David K. Henderson *
Human Immunodeficiency Virus in Health Care Settings
The risk for transmission of bloodborne pathogens in the health care setting became a matter of substantial concern to health care providers in the late 1980s and has remained a concern to the present day. Despite the fact that hospital-associated transmission of hepatitis had been identified as a problem since the late 1940s,1 the epidemic of human immunodeficiency virus (HIV) infection in the United States in the early 1980s focused the attention of health care providers and regulators on the risk for occupational infection with bloodborne pathogens. Since then, investigators have documented that HIV can be transmitted from patient to health care worker, from health care worker to patient, and from one patient to another in health care settings.2 Thus, occupational HIV infections occur uncommonly, iatrogenic infections are exceedingly rare, and carefully designed interventions to prevent exposures (and to manage exposures when they occur) can reduce the risk for transmission in either direction. This chapter describes the epidemiology of HIV infections acquired in health care settings, methods to prevent these infections, and the principles of management for health care–associated HIV exposures.
Occupational HIV Transmission From Infected Patients to Health Care Personnel
Reported Cases of Occupational HIV Infection
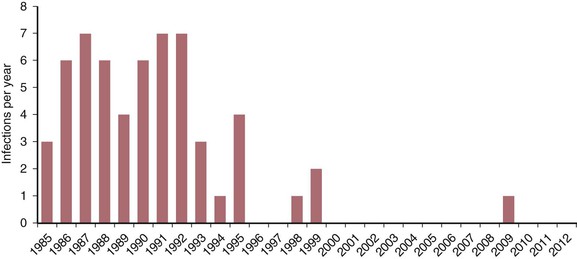
As of December 2012, 58 instances of occupational HIV transmission to health care workers in the United States had been reported to the Centers for Disease Control and Prevention (CDC) (David Kuhar, Division of Healthcare Quality Promotion, CDC, personal communication, March 2013; Fig. 307-1).2,3 In addition, many “possible” occupational infections have been reported worldwide.3,4 Neither baseline HIV serologic tests at the time of known or potential exposures to HIV nor isolate sequencing were performed in these cases, so the relationship between exposure and seroconversion could not be confirmed. However, none of the individuals reported nonoccupational behaviors associated with risk for HIV infection, and all recalled at least one exposure to blood or body fluids before their HIV infections were diagnosed. The demographics of this population suggest that some, but not all, of these individuals probably had confounding community-based risks.5
Mechanisms of Occupational HIV Infection
Most of the occupational HIV infections documented in the United States have been associated with parenteral injuries inflicted by hollow-bore needles used in veins or arteries, but other sharp instruments have also been involved in transmission. Six instances of HIV infection have occurred after either exposure of breaks in skin to HIV-contaminated fluids or exposure of mucous membranes to HIV-contaminated materials. Each of these six instances involved a large-volume exposure, an extended duration of exposure, or both. In one case of mucous membrane exposure in Europe, the inoculum was smaller.6 An additional case was associated with several exposures over an extended time period.7 To date, contamination of intact skin with blood or other infectious material, close personal contact with infected patients, and contact with contaminated environmental surfaces or fomites have not been linked to occupational HIV transmission. Aerosolization of blood occurs during dental, pathologic, laboratory, and surgical procedures, and conventional surgical masks do not prevent inhalation of aerosols. Nonetheless, to date, no data indicate that aerosol exposure is a route of HIV transmission in any setting.
Exposures to blood from HIV-infected patients account for all but 5 of the 58 documented occupational infections in the United States. Of the 5, one infection resulted from exposure to bloody pleural fluid and three involved exposures to concentrated preparations of HIV in scientific laboratories; for the fifth, the source material was not reported.
Definition of Occupational HIV Exposure
The instances of documented occupational HIV transmission have been helpful in developing a definition of what constitutes an exposure associated with HIV transmission risk. As noted in the previous section, exposure routes implicated in occupational HIV transmission include (1) percutaneous injury (e.g., needle puncture or cut caused by a needle or other sharp object); (2) mucous membrane contamination; and (3) contamination of nonintact skin (e.g., skin that is chapped, abraded, or afflicted with dermatitis).8,9 Whereas HIV-infected blood contamination of intact skin has not been implicated in occupational infection, exposures of intact skin to contaminated blood for extended periods (several minutes or longer) or exposures involving extensive areas of skin should be considered potentially infective. This potential exists because unrecognized areas of inadequate skin integrity could serve as portals of entry for the virus.
Sources of HIV that may pose a risk for transmission through these routes include blood; visibly bloody fluids; tissues; and other body fluids, including semen, vaginal secretions, and cerebrospinal, synovial, pleural, peritoneal, pericardial, and amniotic fluids.8,9 In addition, any direct cutaneous or mucosal contact (i.e., without barrier protection) to concentrated HIV in a scientific or research laboratory or production facility should be considered an exposure.
Although nonoccupational HIV transmissions have been attributed to contact with blood-contaminated saliva, these incidents were not analogous to the contact with saliva that occurs during dental or medical care.9,10 In the absence of visible blood in saliva, exposure to saliva from an HIV-infected person is not thought to pose a risk for HIV transmission. Exposure to products that are not visibly bloody (tears, sweat, urine, or feces) from infected patients does not constitute an HIV exposure. Whereas human breast milk has been implicated in perinatal HIV transmission, this route of transmission is not analogous to occupational exposure, and contact with breast milk from a patient infected with HIV does not constitute an occupational exposure.8
Occupational HIV Exposure Transmission Risk
Assessing Infection Risk in Populations of Exposed Health Care Personnel
Worldwide, more than 20 prospective studies have helped to quantify the transmission risk associated with discrete occupational HIV exposures.2,11 In each of these studies, health care workers who sustained occupational HIV exposures were tested for HIV antibody at baseline (i.e., at the time of exposure) and periodically thereafter, at regular follow-up intervals, to detect new infections.
