Hematopoietic stem cell transplantation (SCT) is now commonly used to treat several hematologic and nonhematologic diseases. Central nervous system (CNS) complications post-transplantation occur commonly in the first year and result in increased mortality from infectious, toxic, metabolic, or vascular causes. Infections secondary to aspergillus, toxoplasma and viruses cause many of the complications. Drug-related toxicities arising from conditioning regimens and graft-versus-host disease prophylaxis, as well as intraparenchymal hemorrhage, are not uncommon and can result in increased morbidity. Secondary CNS cancers have a higher incidence 5 or more years after allogeneic SCT.
Key points
- •
Central nervous system (CNS) complications in patients with stem cell transplants result in high morbidity.
- •
Major CNS complications include drug toxicity, infection, and cerebrovascular diseases, and occur most often during the first year after transplant.
- •
There is an increased risk of CNS cancers 5 or more years after allogeneic SCT.
Introduction
Hematopoietic stem cell transplantation (SCT) is used to treat several hematologic and nonhematologic diseases, and involves transfer of stem cells from a donor to a recipient. Although autologous transplants involve the collection of a patient’s own hematopoietic cells to be reinfused later, allogeneic SCT involves transplantation of cells from a healthy related or unrelated donor. Syngeneic transplants are uncommon and are taken from a genetically identical twin donor.
Stem cells can be harvested from bone marrow, umbilical cord, or peripheral blood. For autologous transplantation, hematopoietic stem cells are usually frozen and used within a few weeks. This can be used in older patients because it does not induce graft-versus-host disease (GVHD). Hence, mortality is significantly lower with autologous transplantation compared with allogeneic transplantation; however, the absence of graft-versus-tumor activity in autologous transplantation reduces its effectiveness.
The purpose of conditioning regimens before transplantation is to eradicate cancer and, in allogeneic transplantation, to induce immunosuppression that permits engraftment. Regimens typically involve both radiation and chemotherapy. A better understanding of graft-versus-tumor biology has led to the development of reduced-intensity regimens that are primarily immunosuppressive and depend on the graft to eradicate cancer. For patients with advanced hematologic cancer, however, the low mortality rate associated with reduced-intensity preparative regimens may be offset by high relapse rates. Chemotherapeutic agents in conditioning regimens may include cyclophosphamide, busulfan, and fludarabine. The incidence and severity of rejection and GVHD increase with human leukocyte antigens mismatch. Although cyclosporin A (CsA), tacrolimus, mycophenolate, methotrexate, and antithymocyte globulin can be used for GVHD prophylaxis, infection prophylaxis may include routine use of antibiotics, antifungal, and antiviral agents.
Introduction
Hematopoietic stem cell transplantation (SCT) is used to treat several hematologic and nonhematologic diseases, and involves transfer of stem cells from a donor to a recipient. Although autologous transplants involve the collection of a patient’s own hematopoietic cells to be reinfused later, allogeneic SCT involves transplantation of cells from a healthy related or unrelated donor. Syngeneic transplants are uncommon and are taken from a genetically identical twin donor.
Stem cells can be harvested from bone marrow, umbilical cord, or peripheral blood. For autologous transplantation, hematopoietic stem cells are usually frozen and used within a few weeks. This can be used in older patients because it does not induce graft-versus-host disease (GVHD). Hence, mortality is significantly lower with autologous transplantation compared with allogeneic transplantation; however, the absence of graft-versus-tumor activity in autologous transplantation reduces its effectiveness.
The purpose of conditioning regimens before transplantation is to eradicate cancer and, in allogeneic transplantation, to induce immunosuppression that permits engraftment. Regimens typically involve both radiation and chemotherapy. A better understanding of graft-versus-tumor biology has led to the development of reduced-intensity regimens that are primarily immunosuppressive and depend on the graft to eradicate cancer. For patients with advanced hematologic cancer, however, the low mortality rate associated with reduced-intensity preparative regimens may be offset by high relapse rates. Chemotherapeutic agents in conditioning regimens may include cyclophosphamide, busulfan, and fludarabine. The incidence and severity of rejection and GVHD increase with human leukocyte antigens mismatch. Although cyclosporin A (CsA), tacrolimus, mycophenolate, methotrexate, and antithymocyte globulin can be used for GVHD prophylaxis, infection prophylaxis may include routine use of antibiotics, antifungal, and antiviral agents.
Central nervous system complications
Central nervous system (CNS) complications after allogeneic hematopoietic SCT are common and life-threatening. Recent studies have shown the incidence of significant CNS complications in 9% to 14% of SCT subjects. In an autopsy study of 180 subjects who underwent bone marrow transplant, 90.55% of subjects were found to have CNS abnormalities. However, these complications were the cause of death in only 31 subjects (17%). In another study of 128 subjects, 32% demonstrated structural abnormalities on brain imaging.
CNS complications may be of infectious, vascular, toxic, immune-mediated, or metabolic origin. Disease relapse in the CNS and secondary malignancies can also occur. Neuroimaging is crucial for early diagnosis and treatment of CNS complications, with MRI significantly more likely than computed tomography (CT) to provide specific imaging diagnosis of cerebral lesions.
Drug-Related Toxicity
Busulfan and fludarabine are used in conditioning regimens. Although busulfan has been associated with seizures, in a large study of 954 pediatric subjects, the investigators demonstrated that the incidence of seizures was very low (1.3%) when it was administered along with seizure prophylaxis.
Significant dose-related neurotoxicity has been described with fludarabine including cortical blindness, altered mental status, and generalized seizure with the toxicity seen several weeks after intravenous infusion. In a study of 1596 subjects, 39 subjects (2.4%) were reported to have fludarabine-related neurotoxicity with different manifestations, including posterior reversible encephalopathy syndrome (PRES), acute toxic leukoencephalopathy, and other leukoencephalopathy syndromes. Acute toxic leukoencephalopathy can manifest as fluid-attenuated inversion recovery (FLAIR) hyperintensity and restricted diffusion in the periventricular white matter with no enhancement. Prognosis is poor with fludarabine-related neurotoxicity, with decreased median time of survival.
Calcineurin inhibitors (CsA and tacrolimus [also known as FK506]) are often used for GVHD prophylaxis. In a retrospective study evaluating tacrolimus CsA in 87 SCT subjects, the investigators showed no significant differences in neurotoxicity between the drugs in the first 100 days after transplantation. Seizures were noted in 5 out of 87 subjects and altered mental status occurred in the presence of significant metabolic abnormalities and infection. In another prospective study of 294 subjects undergoing solid organ transplants, major neurotoxicity related to tacrolimus was reported in 16 subjects (5.4%). The common presentations included encephalopathy, seizures, and focal deficits.
The major neurotoxicity associated with calcineurin inhibitors is PRES. Hypertension is commonly associated with CsA, whereas PRES occurs in about 10% of patients who receive CsA. In 1 study, subjects had been taking CsA between 6 days and 5 years before symptom onset. The symptoms included headache, seizures, and visual abnormalities. In another study, 6 of 129 subjects (4.6%) undergoing allogeneic transplants developed CsA-related PRES with the toxicity appearing 3 to 37 days after initiating CsA. PRES secondary to CsA has also been reported in a study of 239 subjects undergoing bone marrow transplants in which 10 subjects (4.2%) developed the syndrome characterized by hypertension, visual disturbances, and seizures.
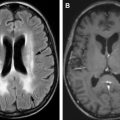
However, in patients with tacrolimus-associated encephalopathy, there is no association with hypertension. Also, serum levels of tacrolimus are found to be elevated in patients with encephalopathy. Fig. 1 shows an example of tacrolimus-associated PRES in a 55-year-old man who developed seizures on day 1 after undergoing allogeneic SCT.
Ictal electroencephalograph in patients with PRES may show rhythmic spikes involving parieto-occipital or temporal-occipital regions. Imaging may be characterized by a posterior-predominant pattern of cortical-subcortical involvement by increased T2 signal and no enhancement. However, frontal and temporal lobes, cerebellum, and basal ganglia may also be involved. Rarely, intraparenchymal hemorrhage may be seen.
Acute toxic leukoencephalopathy has also been described with CsA, 5-fluorouracil, and methotrexate, among other causes.
Infection
In a study of 655 subjects who had undergone allogeneic, syngeneic, or autologous SCT, all CNS infections occurred in allogeneic hematopoietic SCT subjects with a 4% incidence. The most common infections were toxoplasma and aspergillus. Overall mortality of subjects with opportunistic CNS infections was 67%.
Fungal infection
In a study of 230 autologous and allogeneic SCT subjects, 12 subjects were found to have a fungal brain abscess with the most common organism being Aspergillus . The median time of symptom onset was 117 days, with all subjects demonstrating an abnormal CT or MRI. Other studies have shown similar times of onset of cerebral aspergillosis at a median of 110 days (mean 143 ± 94). Lesions related to Aspergillus were located cortically, subcortically, in the basal ganglia and cerebellum, with MRI demonstrating large lesions with intermediate T2 signal, rim enhancement, and associated vasogenic edema.
A study of subjects, most with aspergillus infections, showed that infections in the early post-transplantation period do not show significant vasogenic edema or enhancement when the white blood cell count is low, whereas there is increase in vasogenic edema and associated enhancement as the white blood cell counts increase.
Toxoplasma infection
The incidence of toxoplasma in allogeneic SCT subjects has been variably reported in a range from 0.2% to 3% in different studies. In 1 study, the median symptom onset was 84 days post-transplant (range 51–184 days). The typical MRI features include multiple lesions in the subcortical white matter, basal ganglia, and cerebellum, with focal nodular or rim enhancement present in some lesions.
In an older study of 655 hematopoietic SCT subjects, toxoplasma was diagnosed in 20 subjects with median onset of 72.5 days (mean of 123 ± 150 days). Two subgroups of subjects were identified in this study. The first group showed supratentorial and infratentorial T2-hyperintense lesions with minimal mass effect and no contrast enhancement. The infection in these subjects manifested itself from day 14 to day 75 (mean 45 ± 22) with a mortality rate of 71%. In the second group, the typical MRI appearances were the presence of multiple T2-hyperintense lesions with associated vasogenic edema, mass effect, and rim enhancement. The disease onset in the latter group was between 62 to 689 days (mean 180 ± 184.4 days) with a mortality rate of 36%. The most common locations of lesions were in the subcortical white matter, basal ganglia, and cerebellum.
Bacterial infection
Brain abscess is a rare but severe CNS complication of SCT. In a large study from Brazil of 1000 subjects who underwent SCT, there was 3% incidence of bacterial meningitis and 1% incidence of brain abscess. Other studies do not show bacteria as a significant burden of CNS infection in these subjects, suggesting decreasing incidence of bacterial brain abscess and meningoencephalitis. Similar to aspergillus and toxoplasma infections, bacterial abscesses may not show significant mass effect or enhancement. These atypical imaging characteristics of a brain abscess may portend a poor prognosis.
Viral infection
In a prospective study of 281 subjects who underwent allogeneic SCT, the incidence of herpes virus–associated encephalitis or myelitis was 6.3% with the median time of disease onset being 65 days (range: 22–542) post-transplantation. Causative viruses included herpes simplex virus 1, cytomegalovirus (CMV), varicella-zoster virus, and Epstein-Barr virus (EBV). In another study, the incidence of EBV-associated CNS disease, including encephalitis or myelitis, has been reported to be 3.4%, with the median time of disease onset being 49 days (22–184) post-transplant. Although MRI of the brain may be normal in these subjects, focal or diffuse signal FLAIR hyperintensities or space-occupying lesions may also be seen.
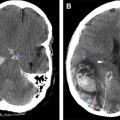
Human herpesvirus (HHV)-6–associated encephalitis in the SCT transplant population is reported to have an incidence of 4% with the median time to initial symptoms being 18 to 21 days (range 6 to 145 days). Patients may present with headaches, altered mental status, and amnesia, with seizures sometimes seen during the disease course. Although the brain MRI within 7 days may be normal, the most common imaging pattern is FLAIR hyperintensity involving the mesial temporal lobes during the early phase, with subsequent hippocampal atrophy. Fig. 2 shows a 55-year-old woman who underwent allogeneic SCT and developed HHV-6 encephalitis 3 months later, with prominent involvement of the mesial temporal lobes on MRI. Nonspecific white matter signal abnormality can also be seen with some patients, demonstrating persistent long-term memory deficits.