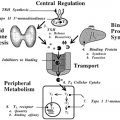
HYPOTHALAMIC-PITUITARY UNIT
EMBRYOLOGY
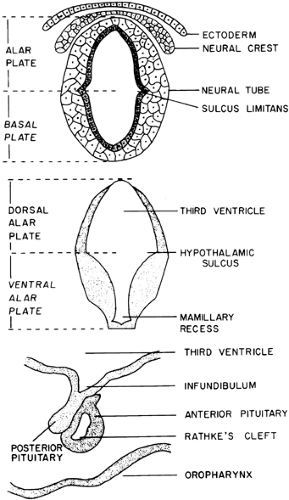
Between the third and fourth weeks of embryonic development, a longitudinal groove, the sulcus limitans, appears in the lumen of the neural tube. This sulcus divides the alar (dorsal) plate from the basal (ventral) plate. The basal plate plays no part in the development of the hypothalamus or pituitary, participating only in the formation of nervous tissue caudal to the diencephalon. At ˜5.5 weeks, the alar plate, in the region that gives rise to the diencephalon, develops a longitudinal groove contiguous with the sulcus limitans. This groove, the hypothalamic sulcus, divides the alar plate into a dorsal portion, which gives rise to the thalamus, and a ventral portion, which gives rise to the hypothalamus3 (Fig. 9-1A and Fig. 9-1B).
During the fifth week of embryonic life, the anterior pituitary begins to form as a diverticulum (Rathke pouch) of the buccal cavity. It expands dorsally to join the basal portion of the forebrain (see Fig. 9-1C) so that by the eighth week, the posterior pituitary, which begins as a diverticulum of the floor of the third ventricle, has contacted the Rathke pouch and been invested by it. By the 11th week, the cavity of the Rathke pouch becomes flattened and loses its connection with the buccal cavity.3 Remnants of its attachment to the buccal cavity form the craniopharyngeal duct, residual cells of which persist in the posterior lobe of the pituitary, the hypophysial stalk, and the basisphenoid. Traditionally, these residual cells are thought to be the origin of craniopharyngioma; however, because craniopharyngiomas occasionally develop in the sella or along the craniopharyngeal duct itself, this idea on the origin of these tumors has been questioned. Careful examination of the superior pharynx in infants and adults almost always reveals a pharyngeal hypophysis at the buccal end of the craniopharyngeal duct. It is composed mainly of undifferentiated epithelial cells with a few chromophobes and chromophils, although rarely this residual pharyngeal hypophysial tissue has been reported to produce excessive pituitary hormone secretion.
FUNCTIONAL NEUROANATOMY
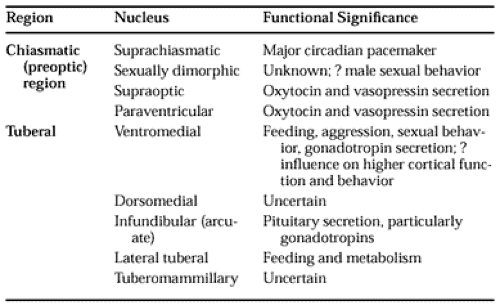
Neuroanatomically, the traditional borders of the hypothalamus are the lamina terminalis (rostrally); the posterior edge of the mammillary body (caudally); the hypothalamic sulcus (dorsally); the floor of the third ventricle (ventrally); and the internal capsule, basis pedunculi, and subthalamus (laterally). The hypothalamus usually is divided into three regions: the chiasmatic (preoptic region), the tuberal region, and the mammillary complex. Of these three, the first two contain the neuronal groups and tracts that are of most significance in neuroendocrine regulation (Table 9-1).
Histologically, the hypothalamus contains two types of neurons: large (magnocellular) and small (parvicellular). Magno-cellular neurons are of two classes: those that secrete arginine vasopressin (AVP) and neurophysin II, and those that secrete oxytocin and neurophysin I. AVP often is colocalized with dynorphin or angiotensin II, whereas oxytocin frequently is colocalized with cholecystokinin (CCK), corticotropin-releasing
hormone (CRH), metenkephalin, or proenkephalin. Parvicellular neurons contain a variety of neuropeptides or biogenic amines, many of which are colocalized.
hormone (CRH), metenkephalin, or proenkephalin. Parvicellular neurons contain a variety of neuropeptides or biogenic amines, many of which are colocalized.
The parvicellular neurons of the hypothalamus that are found in the immediate periventricular region of the third ventricle, from the preoptic area to the mammillary bodies, project to the median eminence, where their neurosecretory products are released to influence anterior pituitary function. Functionally discrete groups of parvicellular neurons have been located in the anterior preoptic region, the paraventricular nucleus, and the arcuate nucleus.
The more medial area of the hypothalamus has collections of neurons that receive inputs from the brainstem and the limbic system. They, in turn, project to the periventricular zone, the limbic system, the brainstem, and the spinal cord in a circuit that is suited to the coordination of endocrine, autonomic nervous, and more complex behavioral responses to ensure homeostasis.
In the lateral hypothalamic area is found the medial fore-brain bundle, a neural pathway that connects the brainstem reticular formation with the septum and the limbic system and also has input into the periventricular secretory neurons.
HYPOPHYSIOTROPIC HORMONES
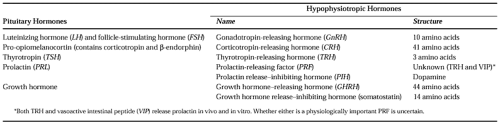
The anterior pituitary is known to produce six peptide hormones (Table 9-2). The secretion of each of these is under the control of one or more hypothalamic neuropeptides and several other classic neurotransmitters. A comprehensive review of these intricate interactions has been published elsewhere.4
In primates, a few neurons containing gonadotropin-releasing hormone (GnRH) are found in clusters above the optic chiasm, medial to the supraoptic nuclei, but most are found in the medial basal hypothalamus.5 Their axons project to the median eminence, where GnRH is released in a pulsatile fashion. In humans, GnRH-positive neurons are found in the arcuate nucleus.4 The GnRH-containing neurons of the medial basal hypothalamus have an intrinsic firing pattern that results in the pulsatile release of GnRH. Norepinephrine, epinephrine, serotonin, acetylcholine, and N-methyl D-aspartic acid all stimulate GnRH release, as do angiotensin II (probably through its action on norepinephrine) and neuropeptide Y.4 Opioid peptides and γ-aminobutyric acid (GABA) inhibit GnRH release.4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree