be associated with these vaccines. In most cases, there was insufficient evidence to establish causation. Table 25.4 summarizes the IOM findings for adverse events in which data was available to reach a conclusion. Subsequently, the IOM findings were reviewed by the Advisory Committee for Immunization Practices (ACIP) who assessed newer data related to Guillain-Barre syndrome. Based on this newer data the ACIP did not confirm a causal relationship between OPV, DTP, or tetanus toxoid and Guillain-Barre syndrome. Further studies failed to substantiate an increased risk for chronic arthritis among women vaccinated with RA 27/3 (rubella) vaccine. Most recently controversy has arisen over the role of measles, mumps, and rubella (MMR) vaccine in autism. Two large studies clearly refuted this suggestion: (i) a cohort study of all children born in Denmark from 1991 to 1998 (440,665 [82%] of 537,303 received MMR) demonstrated a relative risk for autistic disorder in vaccinated children vs. unvaccinated of 0.92 (0.68-1.24); and (ii) a case-control study from the UK General Practice Research Database reported no association between MMR use and pervasive developmental disorder (OR, 0.85; 95% CI, 0.68-1.09). In the later study, findings were similar when restricted to autism alone.
Controlled major infectious diseases
Eradicated smallpox from the world
Saved over 5 million lives annually worldwide
TABLE 25.1 ▪ HISTORICAL COMPARISON OF MORBIDITY AND MORTALITY FOR VACCINE-PREVENTABLE DISEASES: UNITED STATES | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
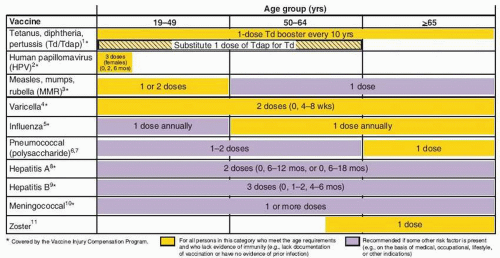
and acellular pertussis [Tdap] should replace a single dose of Td for adults aged <65 years who have not previously received a dose of Tdap); (ii) MMR vaccination ≥1 dose if no documentation of vaccination or prior infection; (iii) influenza vaccine given to high risk groups and considered for all patients; (iv) varicella vaccine given to patients with no history of clinical varicella; and (v) HPV vaccine women ≤26 years who have not completed HPV vaccine series. Age group 50 to 64 years of age should receive: (i) Td booster every 10 years (substitute one-dose Tdap); (ii) influenzae vaccine annually for all persons in this age group; (iii) varicella vaccine in patients with no clinical history of varicella; and (iv) zoster vaccine, a single dose for adults ≥60 years. For those ≥65 years of age it is recommended that they receive influenzae vaccine annually, one dose of pneumococcal vaccine, one dose of zoster vaccine if not given before,
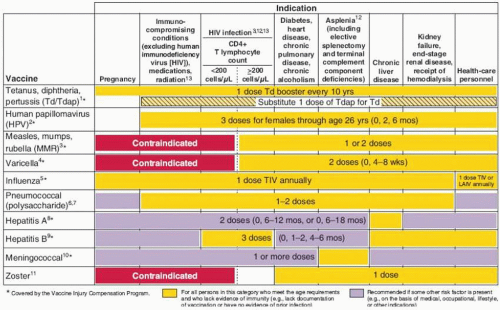
varicella vaccine if no previous infection or vaccination, and Td booster every 10 years. In all age groups, additional vaccines are recommended based on the presence of risk factors (e.g., medical, occupational, lifestyle) (Figs. 25.1 and 25.2). Figure 25.2 summarizes vaccines that are indicated for adults based on medical and occupational indications.
| |||||||||||||||||||||||||||||||||||
TABLE 25.3 ▪ MAJOR VACCINES RECOMMENDED FOR ROUTINE USE IN ADULTS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 25.4 ▪ SUMMARY OF INSTITUTE OF MEDICINE FINDINGS ON THE RELATIONSHIP OF ADVERSE EVENTS TO INDIVIDUAL VACCINESa | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
less expensive. This technology will allow for rapid creation of new vaccines.
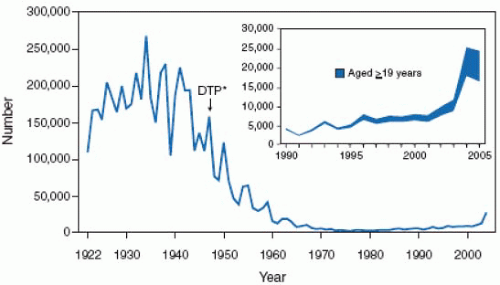
infections and their associated complications worldwide. Measles is a ubiquitous, highly contagious infection, which, prior to vaccine availability, affected nearly 100% of the population by adolescence. In the United States during the premeasles vaccine era there were approximately 500,000 cases of measles reported each year with an estimated actual 4 million cases annually. The morbidity and mortality associated with measles was also extensive with an estimated annual occurrence of 150,000 cases of pneumonia; 48,000 hospitalizations; 7,000 seizure episodes, 4,000 cases of encephalitis of which 25% had permanent neurologic sequelae or deafness; and 500 deaths. Subacute sclerosing panencephalitis (SSPE) is a progressive fatal disease of the central nervous system caused by persistent measles virus infection.
susceptible persons unless there is a contraindication (Figs. 25.1 and 25.2). Persons born prior to 1957 are likely to have been naturally infected with measles, mumps, and rubella and thus can be considered immune. However, in the case of rubella it is critical to ensure that women of childbearing age are immune to rubella. Thus, adult women prior to pregnancy and adult seronegative pregnant women in the postpartum period have been targeted for rubella vaccine administered as a single dose of MMR. In the United States, persons are considered susceptible to measles, mumps, and rubella unless they (i) have documentation of adequate vaccination (two doses after age 12 months); (ii) have laboratory proof of immunity, or (iii) have evidence of physician-diagnosed measles, mumps, or rubella. Among adults, particular groups have been the focus for vaccination of susceptible individuals. These include (i) attendees of college or other higher educational institutions; (ii) healthcare workers; (iii) military recruits; and (iv) international travelers. Susceptible adults should receive two doses of MMR separated by at least 28 days.
TABLE 25.5 ▪ KEY CHANGES IN THE 2006 ACIP RECOMMENDATIONS ON MUMPS | |||||||
|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree