Bone marrow is the essential for function of hematopoiesis, which is vital for the normal functioning of the body. Bone marrow disorders or dysfunctions may be evaluated by blood workup, peripheral smears, marrow biopsy, plain radiographs, computed tomography (CT), MRI and nuclear medicine scan. It is important to distinguish normal spinal marrow from pathology to avoid missing a pathology or misinterpreting normal changes, either of which may result in further testing and increased health care costs. This article focuses on the diffuse bone marrow pathologies, because the majority of the bone marrow pathologies related to hematologic disorders are diffuse.
Key points
- •
Bone marrow disorders or dysfunctions may be evaluated by blood workup, peripheral smears, and marrow biopsy.
- •
Noninvasive techniques such as plain radiograph, computed tomography (CT), MRI and nuclear medicine scan may also be used to evaluate bone marrow disorders.
- •
It is important to distinguish normal spinal marrow from pathology to avoid missing a pathology or misinterpreting normal changes, which may result in further testing and increased costs.
Introduction
Bone marrow is one of the largest organs of the human body. It serves the essential function of hematopoiesis. Its function is of vital importance for the normal functioning of the body as it continuously replenishes the cells required for oxygen delivery, excretion of waste/toxic material, various defense mechanisms, and maintaining the balance between the bleeding and clotting mechanism of the body. Bone marrow disorders or dysfunctions may be evaluated by blood workup, peripheral smears, and marrow biopsy. They may be also evaluated using noninvasive techniques such as plain radiographs, computed tomography (CT), MRI and nuclear medicine scan (single photon emission CT/PET). MRI, owing to its better soft tissue differentiation and higher spatial resolution, can evaluate marrow changes very early, thus giving a lead to the clinician regarding the undergoing disease process.
It is important to distinguish normal spinal marrow from pathology to avoid missing a pathology or misinterpreting normal changes, either of which may result in further testing and increased health care costs. On imaging, bone marrow pathologies may be classified into focal and diffuse ( Table 1 ). In this article, we focus predominately on the diffuse bone marrow pathologies, because the majority of the bone marrow pathologies related to hematologic disorders are diffuse.
| MR Signal Intensity | Focal Lesions | Diffuse Lesions |
|---|---|---|
| T1 hyperintense | Normal variant Focal fatty marrow Solitary hemangioma Degenerative disk disease Paget disease Melanoma metastasis Bone marrow hemorrhage Lipoma | Prior radiation treatment Osteoporosis Multiple hemangiomas Spondyloarthropathy Anorexia nervosa Chronic malnutrition |
| T1 Hypointensity | Degenerative endplate changes Osteomyelitis Amyloid Atypical hemangioma Fracture Malignancy Fibrous dysplasia Metastasis Myeloma Lymphoma Primary bone tumor Fracture | Hematopoietic hyperplasia Neoplasm Renal osteodystrophy Sarcoidosis Spondyloarthropathy Myelofibrosis Mastocytosis Hemosiderosis Gaucher disease Gout |
Bone Marrow Anatomy (Composition of Bone Marrow)
Normal bone structure consists of an outer cortex with an interior network of ossicles, referred to as trabecular, spongy, or cancellous bone. Approximately 80% of total bone volume consists of the compact cortical bone, and the remaining 20% is made up of cancellous or trabecular bone. By definition, trabecular bone refers to the structural network that partitions the space enclosed by the cortical bone. Trabeculae are thin and consist of segments formed by parallel lamellae. Bone marrow is a term used to refer to the tissue occupying the cavities between the trabecular bone.
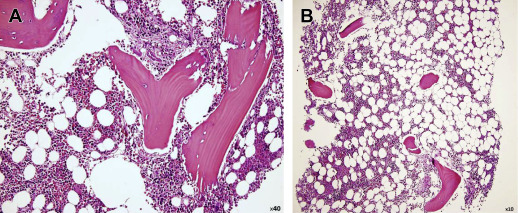
Normal bone marrow is composed of red marrow, yellow marrow, osseous components, and a supporting system. Red bone marrow is the primary organ for the production of mature blood cells, and therefore represents hematopoetically active bone marrow. It is composed of 40% water, 40% fat, and 20% protein ( Fig. 1 ). Red bone marrow has a definitive life span, and must be replenished by the body under normal circumstances. Yellow marrow represents hematopoetically inactive bone marrow. It is composed of 15% water, 80% fat, and 5% protein. Very few capillaries are present in the yellow marrow. With aging, there is a decrease in the number of trabeculae in bone, and subsequent conversion of red to yellow marrow.
Progression of Bone Marrow Changes from Childhood to Adult (Conversion)
At birth, red marrow is present throughout the entire skeleton. Normal physiologic conversion of red to yellow marrow occurs gradually from birth, and continues until adult age. As a general rule, marrow conversion first begins in the distal appendicular skeleton, beginning in the hands and feet and continues into the more proximal bones of the skeleton. By 25 years of age, the adult distribution of red marrow persists predominately in the axial skeleton, proximal humeri, and proximal femora. With advancing age, there may be further conversion of red to yellow marrow, including the axial skeleton. Thus, the normal appearance of the bone marrow on imaging widely depends on the age of the patient. It is worth noting that within long bone, red to yellow bone marrow conversion begins first the diaphysis, then in the distal metaphysis, and finally the proximal metaphysis. The epiphysis and apophyses are cartilaginous at birth, and in the adult contain yellow marrow.
Introduction
Bone marrow is one of the largest organs of the human body. It serves the essential function of hematopoiesis. Its function is of vital importance for the normal functioning of the body as it continuously replenishes the cells required for oxygen delivery, excretion of waste/toxic material, various defense mechanisms, and maintaining the balance between the bleeding and clotting mechanism of the body. Bone marrow disorders or dysfunctions may be evaluated by blood workup, peripheral smears, and marrow biopsy. They may be also evaluated using noninvasive techniques such as plain radiographs, computed tomography (CT), MRI and nuclear medicine scan (single photon emission CT/PET). MRI, owing to its better soft tissue differentiation and higher spatial resolution, can evaluate marrow changes very early, thus giving a lead to the clinician regarding the undergoing disease process.
It is important to distinguish normal spinal marrow from pathology to avoid missing a pathology or misinterpreting normal changes, either of which may result in further testing and increased health care costs. On imaging, bone marrow pathologies may be classified into focal and diffuse ( Table 1 ). In this article, we focus predominately on the diffuse bone marrow pathologies, because the majority of the bone marrow pathologies related to hematologic disorders are diffuse.
| MR Signal Intensity | Focal Lesions | Diffuse Lesions |
|---|---|---|
| T1 hyperintense | Normal variant Focal fatty marrow Solitary hemangioma Degenerative disk disease Paget disease Melanoma metastasis Bone marrow hemorrhage Lipoma | Prior radiation treatment Osteoporosis Multiple hemangiomas Spondyloarthropathy Anorexia nervosa Chronic malnutrition |
| T1 Hypointensity | Degenerative endplate changes Osteomyelitis Amyloid Atypical hemangioma Fracture Malignancy Fibrous dysplasia Metastasis Myeloma Lymphoma Primary bone tumor Fracture | Hematopoietic hyperplasia Neoplasm Renal osteodystrophy Sarcoidosis Spondyloarthropathy Myelofibrosis Mastocytosis Hemosiderosis Gaucher disease Gout |
Bone Marrow Anatomy (Composition of Bone Marrow)
Normal bone structure consists of an outer cortex with an interior network of ossicles, referred to as trabecular, spongy, or cancellous bone. Approximately 80% of total bone volume consists of the compact cortical bone, and the remaining 20% is made up of cancellous or trabecular bone. By definition, trabecular bone refers to the structural network that partitions the space enclosed by the cortical bone. Trabeculae are thin and consist of segments formed by parallel lamellae. Bone marrow is a term used to refer to the tissue occupying the cavities between the trabecular bone.
Normal bone marrow is composed of red marrow, yellow marrow, osseous components, and a supporting system. Red bone marrow is the primary organ for the production of mature blood cells, and therefore represents hematopoetically active bone marrow. It is composed of 40% water, 40% fat, and 20% protein ( Fig. 1 ). Red bone marrow has a definitive life span, and must be replenished by the body under normal circumstances. Yellow marrow represents hematopoetically inactive bone marrow. It is composed of 15% water, 80% fat, and 5% protein. Very few capillaries are present in the yellow marrow. With aging, there is a decrease in the number of trabeculae in bone, and subsequent conversion of red to yellow marrow.
Progression of Bone Marrow Changes from Childhood to Adult (Conversion)
At birth, red marrow is present throughout the entire skeleton. Normal physiologic conversion of red to yellow marrow occurs gradually from birth, and continues until adult age. As a general rule, marrow conversion first begins in the distal appendicular skeleton, beginning in the hands and feet and continues into the more proximal bones of the skeleton. By 25 years of age, the adult distribution of red marrow persists predominately in the axial skeleton, proximal humeri, and proximal femora. With advancing age, there may be further conversion of red to yellow marrow, including the axial skeleton. Thus, the normal appearance of the bone marrow on imaging widely depends on the age of the patient. It is worth noting that within long bone, red to yellow bone marrow conversion begins first the diaphysis, then in the distal metaphysis, and finally the proximal metaphysis. The epiphysis and apophyses are cartilaginous at birth, and in the adult contain yellow marrow.
Imaging techniques
MRI remains the imaging modality of choice in the evaluation of the bone marrow disorders owing to its excellent soft tissue differentiation and early diagnosis of abnormal conditions compared with the other imaging modalities. Single photon emission CT and PET are sensitive but lack specificity and anatomic resolution in the imaging of the bone marrow.
MRI Techniques
MRI appearance of the bone marrow depends on pulse sequence on which it is being evaluated and the relative composition of the marrow (proportion of proteins, water, fat and cells, ie, the proportion of red and yellow marrow). Commonly used MR sequences for the evaluation of bone marrow disorders include T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), a short-tau inversion recovery sequence (STIR), and fat saturation gadolinium-enhanced T1WI ( Fig. 2 ). A T1WI spine echo sequence provides terrific differentiation between the red and yellow marrow components. On T1WI, red marrow will demonstrate decreased signal intensity, lower than subcutaneous fat but higher than disk or muscle. In contrast, yellow marrow demonstrates a hyperintense signal comparable with the subcutaneous fat on T1WI. This is a result of the greater fat content of yellow marrow, which significantly shortens the T1 relaxation time. Owing to longer T2 relaxation of the fat protons, yellow marrow shows high signal intensity on the T2F fast spin echo. Red marrow signal intensity is slightly lower than that of yellow marrow on T2 fast spin echo.
With the STIR technique, the signal from fat is suppressed and the signal from water is preserved, thus providing high tissue contrast, which is very useful in evaluating bone marrow. The STIR sequence produces more homogeneous fat suppression than T2 fast spin echo fat saturation. T2WI spin echo sequences and STIR sequences are very sensitive for evaluation of bone marrow pathology. T2WI is also very sensitive in diagnosing cord-related pathologies.
Fat saturation gadolinium-enhanced T1WI are used routinely in the evaluation of bone marrow. The basic principle of this technique is to highlight the Gd-related enhancement by suppressing the signal from fat. Contrast-enhanced sequencing is very helpful in confirming neoplastic or infective lesions. It is also very helpful in defining the extent of extramedullary spread in the paraspinal soft tissues and within the canal and in evaluating dural or leptomeningeal metastasis.
Less commonly used MR sequences in the evaluation of the spinal pathologies, which may highlight some of the marrow pathologies include T1 fluid-attenuated inversion recovery, diffusion-weighted imaging–apparent diffusion coefficient and in-phase and out-of-phase imaging sequences. T1 fluid-attenuated inversion recovery optimizes the tissue contrast between fatty marrow and abnormal tissue, thus improving the conspicuity of edema and metastatic lesions in the bone marrow. Diffusion-weighted imaging evaluates the molecular diffusion of the protons. Lesions with higher cellular density shows restricted diffusion that is bright on diffusion-weighted imaging and low signal intensity on apparent diffusion coefficient imaging. This property helps in identifying small primary neoplastic or metastatic disease.
Normal Bone Marrow Appearance on MRI from Infant to Adult
Bone marrow conversion in infant (up to 1 year of age)
From birth, there is a high concentration of red marrow present throughout the entire skeleton (both axial and appendicular). In the long bones, the medullary canals within the diaphysis and metaphysic of the long bones will be composed of red marrow and therefore will demonstrate low T1 signal in these areas. The epiphysis of long bones and apophyseal equivalents are unossified at this age, and will appear as intermediate signal. Once the epiphysis and apophysis ossify, they will contain yellow bone marrow, and therefore appear hyperintense on T1WI. At 1 year of age, red marrow predominates within the axial skeleton, and will appear hypointense on T1WI ( Fig. 3 ).
Bone marrow conversion in childhood
In long bones, there is high T1 signal intensity in the diaphysis owing to yellow marrow and intermediate to low T1 signal intensity in the metaphysis. Overall, there is still an abundance of red marrow within the axial skeleton in the first decade of life, and the majority of bone marrow should appear T1 hypointense.
Normal adult bone marrow
By 25 years of age, the majority of normal bone marrow conversion occurs in the appendicular skeleton, with sparing of the proximal femoral metaphyses and proximal humeral metaphysis, which demonstrate intermediate to low T1-weighted signal. Spinal bone marrow has the presence of red marrow throughout life, but the proportions of red and yellow marrow in the axial skeleton vary by age and environmental factors. The marrow conversion pattern in the axial skeleton is less predictable. Several patterns of marrow conversion have been described, including marrow conversion occurring first along the margins of the basivertebral veins, peripheral areas of conversion at the endplates involving the anterior and posterior vertebral body corners, patchy unorganized marrow conversion, or a combination of any of the above. Regardless of the process, there is a gradual decrease in the amount of red marrow in the axial skeleton. Before the age of 40 years, red marrow still dominates in the axial skeleton with only small areas of yellow marrow around the basivertebral plexus. With advancing age, the vertebral bone marrow becomes increasingly replaced with fatty marrow.