Pooled data from these studies suggest that the average risk for HIV transmission associated with percutaneous exposures to blood-contaminated sharp objects that have been used on HIV-infected individuals is 0.32% (21 infections associated with 6498 exposures; 95% confidence interval of 0.18% to 0.46%).2 The estimated risk for mucocutaneous transmission is 0.03% (1 infection associated with 2885 HIV exposures involving mucous membranes or nonintact skin); however, this estimate may be biased because the single transmission event was actually reported before prospective data were collected from the involved institution.12 The risk for infection associated with intact skin exposure to HIV is too low to be detected in these studies.13
Factors Associated with HIV Infection Risk
The average risk for transmission derived from prospective studies is helpful in evaluating populations of exposed persons but does not necessarily reflect the risk associated with the specific exposure experienced by an individual health care worker. Many factors are either known or suspected to affect the infection risks in specific cases, including the route of transmission, the inoculum of infectious virus, and the exposed worker’s immunologic response.
The inoculum of virus is related both to the volume of material involved in the exposure and the titer of virus in that material. Laboratory models of needlestick exposure demonstrate that exposure volume increases with needle size and depth of penetration and that hollow needles generally transmit more blood than do suture needles of comparable size.14,15 In one model, when a needle passed through one or more layers of latex or vinyl gloves before contacting the skin, the volume of blood transferred to the skin was reduced by more than 50% for hollow needles and more than 80% for suture needles.15 However, in all experimental conditions, the blood volume transferred to skin varied by a single order of magnitude. Large volumes of blood—with or without prolonged duration of contact and a portal of entry—are common features in the reported cases of infection through mucosal surfaces or skin, but the number of cases is too small to identify and quantify risk factors associated with increasing risk for mucocutaneous infection.
The amount of infectious virus present in source material may vary by several logarithms, depending on the patient’s stage and severity of HIV infection and the effect of antiretroviral treatments. Thus, viral titer is probably a very important predictor of transmission risk.9,16–18 In general, the titer of HIV circulating in blood is highest at the time of seroconversion and during advanced stages of the acquired immunodeficiency syndrome (AIDS). Both cell-free and cell-associated viruses circulate in the blood of HIV-infected individuals. Tests to quantify cell-free HIV RNA (viral load) in plasma are in widespread use and provide a convenient and reasonably accurate measure of virus replication. However, these tests do not determine what proportion of the plasma virus titer is actually infectious. Quantifying cell-associated virus is much more difficult, although HIV DNA in peripheral blood monocytes can be quantified in a few specialized laboratories. In some studies, higher viral load has been associated with an increased risk for perinatal transmission.19,20 Conversely, HIV transmission from persons with plasma viral loads below the limits of quantification (based on the assays in use at the time the data were collected) has been reported in instances of mother-to-infant transmission19,20 and in one occupational infection.21 As a general tenet, the amounts of cell-free and cell-associated virus present in the circulation of any given patient are highly correlated, but more data are needed to determine which component is more important in predicting transmission risk.
To define factors associated with transmission risk, the CDC conducted a retrospective case-control study of percutaneous exposure to HIV among health care personnel.22 In this study, deep injuries, visibly bloody sharp devices, and devices that had been used in blood vessels were independent predictors of HIV transmission. Each of these factors is probably an indirect measure of the size of the viral inoculum. In this same study, the odds of acquiring infection after percutaneous exposure were six times higher when the source patient had preterminal AIDS (defined as death within 2 months) than when the source patient had earlier stages of infection (Table 307-1). This difference may also be simply a reflection of higher viral inocula. Patients who have advanced HIV disease often have very high titers of circulating HIV. However, the virus strains found in these patients have both phenotypic characteristics (e.g., syncytium induction, macrophage tropism) and genotypic characteristics (e.g., large numbers of HIV quasi-species) that may also contribute to the increased transmission risk associated with advanced HIV disease. The fact that most cases of documented occupational transmission involved exposure to patients who had advanced HIV infection is consistent with the hypothesis that the quantitative or qualitative differences present in these patients do increase the risk. However, patients who have advanced disease are also more likely to be hospitalized and are also more likely to undergo procedures that pose an exposure risk to health care personnel.
TABLE 307-1
Logistic-Regression Analysis of Risk Factors for HIV Transmission after Percutaneous Exposure to HIV-Infected Blood
| RISK FACTOR | U.S. CASES* | ALL CASES† | ||
| Odds Ratio | 95% Confidence Interval‡ | Odds Ratio | 95% Confidence Interval‡ | |
| Deep injury | 13.0 | 4.4-42 | 15.0 | 6.0-41 |
| Visible blood on injuring device | 4.5 | 1.4-16 | 6.2 | 2.2-21 |
| Injuring device used in artery or vein | 3.6 | 1.3-11 | 4.3 | 1.7-12 |
| Terminal illness in source patient§ | 8.5 | 2.8-28 | 5.6 | 2.0-16 |
| Postexposure use of zidovudine | 0.14 | 0.03-0.47 | 0.19 | 0.06-0.52 |
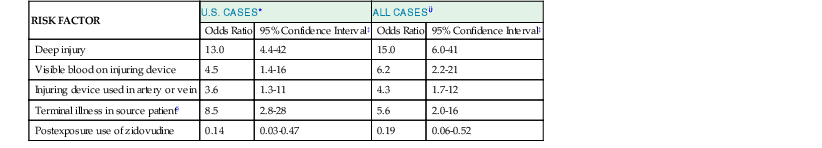
* All were significant at P < .02.
† All were significant at P < .01.
‡ Adjusted odds ratios (95% confidence interval) reflect the odds of seroconversion after exposure in workers with the risk factor, as opposed to those without it.
§ Terminal illness was defined as disease leading to death of the source patient from acquired immunodeficiency syndrome within 2 months after the health care worker’s exposure.
HIV, human immunodeficiency virus.
From Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337:1485-1490. Copyright © 1997 Massachusetts Medical Society. All rights reserved.
The immunologic responses of the exposed health care workers also appear to affect the probability of HIV transmission. At least three outcomes are believed to follow HIV exposure: (1) infection (HIV antibody seroconversion and long-term systemic infection); (2) no infection/ no immunologic response; and (3) “aborted infection” (limited cellular infection detected by T-cell response to HIV antigens, no long-term systemic infection, no HIV antibody seroconversion). Immunologic evidence supporting the concept of “aborted infection” comes from studies of uninfected prostitutes,23,24 studies of sexual partners of infected persons,25–28 studies of children born to HIV-infected mothers,29 patients inadvertently exposed to blood from infected patients during the provision of health care who remain uninfected,30 and studies of occupationally exposed, but uninfected, health care workers.31–33
In the study of the exposed but uninfected hospitalized patients who had a point-source exposure to blood from a patient known to be infected with both HIV and hepatitis B, the investigators were able to obtain postexposure lymphocytes from three of the five patients identified as acquiring hepatitis B in the outbreak. Cryopreserved lymphocytes from these patients were tested for interferon-γ release in response to stimulation with peptides from structural and nonstructural HIV proteins. The investigators also were able to identify circulating HIV-specific CD8 cells by using tetramer staining. T cells from these individuals released interferon-γ in response to stimulation with HIV peptides, suggesting that the cells had been primed in vivo with HIV antigens. These data demonstrate an HIV-specific cell-mediated immune response in patients who did not develop HIV infection, despite a documented intravenous point source exposure to replicating HIV that was significant enough to transmit hepatitis B. None of these patients developed antibody responses to HIV-associated antigens.
Similarly, T lymphocytes derived from the peripheral blood of some uninfected health care workers who were exposed to HIV through needle-related injuries can be stimulated to proliferate and secrete cytokines when exposed to HIV antigens in vitro.31,32,34 The role of cellular immunity in host defense against HIV infection is not delineated, and why some individuals appear to be able to abort infection is unclear. Nonetheless, the observation is consistent with the hypothesis that the cellular immune system is one important determinant of exposure outcome.
Characteristics Associated with Exposures Resulting in HIV Seroconversion in Health Care Workers
For the 51 instances of occupational infection for which data concerning the characteristics and timing of HIV seroconversion have been reported to the CDC, 81% were associated with illnesses compatible with primary HIV infection (i.e., the seroconversion illness) a median of 25 days after exposure.8,35 The clinical syndrome occurring in acutely infected health care workers was indistinguishable from that observed in persons with primary HIV infection acquired through nonoccupational exposures. The median interval from exposure to documentation of a positive HIV antibody test was 46 days (mean, 65 days). This estimate is limited because testing is performed at variable intervals after exposure and the precise date of seroconversion cannot be known with certainty. Overall, of health care workers acquiring infection from occupational exposures, 95% are expected to undergo seroconversion within 6 months of the exposure.35 This estimate is basically identical to that for infection associated with other exposure routes.
Three cases of delayed HIV seroconversion among health care workers have been reported.21,35–37 For each of these health care workers, the result of HIV antibody testing was negative 6 months after an occupational exposure but became positive at some time in the ensuing 1 to 7 months. For one of these cases, DNA sequencing confirmed that the infection was occupationally acquired. Interestingly, two of these health care workers were also infected with hepatitis C virus (HCV) as a result of the index needlestick exposure. In both instances, HCV infection was unusually severe; in one case, the disease was rapidly fatal. Whether coinfection with these two viruses directly influences the timing or severity of either HIV or HCV infection is unclear. Nonetheless, most experts agree that until more data are available, if health care workers are exposed simultaneously to both viruses and develop serologic evidence of HCV infection 6 weeks to 9 months after occupational exposure, they should be carefully monitored for late HIV seroconversion up to a year after exposure.
Iatrogenic HIV Transmission from Infected Health Care Personnel to Patients
Case Reports of HIV Transmission from Infected Providers to Patients
Since the onset of the AIDS epidemic in the early 1980s, only four instances of HIV transmission from infected health care workers to one or more patients have been reported.38–43,44 Of these instances of transmission, one occurred in the United States in 199040–43; two were reported from France38,44,45; and the fourth was reported from Spain.39
The episode in the United States involved six patients whose HIV infections were linked epidemiologically and through DNA sequencing to a dentist who had AIDS. Although the investigation indicated that HIV transmission occurred in the dentist’s office and probably represented transmission from dentist to patient, rather than from patient to patient, precise mechanisms of transmission were never determined. Although the dentist was a patient in his own practice, no deficiency of infection control that would readily explain HIV transmission to the six patients could be identified. The dentist did not report occupational injuries that could have created opportunities for cross-contamination, nor was it proved that the infections were intentionally transmitted. The high rate of transmission in this situation remains unexplained.
The second episode of iatrogenic HIV transmission involved an orthopedic surgeon in France whose HIV transmission to one patient was confirmed through DNA sequence analysis of viral isolates obtained from the surgeon and the patient.38,45 The surgeon in this case probably became infected from an occupational injury sustained during a surgical procedure in 1983. The surgeon was not aware of his infection until AIDS was diagnosed in 1994. Investigators initiated a retrospective investigation of the 3004 patients who had undergone at least one invasive procedure performed by the infected surgeon since 1983. Investigators were able to contact 2458 of these 3004 patients and were able to assess the HIV infection status of 983 of these 2458 patients. One patient, who had a negative result of an HIV antibody test before undergoing the first of three procedures performed by the index surgeon, was found to be infected with HIV when she underwent preoperative testing before a third procedure. Although the precise mechanism of transmission is unknown, the duration of the initial procedure (10 hours) and a presumably high viral titer in the surgeon were hypothesized as contributing factors to the transmission event. No breaches in recommended infection control practices were identified. In the third instance, also in France, the virus was thought to have been transmitted from an infected nurse to a patient (according to the phylogenetic analysis of the isolates from the patient and the nurse), although no route of transmission could be identified.44 In a subsequent retrospective study of 7580 patients of the infected nurse, investigators were able to notify 5308 of the patients concerning the potential for exposure.46 No additional HIV infections were detected in the 2293 (of the 5308) who were tested. The nurse who was identified as the source of the patient’s infection was coinfected with HCV and was found to have both a high HIV viral burden and advanced HCV-induced hepatic disease, including clotting abnormalities.
In the fourth case of iatrogenic transmission of HIV in Spain (about which only limited information has been published),39 a woman was infected with HIV by her gynecologist, presumably during the conduct of a cesarean delivery. Spanish officials conducted a retrospective study in which 250 of 275 of the gynecologist’s patients were tested; no additional infections were identified.39
Investigations of Patients Treated by HIV-Infected Health Care Personnel
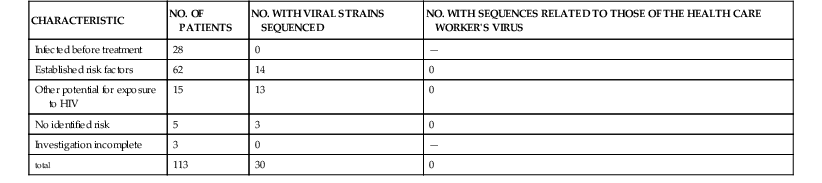
Several investigators have evaluated the risk for HIV transmission for patients of clinical practitioners identified as being HIV infected by studying patients who underwent procedures performed by infected practitioners.2 In March 1992, the CDC developed a database to monitor the results of retrospective investigations of health care workers infected with HIV to assess the risk for this mode of HIV transmission. Excluding the patients from the Florida dental practice (discussed previously), through December 1998, the CDC obtained information from investigations of 66 HIV-infected health care workers in the United States.47,48 The health care workers included 29 dentists and dental students, seven physicians and medical students, 16 surgeons and obstetricians, and one podiatrist. HIV test results were available from patients of 53 of these 66 HIV-infected health care workers. In total, 22,759 patients of these health care workers were tested. No HIV infections were detected among 13,667 tested from the practices of 40 of these HIV-infected health care providers. For the remaining 13 HIV-infected providers (seven dentists and six surgeons and obstetricians), 9,108 patients were tested and 113 were identified with HIV infection. Of these 113 infected patients, follow-up investigations were completed for all but 3. No infections were linked to infected health care providers. Genetic sequence analysis was performed on HIV strains from 3 infected clinicians and 30 of their patients who were infected with HIV, including 3 of 5 patients who had no identified risk for HIV infection. In no instances were viral strains of patients and infected workers genetically related (Table 307-2). These retrospective studies have important limitations, most notably the fact that in virtually all the studies, the follow-up evaluation and testing were incomplete. Despite the limitations, these data are consistent with previous assessments that the risk for HIV transmission from infected health care personnel to patients is extremely low.
Iatrogenic Transmission Detected in the CDC’s HIV/AIDS Surveillance Database
In the United States, persons who have AIDS or, in some states, those infected with HIV who are reported to state and local health departments with no identified risk for HIV infection were historically studied to determine the likely mode of HIV acquisition.49 These studies include a review of medical records, contact with health care providers, and interviews with the patients. Of persons who were identified as having no reported risk for HIV infection, approximately 10% could not be contacted for follow-up, had died, or were otherwise unable to be interviewed. For the remainder, investigations were successful in identifying established modes of infection for more than 95%. With the exception of the Florida dental investigation discussed in detail previously, no cases of HIV transmission from an infected health care provider have been identified through the CDC’s nationwide HIV/AIDS surveillance system.
Provider-to-Patient Transmission Risk Assessment
In general, three conditions are necessary to create a risk for provider-to-patient HIV transmission:
No currently available method reliably estimates the infectivity of individuals infected with HIV, and, at least for now, all infected persons are assumed to be infectious (condition 1), although in time, the circulating viral burden may provide an important “proxy” for condition 1. At present, data are inadequate for establishing a threshold or cutoff for infectivity. Most infected health care personnel pose no risk to patients because they neither perform procedures in which they risk penetrating injuries nor have dermatologic conditions that are a potential conduit of exposure of patients to infected body fluids (condition 2). In addition, most health care providers do not perform the kind of invasive procedures, such as surgical or obstetric interventions, in which an injury could expose the patient to infected blood (condition 3).
The risk for bloodborne pathogen transmission to patients during recontacts is not known but is believed to be lower than that associated with most occupational exposures. Most provider injuries potentially associated with blood exposure to the patient that have been reported in observational studies involved the penetration of a surgeon’s glove by a solid sharp (e.g., suture needle, bone spicule).50,51 In most of these observed cases, no wound or bleeding was evident at the site of the provider’s injury. Many such injuries were not associated with a detectable perforation in the provider’s glove, as measured by the water distention leak test.50 The “recontact” transmission risk may be even lower than is currently perceived, inasmuch as suture needle punctures transfer a smaller volume than do hollow-bore needles and because this blood inoculum may be reduced even further when the needle passes through glove material.15
After years of experience managing HIV infections in the health care setting, physicians now have a substantial body of epidemiologic evidence that demonstrates the risk for iatrogenic HIV transmission to patients from an infected provider to be extremely low, even when all three of the conditions associated with transmission risk are present. Furthermore, the already low risk to patients can be reduced further by adherence to standard infection control practices, prevention of percutaneous injuries during invasive procedures, and changes in surgical practice (see “Primary Prevention”).52,53 Guidelines for managing infected providers are discussed in more detail in a later section (see “Management of Infected Health Care Providers”).
Nosocomial HIV Transmission from Infected Patients to Other Patients
Episodes of nosocomial HIV transmission from one patient to another have most frequently involved breaches in infection control practices and disinfection procedures. Re-use or improper sterilization of blood-contaminated injection needles or syringes has been linked to HIV transmission to hospitalized children in Russia,54 Libya,55,56 Romania,54,57–59 several countries in Africa,55,60–63 and India64 and probably in other developing countries. Medical errors in three institutions (two in the United States and one in the Netherlands) resulted in inadvertent exposures of patients to HIV, as a result of injection of blood from HIV-infected patients during nuclear medicine procedures.65 Contamination of multidose vials frequently has been incriminated as a vehicle for transmission of HIV and other bloodborne pathogens in several instances in both industrialized and developing countries,55,66–69 and one modeling study has demonstrated the clear potential for such transmission.70
Five patients in Australia who underwent minor outpatient surgical procedures necessitating local anesthesia that were performed on the same day by an HIV-negative surgeon were subsequently found to be HIV positive.71 The first infected patient had known risk factors for HIV and was the probable source of infection for the other four patients. The exact mode of patient-to-patient transmission in this practice has not been elucidated. No cases of patient-to-patient transmission of HIV have been reported from hemodialysis centers in the United States. Conversely, HIV transmission to at least nine patients in a hemodialysis center in Colombia has been reported and was attributed to inadequate disinfection and re-use of contaminated access needles; similar cases have been reported from Argentina and Egypt.72–76
Primary Prevention
Standard (Universal) Precautions
In 1985 the CDC recommended that the blood of all persons be regarded as potentially infectious because identification of all patients carrying bloodborne pathogen infections was not possible.77–79 In 1987 the term universal precautions was coined to communicate this concept. Universal precautions were designed to prevent direct contact with blood, bloody body fluids, and certain other fluids (amniotic fluid, semen, vaginal fluid, cerebrospinal fluid, serous transudates and exudates, and inflammatory exudates) that were either known or likely to be associated with bloodborne pathogen transmission. Central to universal precautions is the appropriate use of barriers, such as gloves for procedures associated with a risk for contact with these fluids, tissues, and materials; the use of masks and protective eyewear when the health care worker anticipates the possibility of splash or splatter; and the use of gowns or other protective garments whenever the practitioner identifies a likelihood of soiling of clothing. Body substance isolation (or body substance precautions) is a highly similar, alternative system of infection control.80 In accordance with the body substance isolation approach, the health care worker’s decision about the use of barrier protection is based on the degree of anticipated contact with all body fluids and tissues, irrespective of the patient’s diagnosis. Both universal precautions and body substance isolation include measures to prevent needle-related injuries. In 1996, the CDC recommended the adoption of an infection control system, standard precautions, that effectively merged the most beneficial aspects of the universal precautions and body substance isolation approaches.81 Whereas universal precautions were designed primarily to reduce the risk for patient to provider transmission of bloodborne pathogens, standard precautions are designed to reduce the risk for bidirectional transmission of infectious diseases. Standard precautions apply to all patients and require the use of gloves, protective clothing, and other barriers, as needed, to prevent direct contact with all body fluids (except sweat). As is the case for all of these isolation systems, percutaneous injury prevention is a key component of standard precautions. These guidelines also include standards for the cleaning and reprocessing of equipment used in patient care.
In 1987 the Occupational Safety and Health Administration (OSHA) issued a Joint Advisory Notice designed to enforce compliance with universal precautions for health care personnel.82 In 1991, the U.S. Department of Labor and OSHA implemented a federal standard designed to enforce compliance with universal precautions.83 In addition, many states enacted legislation requiring that universal precautions be implemented as a condition of funding for health care institutions. The 1991 standard presented a hierarchy of control measures that institutions should incorporate into their bloodborne pathogen exposure control plans. These measures included engineering controls (use of equipment and devices designed to be inherently safer), work practice controls (safety procedures), and personal protective equipment.
In several studies that evaluated the efficacy of universal precautions in preventing blood contact, implementation and enforcement resulted in a significant reduction in exposure frequency.84–87 Factors associated with efficacy of these programs in reducing exposures include training, enforcement, and feedback about exposure mechanisms to managers and front-line workers. Implementation of universal precautions has also been associated with a reduction in the frequency of percutaneous injuries caused by needles and other sharp instruments. The fact that reducing occupational exposure will necessarily reduce occupational infection seems intuitively clear; however, because the frequency of occupational HIV infection is so low, the effect of implementing universal precautions or any other intervention program on the incidence of occupational HIV infection cannot be measured. What is known, however, is that occupational HIV infections have been decreasing in the United States, beginning in the 1990s (David Kuhar, CDC, personal communication, March 2013; see Fig. 307-1).3,21 Several factors probably have contributed to the decrease in occupational infections: possible decreased reporting of occupational infections to the CDC; less aggressive case finding; fewer exposures because of the effective use of universal and standard precautions (i.e., primary prevention); the efficacy of antiretroviral therapy in lowering infected patients’ viral burdens, resulting in reduced need for hospitalization of HIV-infected patients and decreased numbers and types of procedures required by HIV-infected patients88; and the presumed efficacy of antiretroviral postexposure prophylaxis (i.e., secondary prevention, discussed later).
Injury Prevention during Routine Patient Care
Needle punctures are the most frequent cause of occupational HIV infection, and priority for their prevention is highest. All health care workers, including those who actually perform or assist with procedures, those employed as housekeepers and laundry workers, and other nonclinicians are at risk for injury and infection. For this reason, prevention efforts must incorporate strategies that prevent injuries while the needle is being used for its intended purpose, as well as strategies that decrease the risk for injuries after use or disposal of the device.
One component of injury prevention that may be overlooked is avoidance of unnecessary needle use. Phlebotomy is a common indication for using needles and is also the procedure most commonly associated with occupational HIV infection. To reduce opportunities for injury, health care workers should avoid “routine” blood drawing that does not contribute to patient care; use better planning to minimize the number of phlebotomies necessary to obtain the necessary blood tests; and use needleless vascular access ports for blood withdrawal and injection of medication. Similarly, avoiding unnecessary placement of intravenous catheters when alternative routes are available for administering therapy will decrease the opportunity for needle-related injuries.
Implementation of needleless or protected-needle infusion systems can reduce the frequency of needle-related injuries.89–92 The effect of this intervention on disease transmission is less certain because most needles used for intravenous infusion are not contaminated with blood. Not every institution that has implemented one of these systems has found it to be effective in preventing disease transmission or cost-effective.93 Needles used for heparin flushes and needles in contact with ports close to the site of intravenous line insertion are more likely to be contaminated with blood and hence are more hazardous. In reality, determining whether an infusion needle is or has been contaminated is often difficult, if not impossible. Preventing injuries associated with intravenous infusions is therefore an important component of risk management, even though such injuries may be substantially less likely to transmit infection. Improving worker safety must not increase the risk for infection or complications among patients; results of several studies have suggested an increased risk for device-associated bacteremia in association with the use of certain needleless intravenous systems.94–96
Safer needle devices that have been engineered to retract, cover, or blunt the needle are now in widespread use. Some of these devices have safety features that are activated while the needle is being used for its intended purpose. Other safety features are activated after withdrawal from the patient. The most effective devices are passive (i.e., do not require the user to activate the safety feature), do not require extensive training, and are cost-effective. A multicenter study conducted and reported by the CDC demonstrated that implementing safer needle devices for phlebotomy procedures is an effective strategy for preventing percutaneous injuries.97–99 Improved product design and lower cost may lead to even more effective programs for protecting workers during procedures that require the use of needles.
All needles and other sharp instruments, with or without safety features, should be discarded in puncture-resistant containers. Such containers should be located as close as possible to the point of use in emergency departments, in operating rooms, and in other patient care areas. Proper disposal also prevents injuries caused by needles that have been carelessly discarded. Needle disposal programs can significantly reduce the incidence of injury. Finally, some investigators have suggested that employees who have certain personality profiles associated with risk-taking may have increased risks for occupational exposures to blood.100
Injury Prevention during Invasive Surgical, Obstetric, Dental, and Radiologic Procedures
Preventing intraoperative and intraprocedural injuries that confer a risk for blood exposure is an important priority for preventing HIV transmission among health care providers and their patients. For operative procedures, data from observational studies indicate that the risk for provider injury is highest during procedures lasting longer than 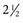 to 3 hours, when intraoperative blood loss exceeds 250 to 300 mL, and during certain categories of major procedures (e.g., intra-abdominal gynecologic procedures, vaginal hysterectomies, major vascular procedures, and orthopedic procedures).51,101,102–109
to 3 hours, when intraoperative blood loss exceeds 250 to 300 mL, and during certain categories of major procedures (e.g., intra-abdominal gynecologic procedures, vaginal hysterectomies, major vascular procedures, and orthopedic procedures).51,101,102–109
Prevention priorities in the operating room are based on the same principles used in other health care settings.110–116 The least invasive surgical approach that will achieve the desired patient outcome is preferable. For example, fiberoptic techniques usually pose a lower risk for injury and blood exposure than do more invasive surgical approaches. Similarly, when patient safety allows, alternatives to needles and other sharp implements (e.g., adhesive tape, staples, and tissue glue rather than sutures; electrocautery rather than scalpels) should be used.
Suture needles are the most frequent cause of injuries in operating and delivery rooms. Curved suture needles with blunted tips are now available and appear to be an acceptable replacement for standard curved suture needles for suturing many types of tissue.98,117–121 Use of these needles is effective in preventing intraoperative injuries. In one multicenter study, 1.9 injuries per 1000 curved suture needles used were observed during gynecologic surgery, but no injuries were associated with the use of blunted suture needles.98 The estimated odds of sustaining an injury with a curved suture needle were reduced by 87% when 50% of the suture needles used during a procedure were blunted. Use of blunted suture needles is also associated with a lower incidence of glove perforation. Overall, surgeons involved in these studies were accepting of the blunted needle and no adverse outcomes among patients were noted.
Another approach advocated to reduce risk for percutaneous exposures during the conduct of invasive procedures is the so-called no-touch technique. Aspects of this technique include using instruments, rather than hands, for retracting and exploring tissue; avoiding the simultaneous presence of the hands of two or more operators in the procedural field; avoiding hand-to-hand passage of sharp instruments by using a “neutral zone” (e.g., emesis basin, Mayo stand, or magnetic pad); and announcing the transfer of sharp instruments from person to person.
Gloves provide an important barrier between potentially infectious materials and health care providers. Sterile surgical gloves prevent microbial contamination of patient wounds and sterile instruments and also protect surgical personnel from cutaneous blood contact. Surgical gloves do not provide a barrier to sharp object penetration, but they may reduce the volume of blood transferred to the skin and hence decrease the risk for infection by a bloodborne pathogen.
Unfortunately, glove perforation is extremely common, especially during major surgical procedures of long duration. Breakdown in glove integrity can cause contamination of exposed tissue and blood contamination of the provider’s hands; and if the provider sustains an injury that results in bleeding (needle puncture) or tissue trauma (e.g., suture-induced “shear injury”), the patient may be exposed to the provider’s blood or interstitial fluids. Double gloving is one strategy that may attenuate these problems. Without exception, in all studies of double gloving, the prevalence of inner glove perforation was significantly lower than that of the outer glove.102,122–130 In addition, double gloving reduces the frequency of visible blood contamination of providers’ hands.
