Human papilloma virus (HPV) is a double-stranded DNA virus. It is a member of the papovavirus family. Papillomaviruses are highly host specific and are characterized by an ability to infect and transform epithelial cells. More than 120 HPV types have been identified, and of these, 35 infect the anogenital tract. The sexual route of transmission for genital HPV infection has been well established and recognized for more than 50 years. HPV is estimated to be the most common sexually transmitted infection in the United States, with 6.2 million new HPV infections occurring each year. More recently, a link between some HPV types and development of anogenital cancer has been identified and HPV DNA is present in >95% of squamous carcinomas of the anogenital tract. The International Agency for Research on Cancer (IARC) noted the pooled odds ratio from 11 case control studies for cervical cancer associated with the presence of any HPV was 158.2 (95% confidence interval [CI], 113.4 to 220.6). In addition, perinatal transmission of HPV has been suggested.
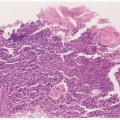
HPV infection may result in either clinically apparent, grossly visible disease (e.g., genital warts) or subclinical disease requiring pap smear, colposcopy, and/or HPV DNA testing for detection. The majority of HPV infections are asymptomatic, unrecognized, or subclinical. The common genital HPV types can be divided into two major categories, based on their oncogenic potential
(Table 6.1). The low-risk group, most commonly 6 and 11, are associated with genital warts and a substantial proportion of cases of low-grade squamous intraepithelial lesions. Fifteen HPV types are considered high-risk oncogenic HPV types (most frequently 16, 18, 33, 35, 45, 52, and 58), which are associated with high-grade squamous intraepithelial neoplasia and invasive cancers. The majority of the clinically apparent lesions are the classic genital warts (condyloma acuminatum).
Over the past two decades, tremendous advances have taken place in our knowledge of HPV. These include (i) modes of transmission and risk factors associated with transmission; (ii) the wide spectrum of infection, with HPV ranging from asymptomatic carrier states to clinical disease including genital warts, intraepithelial neoplasia, and invasive cancer; (iii) the oncogenic potential of certain HPV types and the molecular mechanisms by which they cause cancer. In addition, new diagnostic tests for HPV detection have been developed that result in improved screening for highrisk cervical intra-epithelial neoplasia (CIN) and invasive cancer and for triage of abnormal cervical cytology. Advances in our understanding of the immunology of HPV have resulted in development of new and more effective treatment approaches for HPV infection and the introduction of HPV vaccines.
EPIDEMIOLOGY
Sexual transmission is the primary route for transmission of HPV, resulting in urogenital and anorectal infection. The highest-risk groups for HPV infection are sexually active adolescents and young adults, with 75% of new HPV infections occurring among 15- to 24-year-olds. HPV is highly contagious, and transmission rates are high, with approximately 65% of sexual contacts becoming infected. Although rare, perinatal transmission, especially of HPV types 6 and 11, may occur.
Genital HPV is the most common sexually transmitted infection in the United States, with an estimated 6.2 million new cases annually and an estimated 20 million Americans are infected with HPV
(Box 6.1). This seeming discrepancy is explained by the transient nature and host clearance of >85% of genital HPV infection. The World Health Organization estimates that worldwide an estimated 30 million new cases of genital HPV are diagnosed every year, and approximately 630 million persons are infected with HPV.
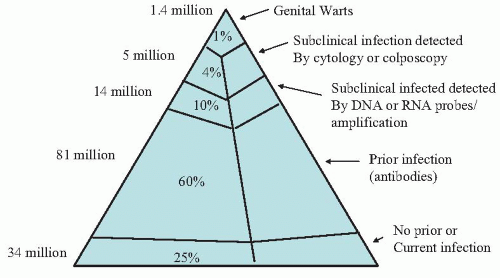
It has been estimated that among sexually active 15- to 49-year-olds in the United States, 1% have clinical HPV infection (e.g., genital warts), 4% have subclinical infection detected by cytology or colposcopy, 10% have subclinical infection detected by DNA or RNA probe/amplification, and 60% have serologic evidence of prior infection with HPV
(Fig. 6.1). Thus, only 25% of sexually active women of reproductive age have not been infected with genital HPV.
Two recent national surveys provide estimates of HPV prevalence in the United States. The National Health and Nutrition Examination Survey (NHANES) 2003-2004, a representative sample of the civilian population in the U.S. using self-obtained vaginal swabs for PCR testing, demonstrated an overall HPV prevalence among females ages 14 to 59 years of 26.8% (95% CI, 23.3% to 30.9%). There was a statistically significant trend for increasing HPV prevalence with each year of age from 14 to 24 years, followed by a gradual decline in prevalence through 59 years
(Table 6.2). The highest prevalence was seen in the 20- to 24-year age group, with a rate of nearly 45%. Based on results from NHANES 2003-2004 (overall prevalence among females ages 14 to 24 years was 33.8%), the prevalence of HPV in the U.S. is much greater than previous estimates, with 7.5 million females in the 14- to 24-year age group.
The second study, the National Longitudinal Study of Adolescent Health (ADHEALTH), used urine specimens from sexually active 18- to 25-year-old women for analysis of HPV DNA and demonstrated that nearly 27% of sexually active women in this age range were infected with any type of HPV and 20% were infected with only a high-risk type.
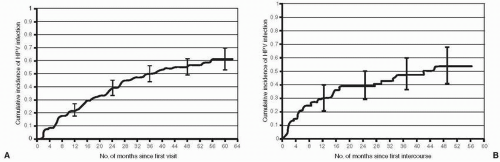
Acquisition of HPV occurs soon after the onset of sexual activity. A longitudinal study of female university students in the state of Washington followed at 4-month intervals with cervical and vulvovaginal specimens collected to detect HPV DNA assessed the cumulative incidence of HPV infection in both virgins and nonvirgins who were HPV-DNA-negative at enrollment. The cumulative 24-month incidence of HPV was 38.89% in women sexually active at enrollment and 38.9% among virgins who initiated sexual activity
(Fig. 6.2). By 48 months, the cumulative incidence was greater than 50% in both groups. Interestingly, any type of nonpenetrative sexual contact was associated with an increased risk of HPV infection in virgins (but not in sexually active women). This study demonstrates the rapid acquisition of and high prevalence of HPV infection with onset of sexual activity, especially with multiple partners or new partners. Comparable cumulative incidences of genital HPV infection among women who were HPV-DNA-negative at enrollment have been noted by studies among adolescents and young women in the United States and the United Kingdom.
Risk factors for HPV infection include young age, early onset of sexual activity, multiple sexual partners, increased frequency of intercourse, exposure to sex partner with genital warts, failure to use condoms, lack of circumcision, and cigarette smoking. Age and sexual behavior are the two major influences on the incidence of genital HPV infection. In the study of university students in Washington state, smoking, oral contraceptive use, and report of a new male partner—in particular, one known for less than 8 months before sex occurred or one reporting other partners—were predictive of incident HPV infection. In addition, pregnancy is associated with increased presence of HPV infection and genital warts and immunosuppressive states (e.g., HIV infection with low CD4+ count) result in increased viral titers of HPV and more rapid progression of HPV disease associated cervical intraepithelial neoplasia.
The economic burden of HPV infection in the United States is substantial. It is estimated that cervical HPV-related disease accounted for total healthcare costs of nearly $4 billion with follow-up of abnormal pap smears and management of CIN, costing $3.6 billion and cervical cancer and genital warts each costing approximately $150 million.
Both squamous cell carcinoma and adenocarcinoma of the cervix are caused by HPV. An estimated 9,710 new cases of cervical cancer occurred in the United States in 2006, with an estimated 3,700 deaths due to cervical cancer. Worldwide cervical cancer remains common with an estimated 510,000 new cases annually; in the developing world cervical cancer is the second most common cancer among women and the second most common cause of cancer deaths, with 288,000 annually.
Whereas most HPV infections of the genital tract are subclinical, many cases are detected cytologically. Based on cytology, a wide spectrum of HPV infection can be detected, including atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesions (LSIL), and high-grade squamous intraepithelial lesions (HSIL). Data from the National Breast and Cervical Cancer Early Detection Program demonstrate that the overall rate of abnormal cervical cytology in the United States was 3.8%. The rates for LSIL and HSIL were 2.9% and 0.8%, respectively. Subclinical HPV infections (presence of HPV DNA) are estimated to be 10 to 30 times more common than cytologically detected infections.
The most common clinically recognized HPV infection of the female genital tract is genital warts (condyloma acuminatum). More than 90% of anogenital warts are associated with HPV types 6 and 11. Genital warts is not a reportable disease in the U.S. and thus its true prevalence and incidence in the U.S. is unknown. Based on surveys of first office visits to physicians by the CDC, estimates of the occurrence of genital warts have been made. Genital warts predominantly affect the young, with the prevalence highest among the 16- to 25-year age group.
In addition, the prevalence of genital warts varies according to the population studied. Among women attending STD clinics, the prevalence of genital warts has been reported as high as 9%. Based on population estimates derived primarily from STD clinics, an estimated 1% of sexually active adolescents and young adults in the United States have genital warts. Among 3.6 million privately insured individuals (Medstat Marketscan database), genital warts were identified in 1.7 cases per 1,000 person-years; the prevalence of genital warts was highest among women ages 20 to 24 years (6.2 cases per 1,000 person-years). The economic burden among this group of privately insured patients was substantial, involving 3.1 physician visits and $436 in cost per episode of genital warts. In an HMO setting, among women presenting for their annual gynecologic examination, the prevalence of genital warts was 0.8% and 0.6%, respectively, for women ages 21 to 29 years and 29 to 39 years, respectively.
Genital warts grow more rapidly during pregnancy and may involve the cervix, vagina, or vulva so extensively that vaginal delivery is precluded. The reason for the increase in size and number of lesions is not known but has been postulated to be the decrease in cell-mediated immunity that occurs during pregnancy. Similarly, immunosuppression (HIV infection, organ transplant) is a risk factor for development of genital warts.
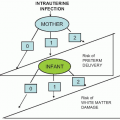
Respiratory papillomatosis (laryngeal papilloma) is a rare disease in the neonate caused by HPV 6 and 11. Laryngeal papillomas can be particularly troublesome because they may produce respiratory distress secondary to obstruction and because recurrence following treatment is common. Some have proposed that juvenile onset recurrent respiratory papillomatosis results from HPV transmission intrapartum. However, other potential routes of transmission of HPV include transplacental and contact during the neonatal period.
The risk for transmission of the virus from maternal genital warts to the neonate has not been established. Because genital papillomavirus infection is so common and respiratory papillomatosis is rare, the risk of intrapartum transmission is low, perhaps on the order of one case of juvenile respiratory papillomatosis per several hundred or per thousand children born to infected mothers. A study in Seattle and Atlanta estimated that the incidence ranges from 0.12 to 2.1 cases per 100,000 children younger than 18 years.
Studies assessing the risk for and incidence of transmission of HPV from mothers to infants have produced conflicting data
(Table 6.3). Initial studies with PCR-based methods for detection of HPV DNA revealed rates of detection of HPV in the first 24 to 48 hours of life, ranging from 4% to 72% among infants born to mothers with genital HPV detected during pregnancy. Studies performed at 6 weeks of life also demonstrated variable rates of detection and demonstrated that the rates of detection of HPV were not always significantly different among infants born to HPV-positive or HPV-negative mothers.
In general, these recent studies demonstrate that the risk of transmission for any given mother-infant pair is low. In addition, up to one third of the HPV-positive newborns was born to mothers who were HPV DNA negative during pregnancy.
PATHOGENESIS
Genital HPV infections are transmitted primarily by sexual activity. The infectivity rate is high with transmission occurring in approximately 65% of sexual partners. The average incubation period is 2 to 3 months.