Imaging of marrow pathologies
Depending on the pathologic mechanism, marrow pathologies are classified into ( Table 2 ):
- a.
marrow reconversion;
- b.
marrow infiltration;
- c.
marrow depletion;
- d.
marrow changes owing to osseous dysplasia and bony abnormalities;
- e.
marrow abnormalities owing to metabolic disorders; and
- f.
miscellaneous.
| Pathologic Mechanism | Disorder |
|---|---|
| Marrow reconversion | Chronic anemia (sickle cell disease, thalassemia, hereditary spherocytosis), neoplastic replacement of normal bone marrow, concurrent administration of granulocyte-macrophage colony-stimulating factor |
| Marrow infiltration | Lymphoma, leukemia, Chronic myeloproliferative disorders metastasis, multiple myeloma |
| Marrow depletion | Radiation therapy, systemic chemotherapy aplastic anemia |
| Marrow changes owing to osseous dysplasia and bony abnormalities | Osteopetrosis, Paget disease, renal osteodystrophy |
| Marrow abnormalities owing to metabolic disorders | Gaucher disease, iron deposition, and iron overload state |
| Miscellaneous | Sarcoidosis gout |
Marrow reconversion
Marrow reconversion refers to replacement of yellow marrow by hematopoietic red marrow, which is a reversal of the normal physiologic conversion of bone marrow. In general, this process occurs as a response when systemic demand for hematopoiesis exceeds the capacity of the existing red marrow stores, for example, in chronic anemia.
Reconversion occurs in the opposite pattern of that of normal physiologic conversion. Thus, reconversion of bone marrow begins in the axial skeleton (flat bones of the pelvis and vertebrae of the spine), and progresses to the appendicular skeleton (individual long bones, followed by the distal extremities). Reconversion within individual long bones also follows the predictable pattern of proximal metaphysis, followed by distal metaphysis, and finally the diaphysis.
Common causes of reconversion of bone marrow include states of chronic anemia (sickle cell disease, thalassemia, hereditary spherocytosis), neoplastic replacement of normal bone marrow, concurrent administration of granulocyte-macrophage colony-stimulating factor during chemotherapy treatment, and nonpathologic circumstances, during which an increased oxygen requirement is present, such as rigorous athletic training and high-altitude dwelling.
Sickle Cell Disease
Sickle cell disease is a hereditary blood disorder characterized by an abnormality of the hemoglobin molecule in red blood cells, leading to an abnormal “sicklelike” shape of the red blood cells rather than the normal biconcave shape. These sickled red blood cells cause vascular occlusion, which in turn leads to tissue ischemia and infarction. When a patient possesses 2 sickle cell hemoglobin genes (Hb SS), it is called sickle cell anemia. Various other combinations seen include sickle cell trait (Hb SA); HbSC; Hb S-thalassemias, and Hb SD. Hb SS ( sickle cell anemia ) accounts for 60% to 70% of the cases of sickle cell disease in the United States and has the severest clinical manifestations of any of the sickle cell disease variants. The majority of the clinical presentations and complications of the sickle cell disease is owing to infarction and infection.
Anemia in sickle cell disease can be owing to several acute or chronic factors, which include vasoocclusive crisis (obstruction of capillaries by abnormally shaped red blood cells), splenic sequestration (abnormally shaped red blood cells being filtered out by the spleen), and hemolytic crisis (accelerated drops in hemoglobin levels owing to faster rate of breakdown of the abnormal red blood cells). These changes can lead to chronic anemia, which leads to excessive intramedullary and extramedullary hematopoiesis. Another complication is ischemic osteonecrosis owing to vasoocclusion. There is also increased susceptibility to infection in the skeleton and other organs of the body.
MRI is very sensitive in the evaluation of marrow conversion, infarction, and infection of the spine. Because of marrow hyperplasia and reconversion, spine marrow is diffusely T1 hypointense, and is slightly hyperintense on T2 fat saturation/STIR sequences ( Fig. 4 ). Widening of osseous medullary spaces leads to osteopenia and thinning of cortical bone, leading to biconcave appearance of the vertebral body and making them vulnerable to pathologic fractures. The central vertebral body compression gives a typical “H-shaped” vertebra, which is seen commonly in thoracic spine and is best appreciated on plain radiograph or reconstructed images of CT ( Fig. 5 ).