous carcinomas |
Cytokeratin | AE1/AE3, CK5/6 (R), CK7, | CK5/6 (S), CK7 (used to differentiate mesotheliomas from sarcomas) | CK7: Most common CK in primary lung cancer (About 100 % in AC, 40 % in small cell carcinoma, about 20 % in carcinoid tumor, and none of SCC arising from lung) |
CK5+ specially in lung SCC | |||
D2-40 | − | + | Usually positive specially in sarcomatoid variants of mesothelioma |
EMA | S (cytoplasmic) | S (membranous) | |
TTF1 | + | − | |
Mesothelin | − | + | |
p63 | − | − | Positive in pulmonary SCC |
pCEA | + | − | |
S100 | + | − | |
SMA | − | 50–60 % | |
SP-A (surfactant protein A) | 50 % | − | |
Thrombomodulin | − | + | |
Vimentin | + | − | |
WT1 | − | 60 % |

Fig. 26.18
Mesothelioma. Adenomatoid type (a) shows immunostaining for mesothelin (b), and tubular type (c) shows immunoreaction for calretinin (d)
26.5 Immunohistochemistry of Gastrointestinal Tumors
Immunohistochemistry is used in gastrointestinal and colon cancers to particularly determine the tumor subtype and origin, especially for poorly or undifferentiated cancers for which morphology alone cannot determine the origin. Generally, it should be noted that definite tissue diagnosis in clinical practice needs combination of IHC results and clinical information, including biopsy site and the patients’ clinical history [110]. Previous studies show that blinded use of an IHC panel for differential diagnosis can primarily identify about 83 % of tumor origins vs. 65.6 % of metastasis. Several publications on IHC studies are available, and each recommends its own IHC panel for differential diagnosis. This makes it clear that there is no single IHC panel, or standard of care, for tissue determination, and pathologists have long known that tissue of origin identification is inherently a multiplex problem [111–113].
Here, the authors have briefly tried to introduce the major and common IHC markers used to differentiate frequent gastrointestinal tumors. It should be noted that the average positivity of a marker in a specific tumor differs from one study to another, as well as in different textbooks. In this chapter the most prevalent and reliable data are provided.
26.5.1 Liver
The most common primary hepatic cancer is hepatocellular carcinoma which is well known to have a wide spectrum of histologic differentiation and a great diversity of appearances. It necessitates the application of IHC as an ancillary aid for better diagnosis of the lesion. It is important to reiterate that IHC is after all an ancillary aid. A significant clinicopathologic correlation seems mandatory for the final diagnosis. If a definitive diagnosis cannot be clinched, at the least, certain differential diagnoses can be excluded [114–118]. Immunophenotype of normal liver is summarized in Table 26.12 (Figs. 26.19 and 26.20).
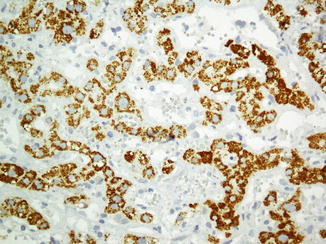
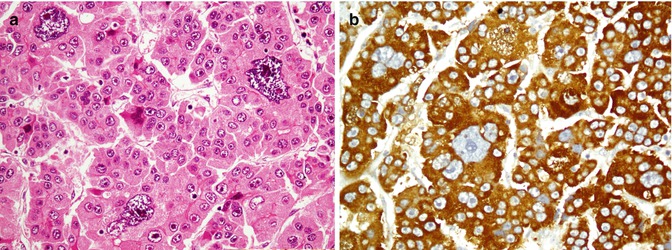
Table 26.12
Immunohistochemistry of normal liver
Normal tissue | Markers | ||||
|---|---|---|---|---|---|
Hepatocellular | Adenocarcinoma | Carcinoma | Canalicular | Others | |
Hepatocytes | HepPar1, TTF1 (cytoplasmic) | MOC31 | CAM5.2 | CD10, pCEA | B-catenin |
Bile duct cells | – | CK7, CK19 (+/−), MUC6 | CAM5.2, CKAE1/AE3, EMA, BerEp4 | – | B-catenin |

Fig. 26.19
Normal liver stains with HepPar1 showing typical cytoplasmic coarse granules of hepatocytes

Fig. 26.20
Hepatocellular carcinoma with huge bizzare giant nuclei making diagnosis simple as malignant (a) exhibits reactivity with HepPar1 (b)
Cholangiocarcinoma is a malignant tumor with characteristics mostly similar to other types of adenocarcinomas. The tumor is usually positive for CK7, CK19, CAM5.2, CK AE1/AE3, pCEA, mCEA (noncanalicular pattern), and MOC31. MUC4, MUC5AC, and MUC6 can also be useful not in diagnosis but in classification and predicting the prognosis. Additionally, CD56 which is positive in benign bile ductular proliferations and negative in cholangiocarcinomas can be useful in differentiating malignant lesions from benign proliferation. The exception for this rule is clear cell cholangiocarcinoma which is positive for CD56. Staining for CK7 and CK19 in cholangiocarcinoma can help to differentiate this tumor from HCC, which is negative for the mentioned markers [119, 120]. Table 26.13 indicates the immunophenotypes of hepatocellular carcinoma and cholangiocarcinoma.
Table 26.13
Immunohistochemistry of hepatocellular carcinoma and cholangiocarcinoma
Tumor | Markers | ||||
|---|---|---|---|---|---|
Hepatocellular | Adenocarcinoma | Carcinoma | Canalicular | Sinusoidal | |
Hepatocellular carcinoma | HepPar1, TTF1 (cytoplasmic) | – | CAM5.2, EMA (−/+) | CD10, pCEA | CD34, FVIII |
Cholangiocarcinoma | – | MOC31, CK7, CK19, MUC4, MUC5AC, MUC6 | CAM5.2, CKAE1/AE3 | pCEA, mCEA (noncanalicular) | – |
26.5.2 Esophagus
The most common esophageal cancers are adenocarcinomas and SCC. Adenocarcinoma of the esophagus is immunophenotypically similar to gastric adenocarcinomas, and there is no IHC panel to distinguish these two. Esophageal SCC is usually positive for most CK markers including CK AE1/AE3, CK 34bE12, CK5/6, CK19 (positivity increases with tumor grade whereas benign squamous lesions are negative for this marker), and p63. Additionally, most SCCs are negative for CK7 and CK20 which can be useful in distinguishing poorly differentiated SCCs from poorly differentiated adenocarcinomas positive for these two CK markers [121–123].
26.5.3 Stomach
Stomach glandular epithelium expresses CK20 and less commonly CK7 (CK7+, CK20+) and MUC5AC, distinguishing it from small intestine and colorectal epithelium. Immunoprofile of normal gastrointestinal mucosa is denoted in Table 26.14. Gastric adenocarcinoma has many histologic variants, but they have almost similar immunophenotyping. It should be mentioned that synaptophysin and chromogranin as neuroendocrine markers can be positive in gastric adenocarcinomas; therefore, positive staining with these markers is not sufficient for the diagnosis of neuroendocrine carcinoma [124–126]. Immunoprofile of gastric adenocarcinoma is demonstrated in Table 26.15 (Fig. 26.21).
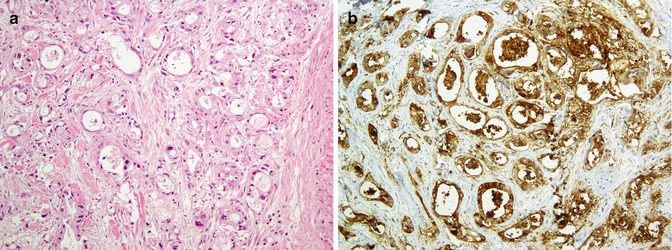
Table 26.14
Immunoprofile of normal gastrointestinal mucosa
Normal tissue | Simple epithelial marker | MUC | CDX2 (intestinal marker) | CD15 | |||||
|---|---|---|---|---|---|---|---|---|---|
CK7 | CK20 | AE1/AE3 | CAM5.2 | CEA | Gastric (MUC5AC) | Intestinal (MUC2, MUC4) | |||
Stomach | +/− | + | + | + | + | + | − | − | + |
Small intestine | − | + | + | − | −/+ | − | + | + | + |
Large intestine/appendix | − | + | + | + | + | − | + | + | + |
Table 26.15
Immunoprofile of gastric, small intestine, and colorectal adenocarcinoma (AC)
Tumor type | Tumor associated marker | MUC | CDX2 (intestinal marker) | CD15 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
CK18/19 | CK7 | CK20 | AE1/AE3 | CAM5.2 | CEA | Gastric (MUC5AC) | Intestinal (MUC2, MUC4) | |||
Gastric AC | + | +/− | −/+ | + | + | + | −/+ | −/+ | −/+ | − |
Small intestine AC | + | +/− | +/− | + | −/+ | −/+ | −/+ | +/− | +/− | − |
Large intestine/appendix AC | + | − | + | + | + | + | −/+ | +/− | + | − |

Fig. 26.21
Adenocarinoma of the stomach with atypical glands and nuclear pleomorphism (a) immunostained with CEA (b)
26.5.4 Small Intestine
26.5.5 Colon
In contrast to older studies which have discussed colon cancers generally, recent studies reveal that colon cancers arise from two different pathways (chromosomal instability of APC gene vs. microsatellite instability (MSI) pathway) with different immunophenotypic features [116, 130–136]. Immunoprofile of normal and colon adenocarcinoma is denoted in Tables 26.14, 26.15, and 26.16.
Table 26.16
Immunoprofile of colon adenocarcinoma based on chromosomal instability and MSI pathways
Chromosomal instability pathway (80–85 %) | MSI pathway (15–20 %) | ||
|---|---|---|---|
CK20 | 100 % | CK20 | Can be negative in about 30 % |
MUC2 | Usually positive | MLH1 | Complete absence of staining with a sufficient internal control is needed for a positive result |
MUC5AC | Usually negative (about 30 % positive, especially in mucinous carcinomas) | MSH2 | |
CAM5.2 | Usually positive | MSH6 | |
MOC31 | Usually positive | PMS2 | |
CDX2 | About 90 % | CDX2 | Can be negative in about 20 % |
CK7 | 5–10 % | ||
CEA | Usually positive specially monoclonal type | ||
CK8 | Usually positive | ||
CK18 | Usually positive | ||
CK19 | Usually positive | ||
CKAE1/AE3 | Usually positive | ||
MSI-related markers | These markers are usually positive in this subtype of colon carcinomas | ||
26.5.6 Anal
The most frequent anal cancers are SCC and adenocarcinoma. Anal SCC is almost similar to SCC of other origins; nonetheless, the role of HPV is highlighted. Adenocarcinomas of the anus are usually positive for CK7 and negative for CK20, CDX2, and CK5/6 which helps to differentiate them from adenocarcinomas of colon origin [135, 137, 138].
26.5.7 Appendix
26.5.8 Pancreas
Pancreas is composed of glandular/ductal, acinal epithelium, and endocrine cells. Pancreatic neoplasms can be roughly divided into two categories of exocrine and endocrine system neoplasms. This part mostly discusses the exocrine system and mostly adenocarcinomas of this area. Additionally, tumor suppressor genes including DPC4 and SMAD4 are inactivated in about 50–60 % of the adenocarcinomas of this site [116, 142, 143]. Immunoprofile of normal pancreas and some pancreatic tumors are summarized in Tables 26.17 and 26.18. Figure 26.22 depicts solid pseudopapillary neoplasm.
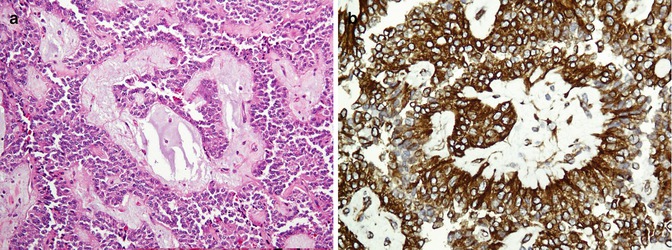
Table 26.17
Immunoprofile of normal pancreas
Marker | Normal tissue | ||
|---|---|---|---|
Exocrine | Glandular/ductal | Epithelial | CAM5.2, AE1/AE3, CK7, CK8/1/8/19 |
MUC | MUC1, MUC6 | ||
ONP | – | ||
Acinar | Trypsin, chymotrypsin, lipase, amylase, elastase | ||
Endocrine | CGN, SYN, NSE | ||
Table 26.18
Immunoprofile of some pancreatic tumors: pancreatic ductal adenocarcinoma (PDAC), acinar cell carcinoma (ACC), neuroendocrine carcinoma (NEC), and solid pseudopapillary neoplasm (SPN)
Marker | PDAC | ACC | NEC | SPN (Fig. 26.22) | ||
|---|---|---|---|---|---|---|
Exocrine | Glandular/ductal | Epithelial | CAM5.2, AE1/AE3, CK7, CK8/18/19, pCEA, PSCA | CAM5.2, AE1/AE3, CK8/18, EMA | CAM5.2, AE1/AE3, CK19 | – |
(Positive for β-catenin, vimentin, PR, CD10) | ||||||
MUC | MUC1, MUC3, MUC4, MUC5AC, MUC6 (+/−) | – | – | – | ||
ONP | CA19.9, CA125, B72.3, DUPAN-2, CECAM1 | – | – | – | ||
Acinar | – | Trypsin, chymotrypsin, lipase, amylase, elastase | – | α1-antitrypsin | ||
Endocrine | – | CGN, SYN | CGN, SYN, NSE, CD56, CD57 | CGN, SYN, NSE, CD56 | – | |

Fig. 26.22
Solid pseudopapillary neoplasm. Papillary projection covered by relatively bland-looking cells supported by a hyalinized stroma (a) highlighted with vimentin (b)
26.5.9 Gastrointestinal Stromal Tumor
Gastrointestinal stromal tumor (GIST) is a soft tissue tumor of the GI wall which is in the differential diagnosis of leiomyoma and fibromatosis. Most GISTs express c-kit (>95 %), CD34, and CD99 (Fig. 26.23). Sometimes weak positivity for S100, SMA, desmin, and synaptophysin (but not chromogranin) can also be found [135, 144, 145].
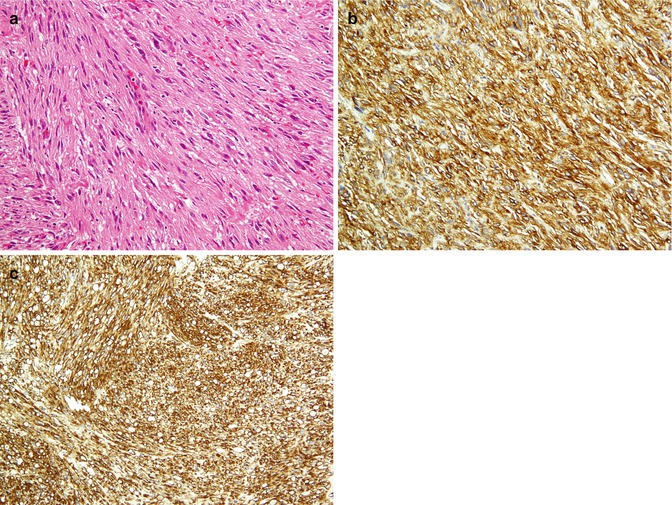

Fig. 26.23
Gastrointestinal stromal tumor. A low-grade intestinal wall tumor shows uniform spindle cells with elongated nuclei (a), with immunoreaction to c-kit (b) and CD34 (c)
26.5.10 Neuroendocrine Carcinomas
Neuroendocrine tumors arise from different organs. Most have similar morphology and tumor marker expression, and the most important diagnostic clues are histologic features, as well as immunostaining for synaptophysin, chromogranin, and NSE (Fig. 26.24). In addition to the mentioned markers, most of neuroendocrine tumors can express the tissue markers in which they originated which help to diagnose the origin of metastatic neuroendocrine tumors [143, 146–148].
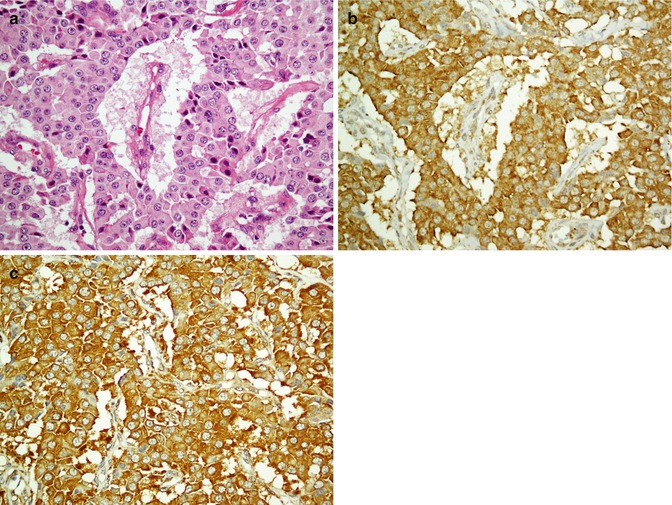

Fig. 26.24
Neuroendocrine carcinoma composed of atypical cells with round nuclei and dusty chromatin (a). Tumor cells are immunostained with chromogranin (b) and synaptophysin (c)
26.6 Immunohistochemistry of the Urinary Tract
26.6.1 Kidney
Renal cell carcinoma (RCC) is the most common tumor of the kidney with variants of clear renal cell carcinoma (CRCC), papillary renal cell carcinoma (PRCC), and chromophobe carcinoma (CC). Commonly used immunohistochemical Abs in the urinary system are summarized in Table 26.19. Immunohistochemistry is an ancillary test used to distinguish variants of RCC as well as tumors with histopathologic similarities including collecting duct carcinoma and urothelial carcinoma of the renal pelvis. Carcinomas with clear cell feature include CRCC (Fig. 26.25), papillary renal cell carcinoma, and transitional (urothelial) cell carcinoma of the renal pelvis. Differential diagnoses of carcinoma with oncocytic appearance are chromophobe carcinoma, oncocytoma, and oncocytic papillary RCC (Fig. 26.26) [149–154]. The immunophenotype of collecting duct carcinoma is 34βE12+, CD10−, and AMACR−, in contrast to PRCC which is 34βE12−, CD10+, and AMACR+ [150, 155]. Considering the histopathologic pattern, the following immunopanels (Tables 26.20 and 26.21) compare the immunohistochemical Abs in these tumors.
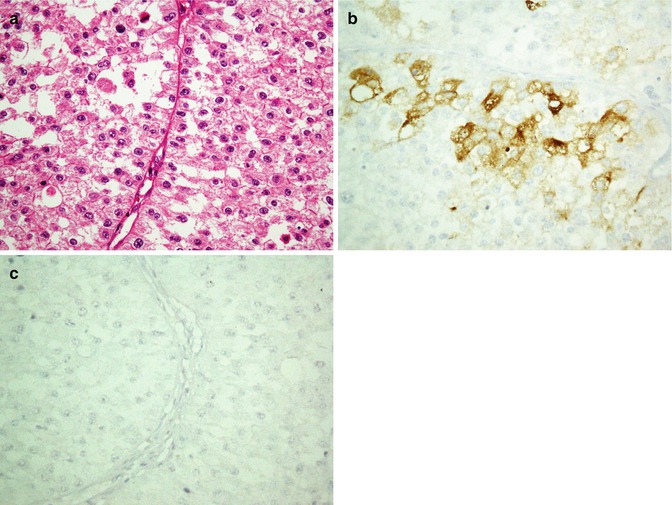
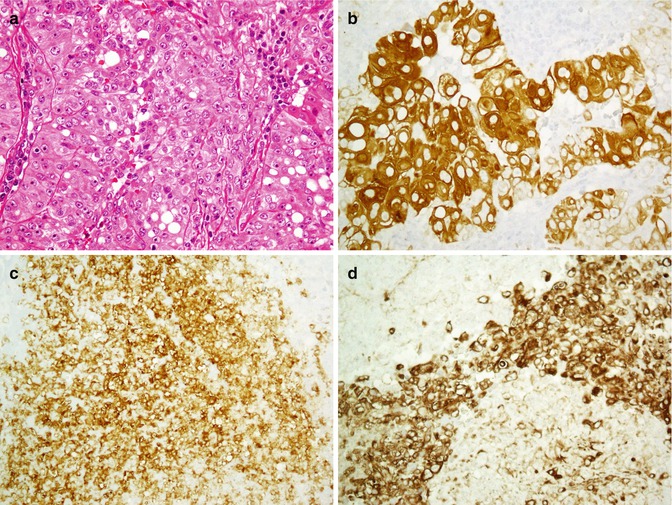
Table 26.19
Immunohistochemical markers in urinary system tumors
Marker | Function | Immunoreaction in tumor |
|---|---|---|
AE1/AE3 | Pan-CK epithelial marker | RCC |
CAIX | Carbonic anhydrase IX: maintenance of intracellular and extracellular pH, regulatory role in cell proliferation | PRCC |
CAM5.2 | Intermediate cytoskeleton filament | RCC, PRCC, CC, CDC |
CD10 (CALLA) | A zinc-dependent cell membrane metalloprotein | RCC, PRCC |
CD117 (c-kit) | Transmembrane glycoprotein receptor tyrosine kinase | CC, CDC, OC |
CK7 | LMWCK (simple epithelia) | PRCC, CC, UC, PAC (+/−) |
CK20 | LMWCH (simple epithelia) | UC (+/−), PAC (+/−) |
34βE12 | HMWCK (CK1, 5, 10, 14) | CDC, UC |
EGFR | Receptor with tyrosine kinase activity | UC (+/−) |
Ep-Cam | Glycosylated transmembrane cell surface epithelial protein in distal nephron | PRCC (+/−), CC, CDC |
HMWCK | Intermediate cytokeratin filaments of prostate basal cell | “Negative” marker in PAC |
Ki-67 (MIB1) | Nuclear protein expressed in all phases of the active cell cycle (G1, S, G2, M) | Proliferative marker |
Ksp-cadherin (kidney-specific) | Calcium-dependent cell adhesion molecule plays an important role in the maintenance of tissue integrity | CC, OC |
p53 | Tumor suppressor protein | UC |
p63 | A member of p53 family transcription factor, marker of basal cells | “Negative” marker in PAC |
P501S (Prostein) | A 553-amino acid protein localized to the Golgi complex | PAC |
P504S (AMACR) | Enzyme mainly localized to peroxisomal structures | PRCC, PAC |
PAX2/PAX8 | Members of the paired box (PAX) gene family expressed in the development of the urogenital tract | RCC, PRCC, CC, CDC, OC (+/−) |
PSA | 330-kD glycoprotein, prostate-specific antigen | PAC |
PSAP | 100-kD glycoprotein, prostate-specific antigen | PAC |
PSMA | 100-kD glycoprotein, prostate-specific antigen | PAC |
RCC | 200-kD glycoprotein expressed in epithelial cells lining the normal renal proximal tubule | RCC, PRCC |
Thrombomodulin | 75-kD glycoprotein, to convert thrombin from a coagulant protein to an anticoagulant | UC |
Uroplakin III | A transmembrane protein unique to urothelium | UC |
Vimentin | Intermediate cytoskeleton filament | RCC, PRCC, CDC |

Fig. 26.25
Renal cell carcinoma, eosinophilic to clear cells (a) is immunostained with CD10 (b) but not with CK20 (c)

Fig. 26.26
Papillary renal cell carcinoma with oncocytic feature (a). Tumor cells are positive for CK7 (b), CD10 (c), and vimentin (d)
Table 26.20
Immunoprofile of kidney carcinoma with clear cell appearance: clear RCC (CRCC), papillary RCC (PRCC), and urothelial carcinoma (UC)
Tumor | CK7 | CK20 | Vimentin | RCC | CD10 | PAX2/8 | AMARC | Uroplakin | p63 |
|---|---|---|---|---|---|---|---|---|---|
CRCC | − | − | + | + | + | + | − | − | − |
PRCC | + | − | + | + | + | + | + | − | − |
UC | + | + | − | − | − | − | − | + | + |
Table 26.21
Immunoprofile of kidney carcinoma with oncocytic cell appearance: oncocytic papillary RCC (OPRCC), chromophobe carcinoma (CC), and oncocytoma (OC)
Tumor | CK7 | CK20 | CAM5.2, EMA, AE1/AE3 | Vimentin | RCC | CAIX | CD10 | CD117 | Ep-Cam | Ksp-cadherin |
|---|---|---|---|---|---|---|---|---|---|---|
OPRCC | + | − | + | + | + | + | + | − | + | − |
CC | + | − | + | − | − | − | − | − | + | + |
OC | − | − | − | − | − | − | − | + | − | + |
26.6.2 Bladder
Normal urothelium exhibits a unique pattern of cytokeratin expression characterized by coexpression of simple epithelium cytokeratin (CK7, CK20, and CAM5.2) and HMWCK (CK5/6 and 34βE12). While CK20 is expressed in umbrella cells of the normal urothelium, in dysplastic urothelium and carcinoma in situ, it is expressed in all layers of the urothelium [150–154, 168, 169]. CD44 is expressed in the basal layer of normal urothelium and shows focal staining of basal layers of the dysplastic urothelium [170]. Urothelial carcinomas are divided into (1) noninvasive papillary carcinoma and (2) invasive carcinoma which can appear as papillary or non-papillary itself (Fig. 26.27). Immunohistochemistry can be helpful to differentiate urothelial carcinoma from direct extension of an adjacent primary carcinoma (prostate, colorectal, cervix, and uterine) as well as metastasis and also to distinguish variants of urothelial carcinoma. Common immunohistochemistry Abs in normal urothelium, urothelial hyperplasia, urothelial dysplasia, and urothelial carcinoma are summarized in Table 26.22.
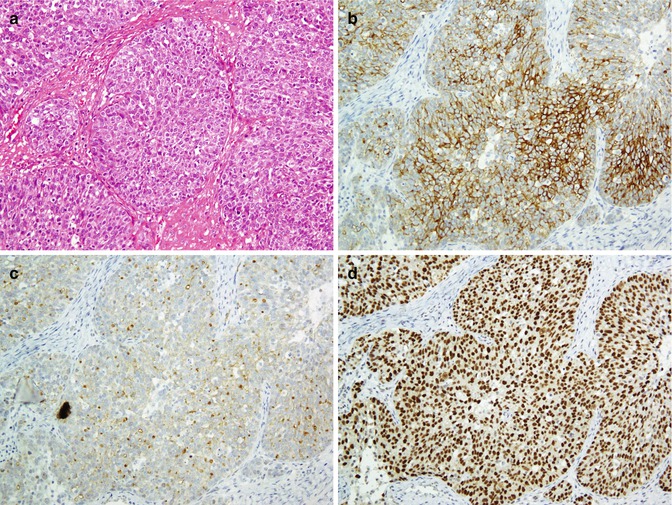

Fig. 26.27
Transitional cell carcinoma, invasive, non-papillary type (a). Tumor cells exhibit immunoreaction with CK7 (b), CK20 (c), and p63 (d)
Table 26.22
Antibody immunoprofile in normal urothelium, urothelial hyperplasia, dysplasia, and carcinoma
Marker | Normal urothelium | Urothelial hyperplasia | Urothelial dysplasia | Urothelial carcinoma |
|---|---|---|---|---|
CK7 | + | + | ND | + |
CK20 | + U | + | + | + |
34βE12 | +B | ND | ND | + |
CD44 | +B | ND | −/+ | ND |
EGFR | −/+ | + | +/− | +/− |
p63 | ND | ND | ND | +a |
UPIII | + U | ND | ND | +a |
TM | + U | ND | ND | +a |
p53 | − | − | + | +a |
26.7 Immunohistochemistry of Female and Male Genital Tumors
26.7.1 Uterine Cervix
The most important and also frequent cervix cancers are cervix SCCs and adenocarcinomas. Cervix SCC markers are similar to those seen in SCCs of other origins. p16 is a unique marker expressed in tumors of the cervix which can help in differentiating this lesion from the same counterparts from uterine or other origins. Adenocarcinomas of the cervix also express most adenocarcinoma markers. One of the advantages of IHC is to differentiate adenocarcinomas of the cervix from the endometrium. Cervix adenocarcinomas usually express p16 and CEA, and are negative for vimentin and ER, whereas endometrium adenocarcinomas have a reverse expression pattern [176–181].
26.7.2 Vulva and Vagina
26.7.3 Uterine Corpus
Uterine tumors are of myometrium or endometrium origin. The myometrial tumors are usually sarcomas and were discussed in the sarcoma section. The endometrium may develop various cancers, but the most frequent one is endometrial adenocarcinoma. Endometrial adenocarcinoma has some variants in which endometrioid adenocarcinoma is the most frequent one. Endometrioid adenocarcinoma usually expresses CK7, CA125, ER, PR, and vimentin but is negative for CEA, CK20, and p16. Some endometrial carcinomas express Her2/neu marker, which along with ER and PR markers can be used in targeted therapies [176–181, 184–187].
26.7.4 Ovary
Except the intestinal type of mucinous adenocarcinoma, all primary ovarian carcinomas are CK7 positive and CK20 negative (Fig. 26.28). This can be used in differentiating primary ovarian carcinoma from metastatic tumors [139–141, 180, 188–191]. The immunophenotype of primary ovarian tumors is described in Table 26.23.
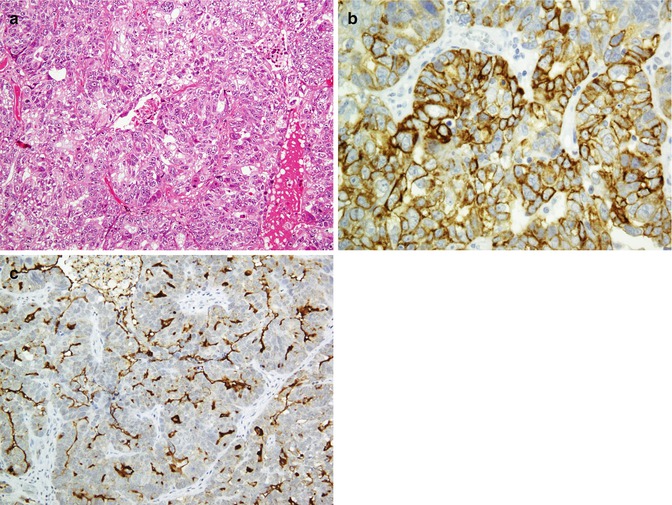

Fig. 26.28
Ovarian serous carcinoma poorly differentiated (a) shows immunoreaction with CK7 (b). CA125 is highlighted in the luminal surface (c)
Table 26.23
Immunophenotype of ovarian cancers
Epithelial tumors | Germ cell tumors | Stromal tumors (almost always negative for EMA) | |||||
|---|---|---|---|---|---|---|---|
Serous (Fig. 26.28) | Mucinous | Dysgerminoma | Yolk sac | Embryonal carcinoma | Choriocarcinoma | Granulosa cell tumor | Sertoli-Leydig cell tumor |
EMA | EMA | PLAP | PLAP | PLAP | HCG | Inhibin | CK |
CK7 | CK7 | CD117 (c-kit) | AFP | Oct-4 | Inhibin | CD99 | CD99 |
CA125 | CK20 | Oct-4 | CK AE1/AE3 | CK AE1/AE3 | CK | WT1 | WT1 |
DPC4 | mCEA | D2-40 | Glypican-3 | CD30 | Calretinin | ||
ER | CDX2 MUC5A | CD56 | |||||
PR | |||||||
WT1 | |||||||
26.7.5 Breast
Breast cancer is one of the most common malignancies with various histopathological types; however, adenocarcinomas and its two subtypes including invasive ductal (IDC) and lobular carcinomas (ILC) comprise the majority. Most breast cancers including IDC and ILC are positive for mammaglobin, GCDFP15, ER, and PR, and some are positive for Her2/neu markers. Additionally, epithelial tumor markers, CK (especially CK7) and EMA, are also positive in these tumors [192–197]. The lack of reaction with myoepithelial markers is in favor of an invasive carcinoma. Both normal (Fig. 26.29) and proliferative glands (Fig. 26.30) and ductal carcinoma in situ (Fig. 26.31) exhibit reactivity with myoepithelial markers. Application of p63 and calponin or p63 and SMA is a good way to evaluate the presence of myoepithelial cells [192, 198]. Immunoprofile of normal breast glands and breast cancers are summarized in Tables 26.24 and 26.25 (Figs. 26.32 and 26.33).
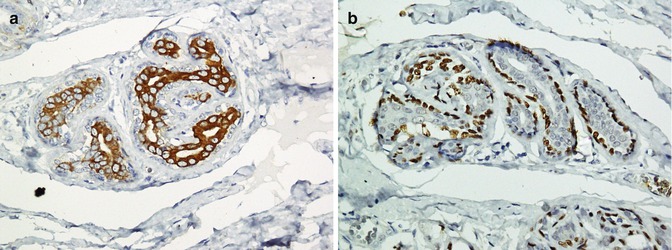
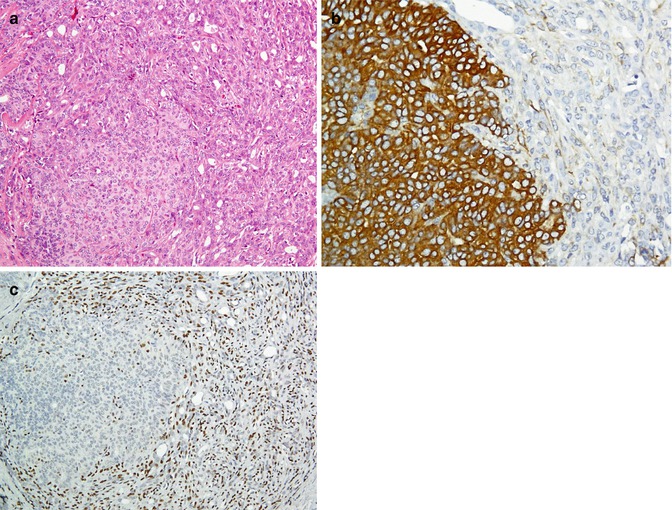
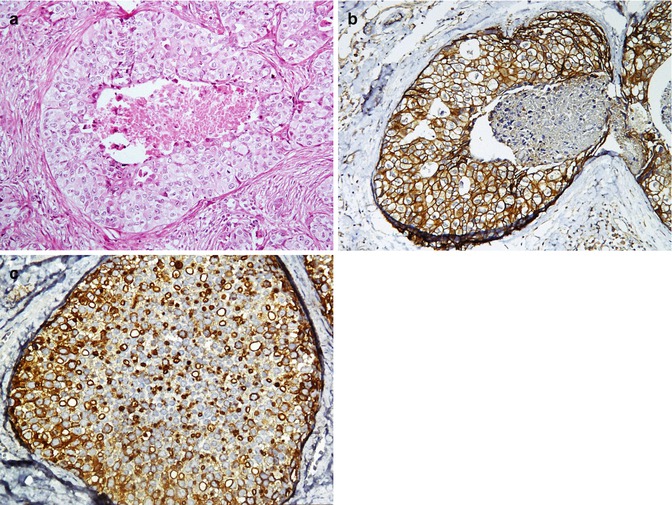
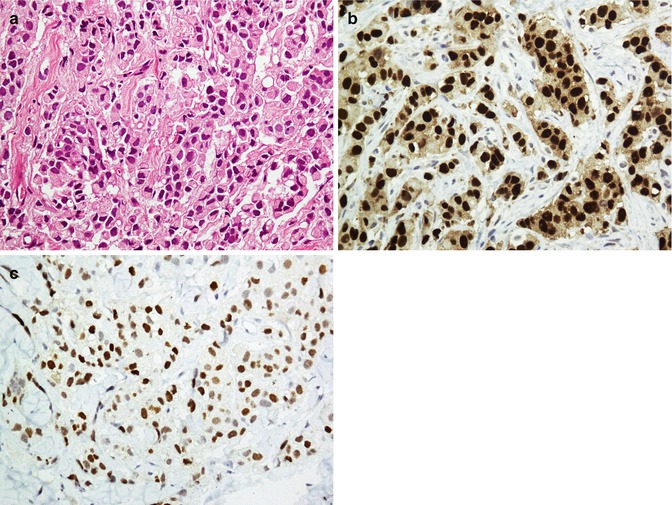
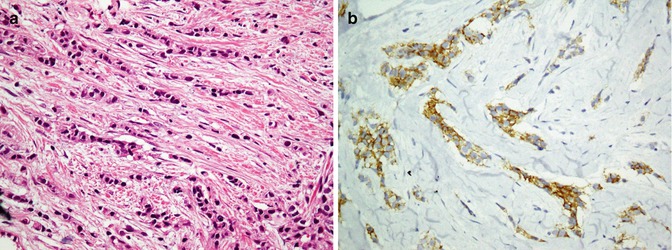