Infection with oncogenic HPV types is the most significant risk factor in the pathogenesis of cervical cancer and its precursors. The most common HPV types associated with cervical cancer are HPV 16, 18, 31, 33, and 45, with HPV 16 and 18 associated with 67% of cervical cancer cases. Low-risk HPV 6 and 11 are responsible for >90% of anogenital warts. Both low- and high-risk HPV types are associated with CIN; most frequently high-risk HPV 16 and 18 and low-risk HPV 6 and 11. In addition, high-risk HPV types are associated with 50% of vulvar and vaginal cancer cases.
HPV is a nonenveloped, double-stranded DNA virus with a small genome (˜8 Kb). The HPV viral genome consists of three major regions: two protein encoding regions (early and late gene regions) and a noncoding upstream regulatory region (URR). These distinct regions produce neoplastic proteins (E6 and E7), virus production proteins (E1, E2 and E3), and virus capsid proteins (L1 and L2). The URR controls transcription of both the early and late regions, resulting in regulation of viral protein and infectious particle production. The early region contains open reading frames (ORFs), which are transcriptional units that encode for a series of proteins designated E1, E2, E4, E5, E6, and E7.
Early region gene expression controls replication, transcription, and cellular transformation of viral DNA. Most importantly, it also plays a role in unregulated cellular proliferation. Whereas E6 and E7 encode proteins involved in viral replication, they are the oncogenic genes and also code proteins critical for host cell immortalization and transformation.
The E6 protein binds to tumor suppressor gene product p53, resulting in its degradation and loss of p53 activity. Reduction in p53 levels results in unregulated cell progression and accumulation of genetic mutations. The E7 gene encodes a protein that binds to tumor suppressor product Retinoblastoma protein (pRb) disrupting the E2-Rb complex, which alters the normal cellular growth control mechanism and allows the released free E2 to stimulate transcription of genes required for DNA replication.
The late gene region contains two ORFs (L1 and L2), which encode structural proteins responsible for production of the viral capsid. The L1 protein is the key component of the recently introduced HPV vaccine and is highly immunogenic.
Acute HPV infection occurs in the genital tract when microtrauma secondary to sexual intercourse allows virus to enter the skin or mucosa of the genital tract. The postpubertal adolescent cervix is characterized by a large transformation zone that is more susceptible to minor trauma during sexual intercourse and whose immature columnar epithelial cells are particularly susceptible to HPV. This may explain why the young sexually active adolescent is at the greatest risk to acquire HPV infection. HPV enters cells in the basal layer of the epithelium and matures as it passes through the parabasal, spinous, and granular layers of the epithelium.
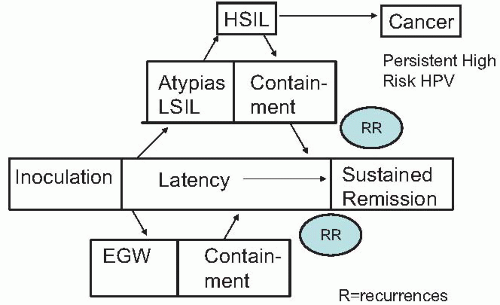
Following acute HPV infection, several clinical scenarios can occur
(Fig. 6.3). Latent viral infection occurs when the
HPV genome is stabilized as a nonintegrated episome and remains in host cells without causing clinical or morphologic changes in the squamous epithelium of the genital tract. Latency can lead to sustained remission, which is the case in the vast majority of HPV infections. Alternatively, active infection may occur dependent on HPV type present. Low-risk HPV types, especially 6 and 11, manifest with proliferation of squamous epithelial cells to form genital warts. In genital warts, the viral DNA is an episome and is not integrated into the host DNA. In contradistinction, high-risk oncogenic HPV types may become integrated into the host genome, resulting in cervical (also vaginal and vulvar) intraepithelial neoplasia (CIN). CIN may progress to precancerous lesions (CIN 2 and 3) and ultimately to invasive cervical cancer. Alternatively, CIN especially CIN 1, and to a lesser extent, CIN 2, may regress and resolve spontaneously.
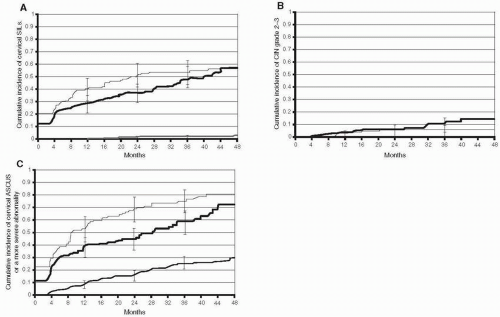
Traditionally, it has been held that squamous intraepithelial lesions (SILs) are uncommon manifestations of HPV infection. Moreover, it was believed that it usually took years for high-grade SILs (HSILs) to develop following initial infection with HPV. Recent studies demonstrated that SILs can be detected more frequently and earlier with frequent screening. In addition, they noted that the duration of lesions is relatively short. In a longitudinal study of HPV infection in 603 female university students, intraepithelial lesions were common early events among women with incident HPV infection and the interval between incident HPV-16 or HPV-18 infection and biopsy-confirmed CIN grade 2-3 was relatively short
(Fig. 6.4). The 36-month cumulative incidence of cervical SILs by cytologic screening was 47.2% (95% CI, 38.9% to 56.4%), and the median time to clearance of cervical SILs was 5.5 months. Among women with incident HPV-16 or HPV-18 infection, the 36-month cumulative incidence of CIN grade 2 was 20% and that of CIN grade 3 was 6.7%. Half of the incident cases of biopsy-confirmed CIN grade 2-3 occurred within 14 months of an incident HPV infection. This study demonstrated that HSILs often are an early manifestation of HPV infection in young women. For genital warts, the 36-month cumulative incidence among women with incident HPV-6 or HPV-11 infection was 46%.
Thus, contrary to the traditional belief that prolonged infection with high-risk HPV types is necessary for progression to high-grade neoplasia, HSILs are often an early manifestation of HPV infection in young women. Half of the incident cases of biopsy-confirmed CIN grade 2-3 occurred within 14 months of an incident HPV infection. These findings are consistent with other recent studies in young women which noted that HSILs occurred within 2 years of detection of HPV infection.
Incident LSILs are detected commonly in young women infected with HPV. Within 3 years of incident HPV infection, 15% to 50% of young women develop LSILs; the higher rates are from studies with more frequent screening. Although the incidence of LSIL is high in young women, these lesions tend to regress quickly (unlike in older women with persistent HPV infection). In a study of women 13 to 22 years of age, the regression rate of LSIL cases was 61% and 91% at 12 months and 36 months, respectively.
Persistent infection with high-risk types is the most important risk factor for CIN 2-3 and cervical cancer. Persistent infection with a high-risk HPV type is associated with a >10-fold risk of CIN 2-3. Infection with greater than 1 type of high-risk HPV has been linked to persistence of high-risk HPV. Recently, high viral load of HPV has also been shown to increase the risk for development of cervical cancer and its precursors. Women with the 20% highest amount of HPV 16 DNA were at 60-fold higher risk of developing carcinoma in situ than women negative for HPV 16.
Coinfection with
Chlamydia trachomatis has also been associated with persistence of high-risk HPV. This finding is similar to several case-control studies that demonstrated a statistically significant increased risk for CIN with chlamydia coinfection
(Table 6.4). The biologic plausibility of this association includes (i)
C. trachomatis is an obligate intracellular organism which is persistent if not treated; (ii)
C. trachomatis up-regulates HPV expression and transactivation; and (iii)
C. trachomatis infection leads to higher HPV viral loads.
Genetic susceptibility may also play a role in the susceptibility to CIN 2-3 and cervical cancer. Gene polymorphisms or single nucleotide polymorphisms (SNPs) in the HLA Class I antigen-processing genes have been associated with development of cervical cancer.