Ann R. Falsey
Human Metapneumovirus
Human metapneumovirus (hMPV) is a recently discovered respiratory pathogen first described by investigators in the Netherlands in 2001.1 This previously unidentified virus was isolated from the nasopharyngeal secretions collected over a 20-year period from 28 Dutch children with upper respiratory infections (URIs). The virus exhibited paramyxovirus-like morphology, and genetic analysis showed it to be most similar to viruses of the Pneumovirinae subfamily, of which respiratory syncytial virus (RSV) is the most prominent member. Serologic analyses indicate that infection with hMPV is nearly universal by age 5 years and that hMPV appears to account for a significant proportion of the respiratory illnesses that were not recognized as being caused by other viral pathogens.
Virus
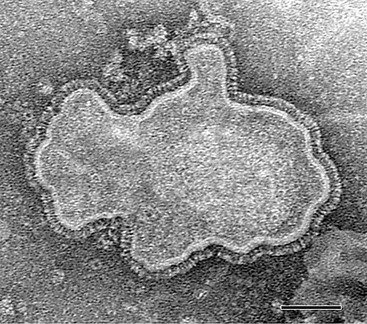
hMPV is a nonsegmented, single-stranded, negative-sense RNA virus belonging to the order Mononegavirales, family Paramyxoviridae, subfamily Pneumovirinae, and genus Metapneumovirus.1 Consistent with the morphology of a paramyxovirus, hMPV particles are pleomorphic, spherical, or filamentous with a lipid envelope and projections on the surface as imaged by electron microscopy (Fig. 161-1).1,2 Within the subfamily Pneumovirinae are the genera Pneumovirus and Metapneumovirus. Members of the Pneumovirus genus include human RSV and a number of animal pathogens, such as bovine, ovine, and caprine RSVs, and pneumonia virus of mice. Until recently, the only member of the Metapneumovirus genus was avian pneumovirus (APV), also known as turkey rhinotracheitis virus. Originally classified as a pneumovirus, APV was placed into a separate new genus, Metapneumovirus because it had a different gene number and gene order and only 40% homology with mammalian pneumoviruses.
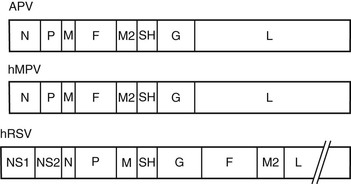
The original genetic analysis of hMPV by van den Hoogen and co-workers1 indicated a gene order of 3′-N-P-M-F-M2-SH-G-L-5′. These eight genes encode for nine proteins and include nucleoprotein (N), phosphoprotein (P), matrix protein (M), fusion protein (F), transcription elongation factor (M2-1), RNA synthesis regulatory factor (M2-2), small hydrophobic protein (SH), glycoprotein (G), and major polymerase subunit (L).3 The lipid envelope of the virus is covered at the interior by the M protein and contains three surface glycoproteins (F, SH, and G) that form spikes of 13 to 17 mm. Within the envelope is a helical ribonucleoprotein complex, consisting of N, P, and L proteins and the hMPV genome.4 The F protein of hMPV is a trimeric, type I membrane glycoprotein and serves both to bind cellular receptors and to mediate fusion.5 Monomers of F are translated as an inactive precursor that is proteolytically cleaved at a monobasic trypsin-like consensus site by host proteases into two disulfide-linked subunits, F1 and F2 to initiate fusion. The infection cycle begins with attachment of the virus to cellular receptors (heparin sulfate and integrins) by the F protein.6,7 For most hMPV strains, fusion occurs at neutral pH, although a few laboratory strains from group A demonstrate enhanced fusion at acidic pH.4 Like RSV, the G protein of hMPV is heavily glycosylated and appears to serve similar functions in the viral life cycle. Current evidence suggests that the G protein helps tether virus particles to the cell surface and confers optimal infectivity, but is not critical for viral attachment.5 The absence of the nonstructural interferon-inhibiting genes NS1 and NS2 is the most striking difference between hMPV and RSV and confirmed its classification in the Metapneumovirus genus (Fig. 161-2).8
Sequence homology of N, P, M, and F genes of hMPV indicate the highest identity with APV serotype C (APV-C), one of four avian pneumovirus types.8 Given the close relationship between hMPV and APV-C, it is speculated that the human virus originated from birds. Phylogenetic analyses of multiple hMPV gene sequences suggest that hMPV diverged from APV about 200 to 300 years ago.9,10 Although current evidence links hMPV with APV, animal challenge studies indicate that hMPV is a primary human pathogen rather than an avian pathogen that incidentally infects humans with species tropism conferred by the F protein.1,11
Genetic variation among hMPV isolates has been observed, and the sequences are found to cluster in two major genotypes (A and B) and four subgroups with two sublineages (A1, A2a, A2b, B1, and B2).12,13 The two isolates representing each of the major genotypes have been completely sequenced, and amino-acid identities between genotypes were 80% and 90%, respectively, similar to the differences found between RSV groups A and B. The greatest diversity is found in two of the surface glycoproteins, G and SH (59% and 37% identity, respectively), which is considerably greater than the diversity observed in the RSV groups.
Pathogenesis and Host Response
The location of hMPV infection has been demonstrated to be type 2 alveolar and bronchiolar epithelial cells in cynomolgus macaques.4 In rodent models, hMPV replicates efficiently in the upper and lower airways, with peak viral titers between days 3 and 5. Lung infection is characterized by alveolar and interstitial inflammation.4 Limited human pathologic data indicate that bronchiolar epithelial cells are infected with hMPV, and prolonged inflammation may be observed. Bronchoalveolar lavage specimens from hMPV-infected children reveal epithelial degenerative changes, eosinophilic cytoplasmic inclusions, and multinucleated giant cells.14 Lung biopsy samples obtained 1 month after initial diagnosis from hMPV-infected children exhibited chronic airway inflammation with foamy and hemosiderin-laden macrophages. Animal data suggest that hMPV can persist in neuronal processes that innvervate the lung, a finding that possibly explains the prolonged pulmonary inflammation observed in children.15 Lower airway involvement in reinfected adults has also been demonstrated.16 Lung tissue from an 89-year-old woman who died during hMPV infection demonstrated diffuse alveolar damage and hyaline membranes with organization, fibrin thrombi, and peribronchial inflammation with hMPV antigens in the cytoplasm of the bronchiolar epithelial cells.
Although hMPV and RSV are closely related viruses, the host immune responses to these two viruses are different.4 Although hMPV lacks the two nonstructural proteins found in RSV that are known to inhibit host interferon (IFN) production, hMPV likely uses other mechanisms to subvert innate immune responses.17 In normal human volunteers, peripheral blood mononuclear cells stimulated with hMPV produce a stronger innate and weaker adaptive cytokine response than RSV.18 In contrast, inflammatory cytokines measured in nasal secretions of babies infected with hMPV are less than infants infected with RSV.19
Similar to RSV, immunity to hMPV is incomplete and reinfections occur throughout life despite the development of an antibody response.20–22 The primary target of neutralizing antibody appears to be the F protein, and antibody directed against F is protective in animal models.23 The other surface proteins, G and SH, are weakly immunogenic, and unlike RSV, antibody to the G protein has been shown to be non-neutralizing.24 Lymphopenia and receipt of cytoxic therapy are risk factors for severe hMPV disease, suggesting that cellular immunity is important for hMPV illness resolution. Animal studies of CD4 and CD8 T-cell–depleted mice indicate that T cells are important for viral clearance but also contribute to disease pathogenesis.25 Specific T-cell epitopes have been mapped to the M2 and N proteins.4
Because of the winter seasonality of hMPV, coinfection with other viral and bacterial respiratory pathogens is common. Several investigators have noted that illness associated with dual hMPV and RSV infection is more severe in young children than that with either pathogen alone, although others have not been able to confirm these observations.26,27 Similar to influenza, hMPV infection has been associated with bacterial pathogens, such as Streptococcus pneumoniae, and bacterial coinfection is felt to contribute in some cases to severe disease.28,29,30 Epidemiologic studies have correlated hMPV activity with invasive pneumococcal disease, and introduction of the seven-valent pneumococcal conjugated vaccine has been shown to significantly reduce severe hMPV infections.31,32 Animal models confirm that influenza and hMPV predispose to severe pneumococcal infection, but mechanisms of disease appear to be different. The presence of neuraminidase on influenza virus appears to promote the adherence of bacteria to respiratory epithelium, whereas replication of hMPV is required to increase superinfection of pneumorovirus in the respiratory tract.33
Epidemiology
hMPV is a ubiquitous pathogen that affects all age groups.1,20,28,34 Seroprevalence studies indicate that by age 5 years, most children have been infected with hMPV. Illness caused by both RSV and hMPV appears to be common in young children, but primary infection with hMPV occurs at a slightly older age.35,36 Whereas nearly 100% of children are infected with RSV by age 2 years, data from the Netherlands show that about 50% are seropositive for hMPV by age 2 years and 100% by age 5 years.1 hMPV accounts for a substantial burden of disease among children in the first 5 years of life, causing 2% to 3% of all symptomatic respiratory infections.34–40 In the United States, annual hospitalization rates are 3 per 1000 children younger than 6 months, 2 per 1000 children 6 to 11 months, and 1 per 1000 children younger than 5 years, resulting in approximately 20,000 yearly hospitalizations.34 As a cause of serious lower respiratory tract disease in children, hMPV ranks second to RSV, with disease burdens similar to those of influenza and parainfluenza.34,41,42 A number of studies indicate that the peak age for hospitalization with hMPV is somewhat older than for hospitalization associated with RSV infection.34–36 In addition to serious illness leading to hospitalization, hMPV accounts for a significant number of outpatient and emergency room visits, with rates of 55 and 13 per 1000 children, respectively. Reinfection occurs throughout life, and about 2% of acute respiratory illnesses in the general adult population are due to hMPV.20
Numerous epidemiologic studies have now documented worldwide hMPV circulation.1, 32,35,39,43–46 In temperate climates, the virus circulates predominantly in late winter and early spring months, frequently overlapping with other seasonal respiratory pathogens.28,38,47 However, low levels of hMPV activity can occur during the summer months.40,44 In the Southern Hemisphere, hMPV circulates in the summer, and in the subtropics, peak activity is in the spring and early summer.35 Studies spanning multiple seasons indicate a fairly regular biannual pattern of alternating large and small outbreaks, which tend to be anticyclic with RSV activity.48 In a 5-year study from Sweden, the average incidence of hMPV infection was 2.9% but ranged from 0.8% to 5.9%, depending on the year.38 Numerous studies from around the world confirm the presence of two major genotypes of hMPV, which, like the RSV groups, often circulate concurrently within the same community.39,47,49 The prevalence of genotypes and subgroups varies significantly each year, suggesting that immune pressure plays a role in the dominant circulating genotype.50
Clinical Manifestations
The clinical manifestations of hMPV infection are similar to those of RSV and range from mild URI to bronchiolitis and severe pneumonia requiring mechanical ventilation.3,28 The spectrum of disease depends on the age and the health of the host. As with most respiratory viruses, the clinical syndrome is not distinct. Fever, cough, and coryza are the most common symptoms. The incubation period between exposure and onset of clinical symptoms is not precisely known, although cases of nosocomial transmission suggest an incubation period of about 5 to 6 days.35
Children
Most young children with hMPV infection exhibit fever, cough, and rhinorrhea (Table 161-1).35,36,51 Fever appears to be more common with hMPV than with RSV, and febrile seizures were noted in 16% of patients with hMPV compared with 3.1% in RSV-infected children in one study.35 Wheezing is also common, with rates ranging from 22% to 83%, depending on the age group studied.36,37 In children younger than 3 years, acute otitis media has been documented in up to 60% of hMPV-infected children, and hMPV RNA can be detected in middle ear fluid in some cases.52,53 Conjunctivitis, pharyngitis, and laryngitis all occur with variable frequencies.36,51,54 Less common symptoms include maculopapular truncal rash and diarrhea.35 Of note, 2 of 26 French children with hMPV infection had diarrhea and high fever without respiratory symptoms.54 Neurologic complications, such as seizures, ataxia, and encephalitis, appear to be approximately 10 times more common in children with hMPV compared with those infected with RSV.55 Although hMPV RNA has been identified in postmortem brain tissue and cerebrospinal fluid (CSF) in some cases, a postinfectious reactive inflammatory demyelinating process has been postulated in others.56,57 Laboratory findings are relatively nonspecific, with lymphopenia and elevated hepatic transaminase values being described.35 Hypoxia and radiographic changes are common in hMPV-infected children, and abnormal chest radiographs have been found in 26% to 53% of hospitalized children.35,51 Radiographic findings, which include peribronchial cuffing, perihilar infiltrates, patchy opacities, and hyperinflation, are similar to those in children with RSV infection (Fig. 161-3). Lobar consolidation has only rarely been described and may be due to bacterial complications.32,58 Clinical diagnoses most frequently associated with hMPV hospitalization in children include bronchiolitis (in 47% to 84%), asthma (in 11% to 25%), and pneumonia (in 11% to 17%).34–36,51,54 The mean length of hospitalization for these children was 3 to 5 days.
TABLE 161-1
Comparison of Signs and Symptoms in Children with hMPV, RSV, and Influenza A
| hMPV (%) | RSV (%) | INFLUENZA A (%) | |
| Fever | 52-80 | 47-57 | 78-81 |
| Cough | 90-100 | 99 | 96 |
| Rhinorrhea | 88-92 | 91 | 84 |
| Retraction | 65-92 | 95 | 82 |
| Wheezing | 22-83 | 23-65 | 5-57 |
| Lacrimation | 25 | 31 | 31 |
| Diarrhea | 8-17 | 17 | 9-27 |
| Vomiting | 10-25 | 8 | 10 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree