The hallmark signs and symptoms of anemia are directly related to a decrease in oxygen delivery to vital tissues and organs and include pallor, fatigue, lightheadedness, and shortness of breath. Neurologic complications are often nonspecific and can include poor concentration, irritability, faintness, tinnitus, and headache. If undiagnosed or untreated, anemia can progress to cognitive dysfunction, psychosis, encephalopathy, myelopathy, peripheral neuropathy, and more focal syndromes, such as stroke, seizures, chorea, and transverse myelitis. Imaging can play an important role in the early diagnosis and treatment of these neurologic and systemic complications associated with anemia, and hence, better outcome.
Key points
- •
The hallmark signs and symptoms of anemia are directly related to a decrease in oxygen delivery to vital tissues and organs and include pallor, fatigue, lightheadedness, and shortness of breath. Neurologic complications are often nonspecific and can include poor concentration, irritability, faintness, tinnitus, and headache.
- •
If undiagnosed or untreated, depending on the cause, anemia can progress to cognitive dysfunction, psychosis, encephalopathy, myelopathy, peripheral neuropathy, as well as more focal syndromes, such as stroke, seizures, chorea, and/or transverse myelitis.
- •
Imaging can play a very important role in the early diagnosis and treatment of these neurologic and systemic complications associated with anemia, and hence, better outcome.
Introduction
Anemia, the most common disorder of the blood, affects more than 3 million Americans. It is defined as a condition marked by a deficiency of red blood cells (RBCs) and/or hemoglobin diminishing the bloods ability to carry oxygen to vital organs leading to symptoms such as fatigue, lightheadedness, and shortness of breath. Anemia is divided into 3 main classes based on pathophysiology, including
- 1.
Blood loss,
- 2.
Increased destruction,
- 3.
Impaired production of RBCs.
More commonly, anemia is classified by various causes and RBC parameters, including size/volume and hemoglobin content of the RBC. This morphologic classification is based on mean corpuscular volume (MCV) and on hemoglobin content of the RBC. The categories include normocytic (MCV between 80 and 100 fL), microcytic (MCV <80 fL), and macrocytic (MCV >100 fL), as well as normochromic (normal hemoglobin content) and hypochromic (low hemoglobin content).
The clinical manifestations of anemia vary markedly due to various causes, rate of progression, and severity. The hallmark signs and symptoms of anemia are directly related to a decrease in oxygen delivery to vital tissues and organs and include pallor, fatigue, lightheadedness, and shortness of breath. Neurologic complications are often nonspecific and can include poor concentration, irritability, faintness, tinnitus, and headache. If undiagnosed or untreated, depending on the cause, anemia can progress to cognitive dysfunction, psychosis, encephalopathy, myelopathy, peripheral neuropathy, as well as more focal syndromes, such as stroke, seizures, chorea, and/or transverse myelitis. Imaging can play a very important role in the early diagnosis and treatment of these neurologic and systemic complications associated with anemia, and hence, better outcome.
Introduction
Anemia, the most common disorder of the blood, affects more than 3 million Americans. It is defined as a condition marked by a deficiency of red blood cells (RBCs) and/or hemoglobin diminishing the bloods ability to carry oxygen to vital organs leading to symptoms such as fatigue, lightheadedness, and shortness of breath. Anemia is divided into 3 main classes based on pathophysiology, including
- 1.
Blood loss,
- 2.
Increased destruction,
- 3.
Impaired production of RBCs.
More commonly, anemia is classified by various causes and RBC parameters, including size/volume and hemoglobin content of the RBC. This morphologic classification is based on mean corpuscular volume (MCV) and on hemoglobin content of the RBC. The categories include normocytic (MCV between 80 and 100 fL), microcytic (MCV <80 fL), and macrocytic (MCV >100 fL), as well as normochromic (normal hemoglobin content) and hypochromic (low hemoglobin content).
The clinical manifestations of anemia vary markedly due to various causes, rate of progression, and severity. The hallmark signs and symptoms of anemia are directly related to a decrease in oxygen delivery to vital tissues and organs and include pallor, fatigue, lightheadedness, and shortness of breath. Neurologic complications are often nonspecific and can include poor concentration, irritability, faintness, tinnitus, and headache. If undiagnosed or untreated, depending on the cause, anemia can progress to cognitive dysfunction, psychosis, encephalopathy, myelopathy, peripheral neuropathy, as well as more focal syndromes, such as stroke, seizures, chorea, and/or transverse myelitis. Imaging can play a very important role in the early diagnosis and treatment of these neurologic and systemic complications associated with anemia, and hence, better outcome.
Imaging modalities
Anemia is a diagnosis made by routine blood workup. Type and associated cause are invariably diagnosed by clinical examination, blood smear, and bone marrow examination. Imaging is not a commonly used technique for evaluation of anemia. However, imaging plays an important role in the early diagnosis of the neurologic and systemic complications associated with anemia. Commonly used imaging techniques include plain radiography, ultrasonography, nuclear medicine, computed tomography (CT), and MRI.
Plain radiography is a fast and relatively inexpensive imaging technique for evaluation of bones, chest, and soft tissues. However, because of its low specificity and sensitivity, compared with the newer imaging techniques, it has lost its importance and application in the evaluation of the early disease process. Ultrasound is inexpensive, is easy to perform, and does not use ionizing radiation. It is predominately used to assess the intra-abdominal and soft organ abnormalities. Bone radionuclide scan is mainly used to assess bony abnormalities, such as metastasis and bone marrow abnormalities. It is very sensitive but lacks specificity to the abnormality. The advent of PET-CT and PET MR, which provide whole body evaluation, has provided a modality that is very useful in staging and assessing the response to various hematologic malignancies following therapy. CT remains the primary modality of choice in the evaluation of various neurologic disorders because of its easy availability and shorter scanning time. It is sensitive in diagnosing various acute neurologic complications, like intracranial hemorrhage, infarctions, and parenchymal and calvarial metastasis. However, it lacks sensitivity to detect posterior fossa lesions, leptomeningeal and dural abnormalities, or metabolic-related disorders. MRI remains the modality of choice for most brain and spine abnormalities because of its excellent soft tissue differentiation and multiplanar acquisition. It is very sensitive in diagnosing abnormalities ranging from neoplastic, metabolic, and inflammatory to infection. In respect to the hematologic disorders, it is very sensitive in diagnosing intracranial bleeds, infarctions, and leptomeningeal and dural abnormalities. It is also very sensitive in the diagnoses of bone marrow abnormalities.
Iron deficiency anemia
Iron deficiency anemia (IDA) is the most common cause of anemia, often related to decreased dietary intake or an overall decrease in iron due to chronic bleeding (eg, gastrointestinal loss, menorrhagia), malabsorption disorders, or increased demand (eg, pregnancy). Iron deficiency is a microcytic (MCV <80 fL), hypochromic (central pallor) anemia consistent with decreased iron, increased total iron binding capacity, and decreased ferritin. The workup for iron deficiency anemia involves identifying the source of the deficiency. Identifying the source of the deficiency often includes a through history, physical examination, complete blood count, blood smear, and stool and urine analysis. Rarely, imaging may be used, particularly to diagnose associated central nervous system (CNS) and musculoskeletal complications.
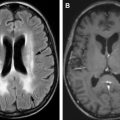
Neurologic symptoms are often nonspecific and include tiredness, fatigue, weakness, poor concentration, irritability, faintness, dizziness, tinnitus, and headache. These findings in an undiagnosed case of anemia are very challenging to link to a specific cause. The most common imaging finding seen in a patient with anemia is marrow reconversion. These findings are best appreciated on an MRI of the spine. Normally as the age advances, yellow marrow, which is predominately fat, replaces the appendicular as well as axial skeleton. Thus, a normal adult spine shows hyperintensity in the vertebral bodies on T1-weighted and fast-spin T2-weighted images ( Fig. 1 ). In cases of marrow reconversion, diffuse hypointensity is seen throughout the axial skeleton due to red marrow ( Fig. 2 ). These changes may be associated with diffuse dull back pain.
Various case reports have also shown an association of IDA with benign intracranial hypertension and cerebral venous sinus thrombosis. Waxing and waning of the benign intracranial hypertension symptoms have been documented with recurrence and resolution of the iron deficiency anemia. On the background of carotid vessels atherosclerosis, iron deficiency anemia may cause focal neurologic signs. These changes are particularly seen in elderly patients with severe anemia. These symptoms may rapidly improve following blood transfusion and an increase in hemoglobin. Restless legs syndrome has also been shown to be strongly associated with IDA.
Some patients may present with thrombocytosis due to repeated stimulation of the bone marrow from chronic anemia. This increase in the platelet mass may cause transient blockade of the intracranial vessels, giving rise to transient cerebral ischemic attacks (TIAs), amaurosis fugax, or cerebral infarction. Diffusion-weighted imaging–apparent diffusion coefficient images are very sensitive for the diagnosis of this tiny lacunar infarction in the supratentorial and infratentorial brain parenchyma. Profound anemia, if associated with thrombocytopenia (mostly in adults) and erythroblastopenia (in childhood), frequently presents with ocular symptoms. These ocular symptoms may include papilledema, cotton-wool exudates, flame-shaped hemorrhages, retinal edema, and very rarely, detachment.
Thalassemia
Thalassemia is an autosomal-recessive disorder that results in a microcytic, hypochromic anemia similar to that of iron deficiency anemia ( Fig. 3 ). Normal adult hemoglobin consists of 98% hemoglobin A (HbA; 2 α-globin chains and 2 β-globin chains) and 2% HbA2 (2 α-chains and 2 δchains). In thalassemia, a genetic defect causes the patient to produce a deficient amount of either α- or β-globin, resulting in a quantitative problem of globin synthesis. Thalassemia is classified depending on the globin chain (αor β) that is reduced. The diagnosis of thalassemia can be confirmed via hemoglobin electrophoresis.
The clinical presentation of these patients widely depends on the severity of the disease. The ineffective erythropoiesis leads to anemia, hepatosplenomegaly, and extramedullary erythropoiesis with skeletal deformities. Skeletal abnormalities are often due to the expansion and invasion of erythroid bone marrow, which widen the marrow spaces, attenuate the cortex, and produce osteopenia/osteoporosis. These changes are typically seen in the spine, the skull, and the facial bones, as well as the ribs and the metaphyses of the long bones.
The spine shows diffuse marrow conversion, osteopenia, and a decrease in the subchondral bone thickness leading to multisegmental end-plate compression fractures. On imaging, vertebrae are biconcave or wedge shaped. As the disease progresses, compression fractures as well as paravertebral expansion of extramedullary masses become more prominent. These changes can progress to back pain, spinal asymmetry and scoliosis, cord compression from intraspinal collections of hematopoietic tissue, and intervertebral disc degeneration. Organs such as the liver, spleen, and kidneys are often enlarged due to extramedullary hematopoiesis.
Marrow hyperplasia widens the diploic space with thinning of the outer skull, reduction of trabeculae, granular osteopenia, and solitary or multiple circumscribed osteolytic areas. The classic “hair-on-end” appearance is due to a periosteal reaction that manifests as perpendicular trabeculations interspersed with radiolucent marrow hyperplasia along the skull vault. The expansion of the facial bones inhibits the pneumatization of the paranasal sinuses leading to lateral displacement of the orbits and maxillary protrusion with ventral displacement, ultimately producing the characteristic “chipmunk facies” ( Fig. 4 ).
Extramedullary hematopoiesis (EMH) is a compensatory process associated with chronic hemolytic anemia that occurs in the hematopoietic elements outside the bone marrow when the bone marrow is unable to maintain sufficient RBC production to suffice for the body’s demand. It often occurs in patients with a myeloproliferative disorder or hemoglobinopathies such as sickle cell disease (SCD), thalassemia, or hereditary spherocytosis (HS). Common sites include the spleen, liver, and lymph nodes leading to organomegaly. EMH is commonly seen in the paraspinal region, which may extend into the spinal canal, leading to cord compression and myelopathic changes. These masses are isointense on both T1- and T2-weighted images. In addition, vertebral bodies are found to have low- to intermediate-signal intensity due to the displacement of fatty marrow with hematopoietic marrow.
Patients that are adequately treated with repeated transfusions have less medullary expansion but high serum iron levels. This iron overload gets deposited in the various organs of the body, including bone marrow. MRI in these patients shows low signal intensity of the axial, appendicular skeleton and other soft tissue organs on T2 and gradient echo sequence ( Fig. 5 ).
Vitamin deficiency anemia
Vitamin B12 Deficiency
Vitamin B12 deficiency causes a characteristic megaloblastic anemia (MCV >100 fL) and an ineffective erythropoiesis. Pernicious anemia, or the absence or deficiency of intrinsic factor, is the most common cause of vitamin B12 deficiency. Other causes include nutritional deficiency (vegans, elderly, alcoholics), malabsorption syndromes, type B atrophic gastritis, abnormality of the distal ileum (Crohn, resection, Whipple), colonization of small bowel with bacteria or intestinal parasite, and chronic infections, such as human immunodeficiency virus (HIV), congenital syndromes (Imerslund-Grasbeck syndrome), and medications.
Vitamin B12 deficiency may take decades to develop. Patients may be asymptomatic or present with a wide spectrum of hematologic and neuropsychiatric manifestations. Vitamin B12 deficiency causes a typical pattern of degeneration of the white matter in the CNS that can be manifested clinically as encephalopathy, myelopathy, peripheral neuropathy, and optic neuropathy.
Subacute combined degeneration of the cord is associated with vitamin B12 deficiency, due to demyelination and axonal degeneration of the most heavily myelinated fibers, such as posterior and lateral columns of the cord. It is most commonly seen at the midthoracic level. Clinical presentations include paresthesia in the hands and feet, with progression to sensory loss, gait ataxia, and distal weakness, especially in the legs.
On MRI, linear T2 hyperintensity is seen on the posterior aspect of the cord, specifically on sagittal T2 images. On an axial plane, symmetric bilateral areas of T2 hyperintensity are seen in the dorsal columns, giving an “inverted V sign” appearance ( Fig. 6 ). The signal changes typically begin in the upper thoracic region, with ascending or descending progression. The lateral corticospinal tracts and sometimes lateral spinothalamic tract may also have similar signal intensity changes. Unfortunately, symptoms often precede months or years before any imaging abnormality is identified. Therefore, spinal cord abnormality on MRI is not very sensitive as an early test for subacute combined degeneration.