Imaging of Transitional Cell Carcinoma
Efrén J. Flores
Mukesh Harisinghani
Transitional cell carcinoma is the predominant histologic type of urothelial cancer in the United States and western Europe, accounting for approximately 90% to 95% of urinary bladder and upper urothelial malignancies, with the remaining being squamous cell and adenocarcinomas or rare sarcomas and metastases from primary tumors (1,2). The typical clinical presentation of transitional cell carcinomas is usually painless hematuria with radiology playing an important role in the diagnosis of transitional cell carcinoma. Recent advancements in diagnostic imaging, such as in multidetector computed tomography (MDCT) and magnetic resonance imaging (MRI), have allowed imaging to provide more accurate detection and be a key component in grading and staging
The purpose of this chapter is to provide an overview of diagnostic imaging assessment for transitional cell carcinoma and to provide an overview of how the most recent advancements have improved staging and grading with a focus on cross-sectional imaging modalities of MDCT and MRI.
PLAIN RADIOGRAPHY
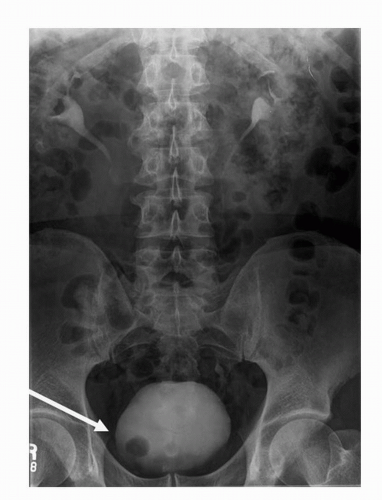
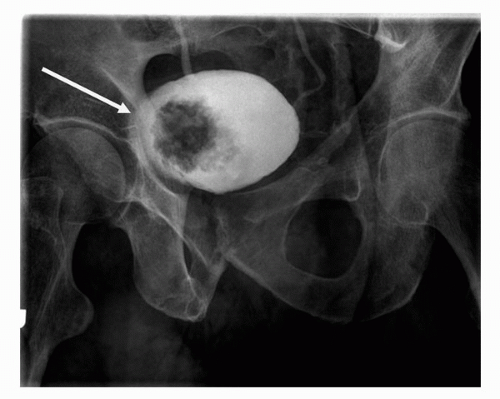
Plain radiography as a modality has high spatial resolution, but extremely poor soft tissue contrast. As a result, plain radiography remains a poor means of diagnosing transitional cell carcinomas. Plain radiography may detect the presence of mottled or irregularly shaped calcification, which is present in 2% of renal transitional cell carcinoma and 0.7% to 6% of bladder transitional cell carcinoma. Although the etiology of the calcification maybe secondary to malignancy, it is often secondary to superimposed infection with dystrophic calcification, cystic degeneration, necrosis, or hemorrhage (3,4,5,6). Plain radiography has largely been replaced by MDCT due to increased sensitivity for detection and localization of calcification (Fig. 20.1).
ULTRASONOGRAPHY
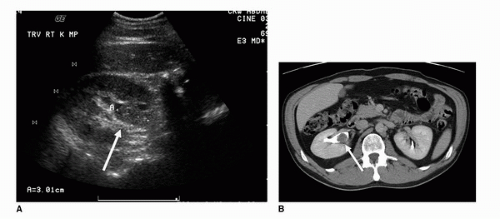
Ultrasound examination of the kidneys and urinary bladder is a noninvasive imaging modality, whose precise role in the evaluation of transitional cell carcinomas remains undetermined. The benefits and advantages of ultrasound in the evaluation of hematuria, which is the most common presenting sign in patients with bladder and upper tract transitional cell carcinoma, include the following: (a) Ultrasound is readily available, cost effective, and requires no patient preparation. (b) Ultrasound examination of the urinary bladder does not require any use of intravenous contrast and therefore minimizes the associated risk that accompanies intravenous, iodinated contrast. The disadvantages include poor spatial resolution, poor soft tissue contrast, operator dependence, and lack of full volumetric coverage. As a screening modality, ultrasound is effective in detecting indirect signs of TCCs such as obstruction of the collecting system or a heterogeneously echogenic soft tissue mass in the renal pelvis (7). In the setting of mild pelvocaliectasis, filling defects within the proximal collecting systems may be visualized against the background of anechoic urine (Fig. 20.2). In the absence of obstructive hydronephrosis and hydroureter, the ureters are not well visualized with ultrasound; therefore, subtle infiltrating lesions may go unnoticed (Fig. 20.3). However, developments in high-resolution endoluminal US performed during ureteroscopy have shown promise in the evaluation of upper tract TCC (7,8).
Within the bladder, ultrasound has demonstrated increased sensitivity secondary to the contrast in the background of an anechoic bladder (Fig. 20.4). In a study of 1,007 patients complaining of hematuria, Datta et al. demonstrated that the sensitivity of ultrasound with respect to bladder cancer was 63% and specificity was 99%. The odds ratio of diagnosing cancer in patients with visible hematuria compared to microscopic or unspecified hematuria was 3.3 (9). Dibb et al. retrospectively analyzed the ultrasonographic features of bladder tumors during transabdominal ultrasonography. Of 109 patients reviewed, 104 had transitional cell carcinoma, 3 adenocarcinoma, 1 carcinosarcoma, and 1 prostatic carcinoma. The most common appearance was a polypoid lesion arising from the trigone, but there was much variation in the ultrasonographic features of bladder tumors (10).
Transabdominal ultrasound imaging is limited in diagnosing tumors of the bladder neck, dome, and anterior wall. Transrectal and transvaginal approaches, secondary to both the improved proximity to the urinary bladder and the increased probe frequency used, have significantly improved the detection sensitivity in this regard. Furthermore, endoluminal (transurethral) ultrasound techniques have evolved and provide intravesicular imaging with high-frequency (20 MHz) miniature probes. Although limited, these techniques have demonstrated excellent correlation with stage pTa or pT1 disease, but decreased sensitivity in Stage 2 disease, likely secondary to lack of penetration with the high-frequency probe. Furthermore, these techniques suffer from being invasive and often require sedation for administration. In some studies endoluminal, transurethral ultrasound demonstrated lower sensitivity than cystoscopy, bimanual examination, and biopsy (11).
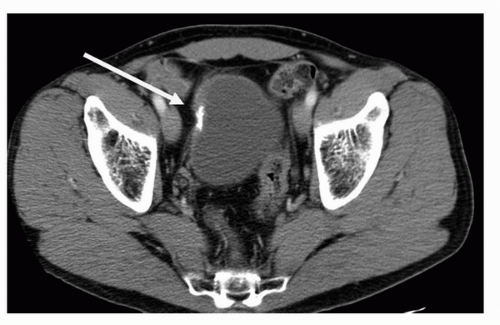 FIGURE 20.1. Axial contrast-enhanced CT image demonstrates dystrophic calcification within the right aspect of the bladder wall. Biopsy confirmed the diagnosis of TCC. |
CONTRAST-ENHANCED UROGRAPHY
Intravenous, Antegrade and Retrograde Urography
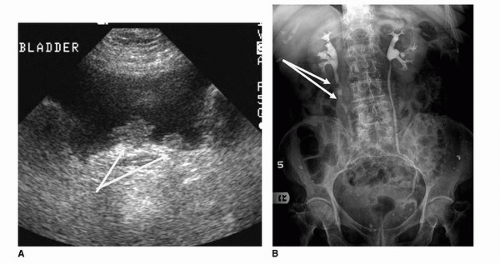
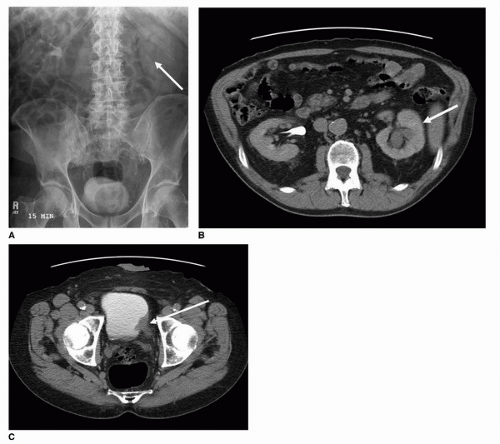
Until recent advancements in diagnostic imaging, intravenous urography or pyelography was considered the most sensitive initial screening method for patients presenting with painless hematuria or with cytology suspicious of malignancy. The intravenous pyelogram is performed by injecting iodinated contrast, followed by imaging with both plain radiography and tomography, of the contrast-filled collecting system, ureters, and bladder. As 6% of transitional cell carcinoma appears within the upper urothelial tract, it is important to investigate not only the bladder but also the collecting system and ureters (Fig. 20.5) (12). Masses may appear as nodular, well-demarcated filling defects or frondlike and irregular filling defects within the collecting system, ureter, or bladder
(Figs. 20.6 and 20.7). Asymmetric thickening of the bladder and ureters should also be viewed with some level of suspicion, and should be further investigated cystoscopically. Masses within the infundibulum or proximal collecting system may also present with asymmetric enhancement of the entire renal parenchyma with obstructive hydronephrosis, or part of the kidney with infundibular stenosis during the nephrographic phase (Fig. 20.8).
(Figs. 20.6 and 20.7). Asymmetric thickening of the bladder and ureters should also be viewed with some level of suspicion, and should be further investigated cystoscopically. Masses within the infundibulum or proximal collecting system may also present with asymmetric enhancement of the entire renal parenchyma with obstructive hydronephrosis, or part of the kidney with infundibular stenosis during the nephrographic phase (Fig. 20.8).
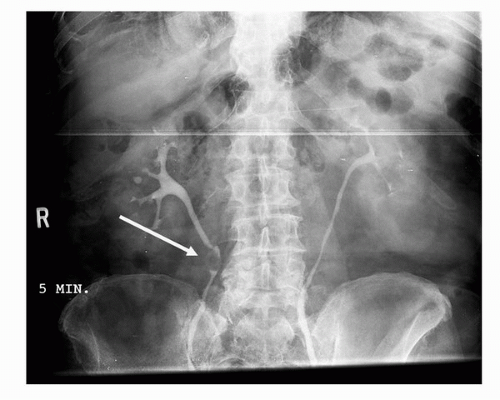
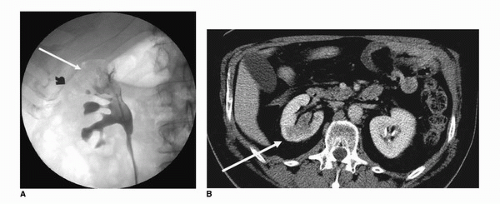
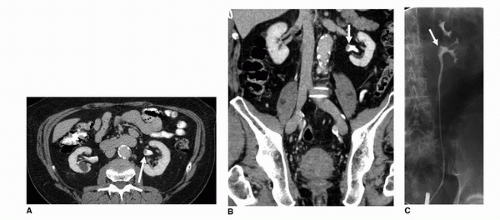
Retrograde pyelography is an invasive means of examining the collecting system, ureter, and bladder in patients allergic to intravenous contrast. Being invasive, this technique requires catheterization and cystoscopic selection of the ureters. The morphologic findings associated with malignancy are similar to those with IVP and include filling defects within the bladder, ureters, and collecting system (Fig. 20.9).
Although urography remains sensitive to large massoccupying lesions, it is insensitive to smaller lesions or carcinoma in situ. Furthermore, the depth of invasion, which is the most important prognostic indicator and requisite for accurate staging of TCC, is not accurately assessed. Only with associated sequelae such as hydronephrosis and hydroureter is muscular invasion inferred. This sign as well is anatomically dependent and is only noted in 92% of cases (13,14).
The improvement in imaging technology, most notably within MRI and MDCT, has improved the detection sensitivity and the staging of TCC.
MULTIDETECTOR COMPUTED TOMOGRAPHY
In addition to urinalysis, the evaluation of painless hematuria usually includes a combination of diagnostic imaging and cystoscopy (15). Until very recently, the radiologic evaluation of painless hematuria involved contrast urography, or a combination of retrograde urography and cystoscopy in patients who are intolerant of intravenous contrast. Nevertheless, many studies have demonstrated the superiority of MDCT in the evaluation of both urinary tract calculi as well as renal masses (16,17,18,19). The spatial resolution in the axial dimension, concurrent with improvements in computing technology, and multiplanar reconstructions have demonstrated similar improvements in the evaluation of the urothelium in delayed, urographic phase MDCT imaging (MDCT urography). Studies have demonstrated comparable sensitivity of excretory phase MDCT with intravenous urography in detecting the etiology for patients presenting with painless hematuria (20,21).
The evaluation of painless hematuria has now shifted in many institutions to utilizing contrast-enhanced MDCT as the primary screening modality. A typical hematuria protocol
is detailed in the appendix. Advancements in MDCT allow postprocessing very thin slice thickness (e.g., 0.625 mm) and multiplanar reconstructions in sagittal (Fig. 20.10) and coronal planes of the excretory phase images. MDCTU offers several advantages for imaging of the urinary tract: single breath-hold coverage of the entire urinary tract with absence of respiratory misregistration and rapid imaging with optimum contrast medium opacification. Additionally, acquisition of multiple thin overlapping slices provides excellent two-dimensional (2D) and three-dimensional (3D) reformations, such as maximum intensity projection (MIP; Fig. 20.11) and volume rendered images, which facilitate virtual cystoscopy (22,23).
Recent studies have demonstrated that MDCTU is not limited to evaluation of the bladder, but is also effective in evaluating abnormalities in the upper urinary tract (24,25,26).
is detailed in the appendix. Advancements in MDCT allow postprocessing very thin slice thickness (e.g., 0.625 mm) and multiplanar reconstructions in sagittal (Fig. 20.10) and coronal planes of the excretory phase images. MDCTU offers several advantages for imaging of the urinary tract: single breath-hold coverage of the entire urinary tract with absence of respiratory misregistration and rapid imaging with optimum contrast medium opacification. Additionally, acquisition of multiple thin overlapping slices provides excellent two-dimensional (2D) and three-dimensional (3D) reformations, such as maximum intensity projection (MIP; Fig. 20.11) and volume rendered images, which facilitate virtual cystoscopy (22,23).
Recent studies have demonstrated that MDCTU is not limited to evaluation of the bladder, but is also effective in evaluating abnormalities in the upper urinary tract (24,25,26).
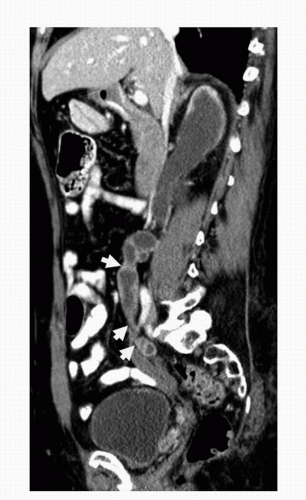
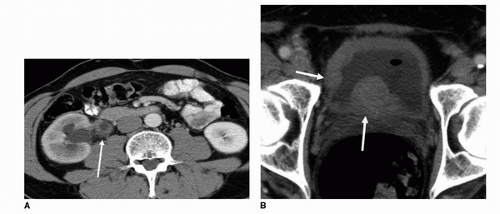
Criteria for mass lesions applied to intravenous urography are similar for MDCTU. Images should be reviewed with narrow and wide windows in two planes (axial and coronal), as small bladder filling defects may be missed if only narrow (soft tissue) windows are used (27). Primary tumors appear as focal filling defects within the ureters (Fig. 20.12), infiltrating asymmetric soft tissue density within the collecting system and distal ureters (Fig. 20.13), or multicentric thickening of the ureters or bladder wall (Fig. 20.14). Furthermore, as a result of approximately 5% of transitional cell carcinomas containing calcification (28), nodular or arched surface calcifications
may be visualized on noncontrast or within the early phase of a MDCTU examination (Fig. 20.1).
may be visualized on noncontrast or within the early phase of a MDCTU examination (Fig. 20.1).