Overall, the thumb, index, and middle fingers of the nondominant hand are the most common glove perforation sites.51,131,132 Reinforcement of these areas is one approach to prevent perforation.110,133–136 The use of gloves that increase the thickness of the barrier between a patient and the provider creates concern about manual dexterity and tactile sensitivity.134,136,137 Nonetheless, in a study that measured two-point discrimination and the ability to tie surgical knots, double gloving did not affect performance. Some measures of tactile sensitivity are reduced, but not the ability to discriminate between suture pairs. In a subjective assessment, surgeons reported that double gloving did impair comfort, sensitivity, and dexterity, but acceptance was better if the inner glove was larger than the outer glove.137
The benefits of double gloving, glove reinforcement, and new glove materials in preventing disease transmission have not been proved. Nevertheless, double gloving decreases inner glove perforation and reduces blood contamination of operators’ hands. Most authorities now recommend routine double gloving during invasive surgical and obstetric procedures.52,131
As emphasized previously, preventing intraoperative injuries to surgical care providers is the most important strategy for preventing the transmission of HIV and other bloodborne pathogens to patients. In two studies of intraoperative provider injuries, 11.4% to 29% of the sharp objects that injured a provider subsequently recontacted the patient.50,51 These exposures are preventable by immediately replacing the contaminated suture needle or other sharp object before re-use. Recontacts can also occur when the provider is injured by bone spicules or materials permanently embedded in the patient’s body.50,51 Exposure to these types of sources might be prevented by the use of reinforced gloves,138,139 liners, or other devices or materials to protect the provider’s hands.110,134–136,138,140 Gloves constructed of monofilament polymers or other materials resistant to tears have become available for use when manipulation of bone fragments or of suture wires is needed, but, as noted previously, their use is not universal because of the associated decrease in tactile sensation.
The frequency of blood exposure among dental personnel has declined since the 1990s. Surveys conducted at annual meetings of the American Dental Association revealed that the mean number of injuries involving blood or body fluid contact reported by dentists decreased from 12.0 to 2.2 per year between 1986 and 1993.141 This impressive decline may be the result of the widespread implementation of universal precautions in dental practices, safer instrumentation, and educational programs for dental professionals and patients. Nonetheless, a significant number of exposures continue to occur in the dental health care setting.142
Specific practices designed to prevent injuries include use of the one-handed “scoop” technique and mechanical devices for recapping needles used to administer local anesthetic; restricting the use of fingers during suturing and administration of anesthetic; controlling the placement of sharp instruments (e.g., scalers and laboratory knives); and improvements in the ergonomic design of dental operatories.143 Safer devices such as self-sheathing anesthetic needles, dental units designed to shield burs in handpieces, and plastic finger guards might also contribute to safer dental care.
Today, most injuries to dental personnel actually occur outside the patient’s mouth, involve very small amounts of blood, and are unlikely to pose a risk to patients.141,143–145 In a 7-month observational study of dentists and of oral and maxillofacial surgical residents in two New York City teaching hospitals, injuries were observed during 0.1% of dental procedures, and 86% of these injuries occurred outside the patient’s mouth.144 Only one needle puncture was observed during 16,000 anesthetic injections.
Low exposure rates have been observed during outpatient oral surgical procedures as well. However, oral procedures performed in the operating room are associated with injuries caused by surgical wires during fracture reduction.123,146,147 The use of small plates instead of wires during the surgical treatment of some mandibular fractures, as well as reinforced gloves, may help prevent some of these injuries.
Interventional radiologists, stimulated by increasing awareness of bloodborne pathogen risks, have developed similar approaches to risk aversion in the interventional radiology suite,148–150 a venue in which particular attention must be paid to the risk for splashes, spattering, and mucous membrane exposures.151
Management of Occupational Exposures to HIV in the Health Care Setting
Initial Exposure Management
Exposure Reporting
Employers of health care workers and other employees at risk for occupational HIV exposure are required to provide a system for reporting exposures and prompt access to medical care.82,83 Many institutions have developed “needlestick hotlines” or other rapid-response systems to direct exposed persons to triage and to initiate immediate treatment.152,153 However, even in facilities with excellent reporting mechanisms and on-site clinical expertise, many exposures are not reported. Underreporting remains a problem in myriad clinical settings and in health care institutions around the world.153–161 All persons at risk must be informed of the importance of immediate reporting to ensure that preventive care can be initiated in time to be effective.
Exposure Site Management
Wounds and skin sites that have been in contact with blood or body fluids should be washed with soap and water.8,162 Exposed mucous membranes should be flushed with tap water. Eyes should be flushed with sterile water or a commercial eye irrigant when available or else with clean tap water. Antiseptics can be used to flush the wound, but they are not known to reduce the incidence of infection, and decontamination should not be delayed until they are obtained.
Counseling and Triage
The emotional impact of a known or suspected HIV exposure is usually significant, especially in the first hours to days after the episode.163,164 During this time, it is helpful to have access to supportive counseling by experienced clinicians who are familiar with the special medical and psychological needs of exposed persons. The clinician must function as an effective translator. Objective information about exposure risk and the pros and cons of chemoprophylaxis must be communicated to an individual who is usually preoccupied with very subjective emotions.152,165 Although trying to talk an exposed worker out of “irrational” fear when objective data indicate that the risk is low may seem intuitively tempting, such reassurance is rarely successful. To the worker, any exposure risk may feel like 100%, and no amount of epidemiologic data is likely to change this impression in the short run. The most important initial messages to communicate are probably empathy (e.g., “I can see how frightening this is for you”), validation (e.g., “Most people in your situation feel the way you do now”), and reassurance (e.g., “This is difficult, but I’ll help you get through it”). Because the exposed individual is very likely to be preoccupied, the counselor should be patient and prepared to answer the same questions repeatedly.