Fig. 26.29
Cytokeratin (a) stains epithelial cells and p63 (b) stains myoepithelial cells of normal breast glands

Fig. 26.30
Breast proliferative lesion (a). Presence of myoepithelial cells confirmed by immunoreaction to HMWCK (b) and p63 (c) which is indicative of a benign process

Fig. 26.31
Ductal carcinoma in situ (a) is immunostained with Her2neu (b) and CA15.3 (c)
Table 26.24
Immunoprofile of normal breast gland tissue
Normal epithelium | Immunoreactive antibodies |
|---|---|
Luminal cells (LC) | CK8/18, CK19 |
Myoepithelial cells | CK5/6, CK14, CK17, p63, SMA, calponin, CD10 |
Both LC and MC | Pan-CK, AE1/AE3, CK7, S100 |
Marker | IDC | ILC |
|---|---|---|
Mammaglobin | −/+ | +/− |
ER | +/− | + |
GCDFP15 | −/+ | −/+ |
E-cadherin | + | − |
p120 | + | + |
34βE12 | − | + |

Fig. 26.32
Invasive ductal carcinoma (a) with ER (b) and PR (c) immunoreaction

Fig. 26.33
Infiltrating carcinoma with Indian file pattern simulating lobular carcinoma (a), revealing immunoreaction with E-cadherin which is in favor of invasive ductal carcinoma (b)
26.7.6 Prostate
Prostate gland is composed of two layers, epithelium and basal cell layer. Normal prostate epithelium exhibits immunoreactivity with prostate-specific antigen (PSA), prostate-specific membrane antigen (PSMA), prostate-specific acid phosphatase (PSAP), prostein (P501S), and α-methylacyl-coenzyme-A racemase (AMACR) enzyme, whereas prostate basal cells display immunostaining with HMWCK (34βE12), p63, and S100A6 (Fig. 26.34) [149–154]. Immunolabeling for basal cell markers is usually used in a mode of “negative” diagnostic marker in order to show the absence of basal cells in prostate carcinoma (Fig. 26.35). Basal cell cocktail is a mixture of basal cell markers (HMWCH and p63 or CK5/6 and p63) used to highlight the presence of basal cells in normal glands which differentiates benign lesions from prostate intraepithelial neoplasia (PIN) and prostate adenocarcinoma [201]. Metastatic carcinoma of prostate origin exhibits reactivity to CK 7 and CK20 as well as PSA (Fig. 26.36). Table 26.26 summarized the immunoprofile of normal prostate glands as compared with PIN and adenocarcinoma.
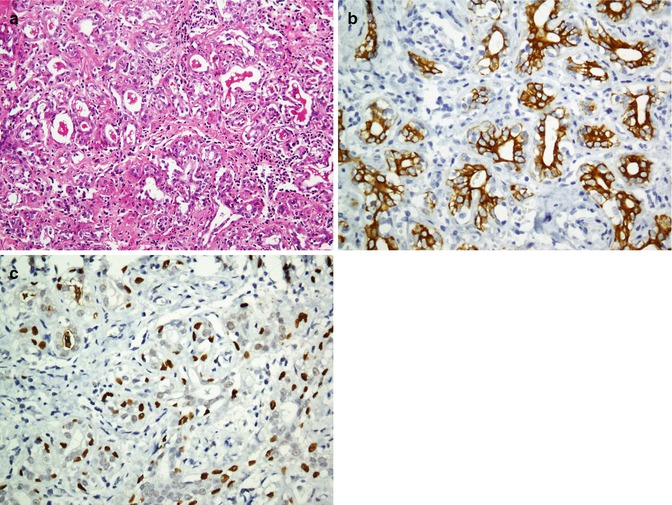
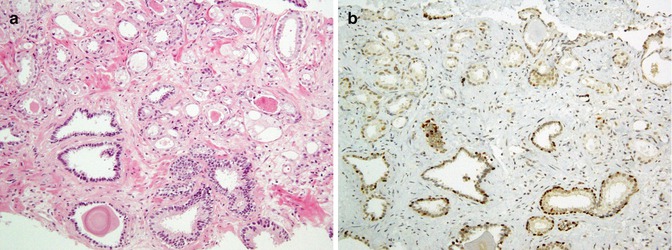
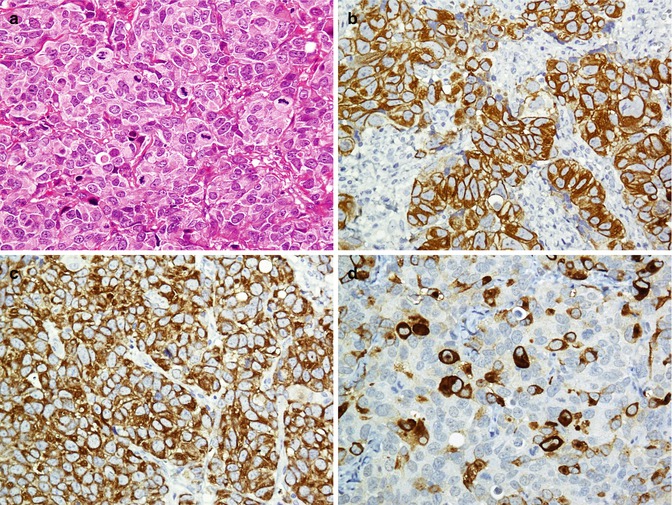

Fig. 26.34
Normal prostate tissue (a). The epithelium is immunostained with PSA (b), and basal cells are immunoreacted with p63 (c)

Fig. 26.35
Atypical prostate glands in the top of the picture which are highly suspicious of adenocarcinoma (a) show negative reaction to p63 (b). Some normal glands at the bottom of the picture exhibit reaction with p63

Fig. 26.36
An undifferentiated carcinoma from the pelvis with high mitotic rate (a) demonstrates cytoplasmic reaction with CK7 (b), CK20 (c), and PSA (d) which support the origin of this tumor as prostate
Table 26.26
Immunoprofile of normal prostate (NP), high-grade prostate intraepithelial neoplasia (HGPIN), and prostate adenocarcinoma (PAC)
Marker | NP | HGPIN | PAC | Application |
|---|---|---|---|---|
PSA | +E | + | + | Weak reaction in HGPAC or metastatic carcinoma, to differentiate HGPAD from other undifferentiated carcinoma (colon, urothelium) |
PSAP | +E | + | + | Similar to PSA |
PSMA | +E | + | ++ | Correlated with grade and stage, more intense in HGPAC |
P501S | +E | + | + | To differentiate high-grade PAC from other high-grade adenocarcinomas (colon, urothelium) |
P504S (AMACR) | − | ++ | ++ | Combine with basal cell markers to differentiate HGPIN and PAC from normal prostate |
HMWCK (34βE12) | +B | Partial loss | − | Complete loss in PAC (“negative” marker) |
p63 | +B | Partial loss | − | More sensitive than HMWCK (“negative” marker) |
CK5/6 | +B | Partial loss | − | More sensitive than HMWCK (“negative” marker) |
26.7.7 Testis
Testicular tumors are classified into germ cell tumors and sex cord stromal tumors. Germ cell tumors are the most common type with classic seminoma subtype comprising the majority. The definite diagnosis of these tumors is dependent on proper application of the immunohistochemistric markers and histopathologic evaluation of the biopsy (Figs. 26.37, 26.38, and 26.39). Table 26.27 summarized the immunophenotype of testicular tumors.
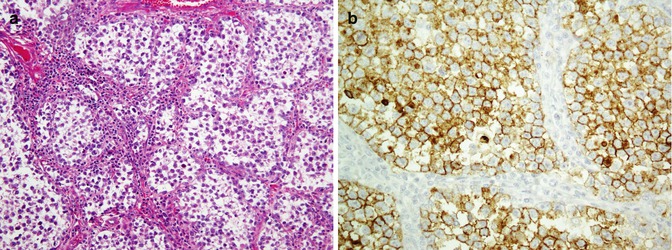
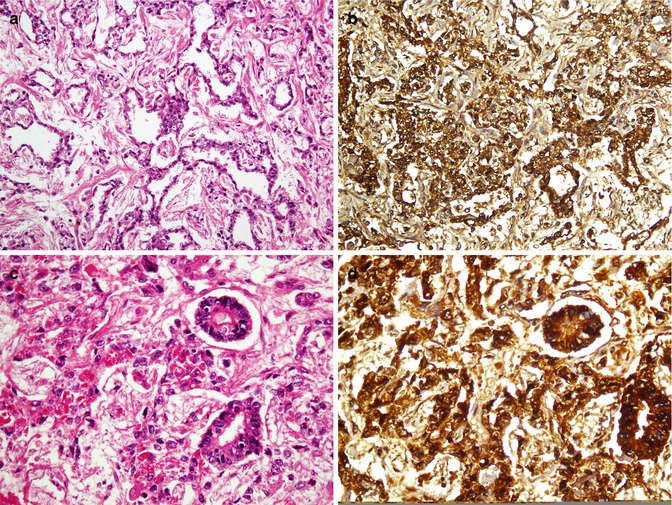
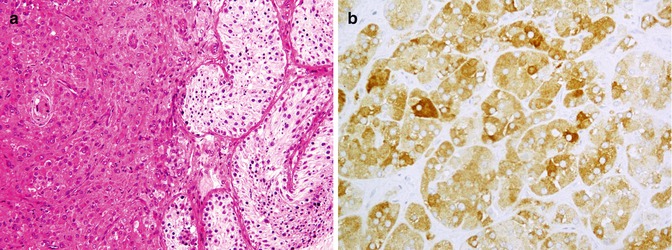

Fig. 26.37
Classic seminoma with polygonal cells and abundant watery cytoplasm (a) shows immunostaining with PLAP (b)

Fig. 26.38
Yolk sac tumor with tubuloglandular structures exhibits immunostaining with AFP (a, b) and glandular structures with numerous hyaline globules which are positive for AFP (c, d)

Fig. 26.39
Leydig cell tumor. Eosinophilic polygonal cell growth in the adjacent of seminiferous tubules (a) show immunoreaction with inhibin A (b)
Table 26.27
Immunophenotype of testicular tumors: classic seminoma (CS), spermatocytic seminoma (SS), embryonal carcinoma (EC), yolk sac tumor (YST), choriocarcinoma (CC), Sertoli cell tumor (SCT), and Leydig cell tumor (LCT)
Germ cell tumors (PLAP+, inhibin−) | Sex cord stromal tumors (PLAP−, inhibin+)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||||
|---|---|---|---|---|---|---|