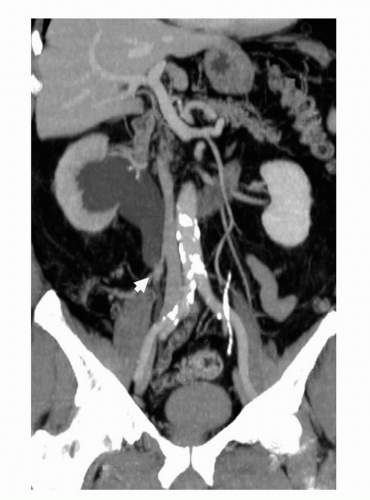
A typical hematuria protocol images the collecting system, ureters, and bladder in two different phases of contrast administration that varies the amount of contrast within the bladder and allows contrast opacification of the urinary system. Bladder masses are usually noted as filling defects and are most often seen in a later phase of imaging when the bladder is filled with contrast (Fig. 20.15). Masses can also show changes in relative contrast depending on the phase of imaging utilized, as well as the amount of urine present within the bladder which can dilute the contrast. This is well demonstrated in Figure 20.16, where a mass at the right ureterovesical junction (UVJ) has penetrated and extended into the distal right ureter. These changes in enhancement and the contrast phase, at which images are acquired, could potentially mask a lesion if images are not acquired with appropriate timing. For instance, some masses are more easily distinguished during the early phase of contrast administration, and could easily be missed during a later phase of imaging, when there is more contrast within the bladder. This is illustrated in Figures 20.17 and 20.18. At our institution, the split-bolus MDCTU technique is used, where intravenous contrast (i.e., 40 mL) is given just after the unenhanced images are obtained along with 250 mL of intravenous normal saline and the patient is taken off the table. Once the saline drip is finished, the patient is placed back on the table and a second bolus of intravenous contrast (i.e., 80 mL) is given after a second delay of approximately 100 seconds and images are acquired in prone position. More delayed images of the bladder are acquired to get
adequate contrast opacification. The purpose of the split-bolus technique is to image the kidneys during synchronous nephrographic and excretory phases, as well as getting adequate contrast opacification of the bladder (29).
adequate contrast opacification. The purpose of the split-bolus technique is to image the kidneys during synchronous nephrographic and excretory phases, as well as getting adequate contrast opacification of the bladder (29).
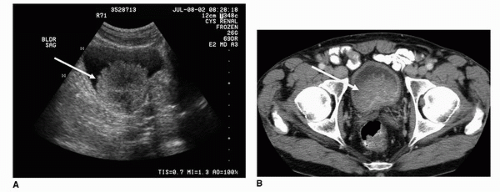
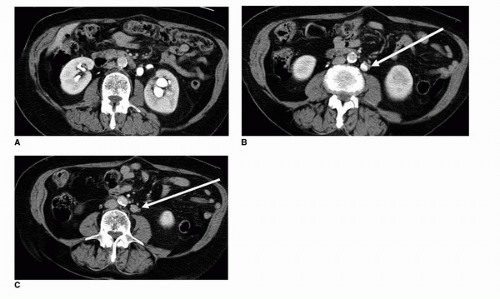
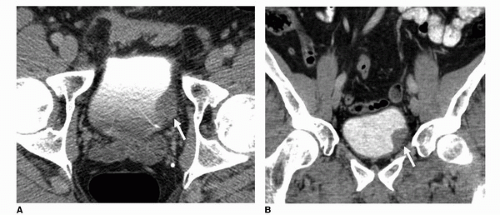 FIGURE 20.15. (A) Axial and (B) coronal images of the bladder from a contrast-enhanced MDCTU demonstrate a filling defect along the left posterolateral bladder wall (arrow).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|