Health care workers who are too upset or confused to make decisions about chemoprophylaxis can sometimes be helped by suggesting that treatment be started immediately, with the option to stop it later (e.g., “Start treatment now, and then tomorrow we can decide whether continuing is your best option”). Buying some time in this manner alleviates the additional pressure to make an immediate decision about initiating the full 4-week course of treatment; empowers workers to be able to change their minds about treatment when they are able to evaluate the risks and benefits more objectively; and (on the basis of animal data) provides the best opportunity for therapeutic efficacy.152,165,166
Several often overlooked points should be included in counseling any health care worker who is facing a decision about postexposure chemoprophylaxis, among them: (1) most persons exposed to HIV do not become infected, even if no treatment is administered; (2) treatment can be stopped at any time; (3) data about the efficacy and safety of chemoprophylactic regimens are incomplete; (4) to date, zidovudine is the only drug for which there are any data suggestive of efficacy in preventing HIV transmission in humans; and (5) despite the logic associated with administering two or three agents for chemoprophylaxis, no data prove that combination treatment is more effective than single-drug therapy for HIV prevention.153
Health care workers who sustain exposure to HIV should be counseled to avoid transmission to others during the follow-up period, especially during the first 6 to 12 weeks after exposure, when seroconversion is most likely to occur.8–10 Recommended practices to avoid transmission include sexual abstinence or the use of condoms to prevent sexual transmission, as well as the avoidance of blood and organ donation. If the exposed person is breast-feeding, discontinuation of breast-feeding should be considered, especially for high-risk exposures. Modifying an exposed health care worker’s responsibilities for patient care to prevent transmission to patients is not necessary.
Counselors should also provide reassurance, review information about the degree of risk present, and inform the worker about procedures to protect the confidentiality of the exposure medical records. As noted earlier, the most important message to communicate to most workers is that occupational HIV transmission is very unlikely; 99.7% of exposures do not result in HIV infection, even if chemoprophylaxis is not administered. Continued reassurance from a supportive clinician, coupled with practical advice about measures to prevent future exposure, enables the worker to cope successfully with the exposure and its aftermath, although some exposed workers have major difficulty adjusting.167 Counselors should also be alert to the concerns of sexual partners, co-workers, family, and friends of the exposed worker. Referral for ongoing supportive therapy during the follow-up interval is helpful for the minority of exposed persons who experience difficulty in adjusting to the stress inherent in waiting the 6 months for testing to be complete. For such individuals, testing by using one of the newer fourth-generation platforms, which detect P24 antigen and HIV antibodies, may be beneficial, if available. Finally, adherence to chemoprophylaxis regimens may be enhanced if skilled counselors provide advice to drug recipients. In the San Francisco Post Exposure Prevention Project, the frequency of side effects was similar to those in health care worker studies; however, nearly 80% of participants completed 4 weeks of therapy. The authors ascribed this success largely to the intensive, skilled counseling that enrollees received.168,169
Exposure History
When an exposure is reported, the first priority is to evaluate the risk for infection and the need for immediate wound care and prophylactic treatment. After these issues have been addressed and the exposed worker is calm enough to engage in a more detailed discussion, the interviewer can elicit additional details about the exposure. Information should be recorded about when, where, and how the exposure occurred, the type of device involved, the presence or absence of safety features (and, if present, their state of activation), and when in the course of handling the device the exposure occurred (e.g., during use, after use, during disposal). Each institution should pool these data and should periodically evaluate them systematically to identify common circumstances or flawed processes that may be modified to reduce these risks and to increase patients’ and workers’ safety.
If exposure to HIV (or hepatitis B or C virus) (see Chapters 305 and 306) has occurred, the risk for transmission for any or each of the pathogens to which the individual is exposed should be assessed. If the source patient is known to be infected with HIV, the interviewer should determine the stage of illness, recent results of viremia testing (if available), and recent antiretroviral treatment history.8,9,152,165 If the source patient’s HIV status is not known, information relevant to the probability of infection (e.g., presence or absence of risk behaviors), as well as clinical and epidemiologic clues that suggest undiagnosed HIV infection, should be recorded and considered when recommendations for follow-up management are made.
For needle punctures or similar percutaneous injuries, information about the source material and exposure characteristics known to be associated with increased risk for HIV transmission (deep injury, visibly bloody device, device used in an artery or vein) should be carefully collected and recorded.8,9,22 In addition, the practitioner should inquire about factors likely to increase the transmission risk (e.g., injection of a volume of blood, exposure to hollow-bore needle, exposure to large-gauge needle).
For mucosal exposure, the body fluid or material involved and the exposed site, volume of material, and duration of contact before decontamination should be recorded. In addition to these data, for reported skin contacts, the condition of the skin at the site of contact should be evaluated to detect lesions that could provide a portal of entry and influence the risk for infection. For intact skin exposures without an obvious portal of entry, infection is so unlikely that further evaluation and treatment are not necessary unless the contact is prolonged or involves a large area of intact skin. Even then, the risk for HIV infection is extremely small.13
Human bites rarely transmit HIV (see later discussion).170–173 The person who inflicted the bite may sustain a mucosal blood exposure to HIV, but only if the skin was penetrated, the bite wound bled, or both. The person who is bitten is usually not at risk for HIV infection unless blood or visibly bloody saliva was in direct contact with the bite wound. Penetrating bite wounds do pose a risk for bacterial wound infection, and appropriate wound care and antibacterial prophylaxis should be provided, when indicated (see Chapter 320).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree