HORMONE RECEPTORS
Part of “CHAPTER 4 – HORMONAL ACTION“
GENERAL FEATURES
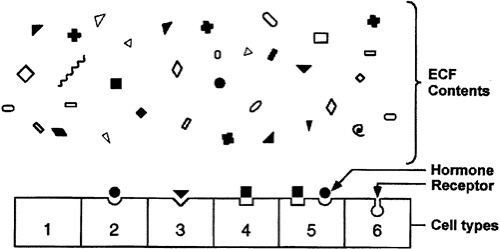
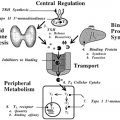
One of the major challenges in making the hormone-based communication system work is depicted in Figure 4-1. Hormone concentrations are very low in the extracellular fluid, generally in the range of 10-15 to 10-9 M. This is much lower than that of the many structurally similar molecules (e.g., sterols, amino acids, peptides) and other molecules that circulate at concentrations in the 10-5 to 10-3 M range. Target cells must identify the various hormones present in small amounts and differentiate a given hormone from the 106- to 109-fold excess of other, often closely related, molecules. This high degree of discrimination is provided by cell-associated recognition molecules called receptors. Hormones initiate their bioeffects by binding to specific receptors, and because any effective control system must provide a means of stopping a response, hormone-induced actions usually terminate after the effector dissociates from the receptor.
A target cell is defined by its ability to bind a given hormone selectively by means of a receptor, an interaction that is often quantitated using radioactive ligands that mimic hormone binding. Several features of this interaction are important. The radioactivity must not alter the bioactivity of the ligand. The binding should be specific, in which case the ligand is displaceable by unlabeled agonist or antagonist. Binding should be saturable. Binding should occur within the concentration range of the expected biologic response.
RECOGNITION AND COUPLING DOMAINS OF RECEPTORS
All receptors, whether for polypeptides or steroids, have at least two functional domains, and most have several more. A recognition domain binds the hormone, and a second region, the coupling domain, generates a signal that links hormone recognition to some intracellular function. The binding of hormone by receptor implies that some region of the hormone molecule has a conformation that is complementary to a region of the receptor molecule. The degree of similarity, or fit, determines the tightness of the association; this is measured as the affinity of binding. If the native hormone has a relative affinity of 1, other natural molecules range between 0 and 1. In absolute terms, this actually spans a binding affinity range of more than a trillion. Ligands with a relative affinity of more than 1 for some receptors have been synthesized and are used to study receptor biology.
Coupling (i.e., signal transduction) occurs in two ways. Polypeptide and protein hormones, and the catecholamines, bind to receptors located in the plasma membrane, and thereby generate signals that regulate various intracellular functions. Steroids, thyroid hormones, retinoids, and other hormones of this class interact with intracellular receptors, and this complex provides the initial signal.
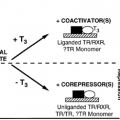
The amino acid sequences of the recognition and coupling domains have been identified in many polypeptide hormone receptors. Hormone analogues with specific amino acid substitutions were used to change binding and alter the bioactivity of the hormone. Steroid hormone receptors also have these two functional domains; one site binds the hormone and the other binds to specific DNA regions. They also have other domains important for their function, which are described later. Several receptors have been characterized by recombinant DNA techniques, and structural analysis shows that these domains are highly homologous. This homology has been used to isolate cDNAs encoding several receptors that had not been obtained through classic protein purification procedures. The investigations have shown that these nuclear receptors are part of a large family of related proteins.17 This family of proteins is thought to regulate gene transcription, often in association with other transcription factors and coregulatory molecules. The ligands for these are called orphan receptors.
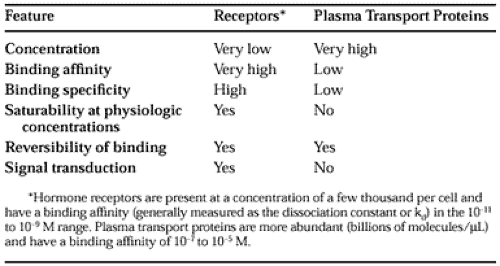
The dual functions of binding and coupling ultimately define a receptor, and it is the coupling of hormone binding to signal transduction, called receptor-effector coupling, that provides the first step in the amplification of the hormonal response. This dual purpose also differentiates the target cell receptor from the plasma carrier proteins that bind hormone without generating a signal. It is important to differentiate the binding of hormones to receptors from the association that hormones have with various transport or carrier proteins. Table 4-1 lists several features of these functionally different classes of proteins.
RECEPTOR OCCUPANCY AND BIOEFFECT
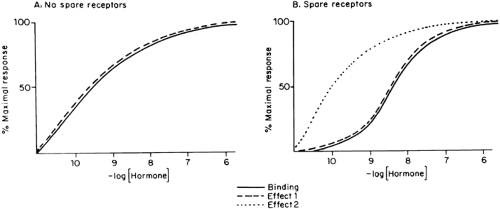
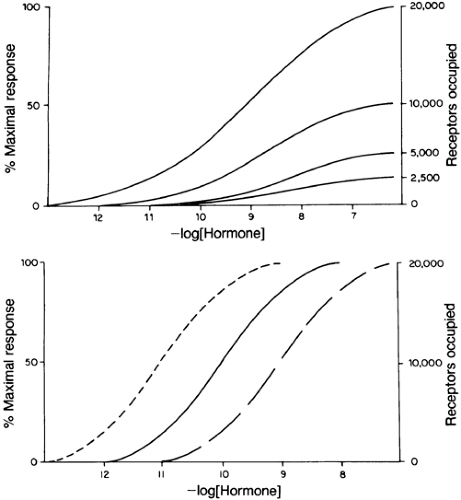
The concentrations of hormone required for occupancy of the receptor and for elicitation of a specific biologic response often are similar (Fig. 4-2A). This is especially true for steroid hormones, but some polypeptide hormones also exhibit this characteristic. This tight coupling is remarkable, considering the many steps that must occur between hormone binding and complex responses, such as transport, enzyme induction, cell lysis, or cell replication. When receptor occupancy and bioeffect are tightly coupled, significant changes in the latter occur when receptor occupancy changes. This happens when fewer receptors are available (Fig. 4-3A) or the affinity of the receptor changes but hormone concentration remains constant (see Fig. 4-3B). Otherwise, there is a marked dissociation of binding and effect, and a maximal bioeffect occurs when only a small percentage of the receptors are occupied (see effect 2 in Fig. 4-2B).
Receptors not involved in the elicitation of the response are called spare receptors. They are observed in the response of several polypeptide hormones and are thought to provide a means of increasing the sensitivity of a target cell to activation by low concentrations of hormone and to provide a reservoir of receptors. The concept of spare receptors is operational and may depend on which aspect of the response is examined and which tissue is involved. For example, there is excellent agreement between LH binding and cyclic adenosine monophosphate (cAMP) production in rat testis and ovarian granulosa cells (there generally are no spare receptors when any hormone activates adenylate cyclase), but steroidogenesis in these tissues, which is cAMP dependent, occurs when fewer than 1% of the receptors are occupied (see effects 1 and 2 in Fig. 4-2).18 Transcription of the phosphoenolpyruvate carboxykinase gene is
repressed when far fewer than 1% of hepatoma cell insulin receptors are occupied, but there is a high correlation between insulin binding and amino acid transport in thymocytes.19 Other examples of the dissociation of receptor binding and biologic effects include the effects of catecholamines on muscle contraction, lipolysis, and ion transport.20 These end-responses presumably reflect a cascade or multiplier effect of the hormone.
repressed when far fewer than 1% of hepatoma cell insulin receptors are occupied, but there is a high correlation between insulin binding and amino acid transport in thymocytes.19 Other examples of the dissociation of receptor binding and biologic effects include the effects of catecholamines on muscle contraction, lipolysis, and ion transport.20 These end-responses presumably reflect a cascade or multiplier effect of the hormone.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree