INTRODUCTION
Hepatobiliary malignancies comprise a diverse group of tumors, including hepatocellular carcinoma (HCC), variants such as fibrolamellar HCC (FLHCC) and cholangiocellular carcinoma, cholangiocarcinoma, carcinoma of the gallbladder, and rare cancers such as sarcoma, angiosarcoma, and hepatoblastoma. The relative frequency of these tumors is shown in Table 22-1. The estimated new cases and deaths from liver and intrahepatic bile duct cancer in the United States in 2014 totaled 33,190 and 23,000, respectively (1).
The majority of primary liver tumors are HCC or cholangiocarcinoma. These tumor types have different etiologies, epidemiology, clinical presentations, and treatment options. Thus, they are discussed separately.
HEPATOCELLULAR CARCINOMA
Hepatocellular carcinoma is a malignancy of worldwide significance and has become increasingly important in the United States. It is the most common primary liver malignancy, the sixth most common cancer, and the third most common cause of cancer-related deaths worldwide (2). Eighty percent of new cases occur in developing countries, but the incidence is rising in economically developed regions, including Japan, Western Europe, and the United States (3,4,5,6). The worldwide distribution of HCC and its associated etiologies are summarized in Table 22-2. Liver cirrhosis is the seventh leading cause of death in the world, the tenth most common cause of death in the United States, and acknowledged as a premalignant condition for developing HCC (7,8,9).
| Incidencea | ||||
|---|---|---|---|---|
| Region | Men | Women | Number of Cases | Principal Associations |
| Asia, sub-Saharan Africa | 30-120 | 9-30 | >500,000 cases per year | HBV, aflatoxin exposure |
| Japan | 10-30 | 3-9 | HCV | |
| Southern Europe, Argentina, Switzerland | 5-10 | 2-5 | HCV | |
| Western Europe | <5 | <3 | HCV | |
| United States | <5 | <3 | 19,000 predicted for 2004 | HCV, alcohol |
In the United States, hepatitis C virus (HCV), alcohol use, and nonalcoholic fatty liver disease (NAFLD) are the most common causes of cirrhosis (9). The incidence of HCC doubled during the period 1975 to 1995 and continued to rise through 1998 (10,11). This trend was previously expected to continue due to the estimated 4 million US individuals who are hepatitis C seropositive and the known latency of HCC development from the initial HCV infection, which may take two to three decades (11). However, given the improved treatment regimens now available for patients with chronic hepatitis C, HCV-related HCC incidence may decrease in the next few years (12). It is also known that NAFLD-associated cirrhosis is on the rise in the United States (13,14,15). A majority of patients present with advanced disease that is not amenable to curative procedures. Overall, HCC has a very poor prognosis, with a 5-year survival rate of 5%.
EPIDEMIOLOGY
As shown in Table 22-1, HCC represents approximately 85% of all primary liver cancers (16). The distribution of HCC varies significantly by geography; it is endemic in parts of the world where hepatitis B virus (HBV) is also endemic. In Western countries, HCV infection and alcoholic cirrhosis are the principal risk factors for HCC. Due to rising incidence of HCV infection in American subpopulations, the incidence of HCC is projected to increase fourfold by 2015 (11). Moreover, HCC incidence increases with age, with the age of peak incidence varying somewhat with population. The median age group of HCC is between the fifth and sixth decades. The disease is also seen in children and young adults in areas where HBV is endemic, and most of these infections occur perinatally. In all populations worldwide, there is a strong male predominance in HCC incidence. In the United States, the male-to-female ratio is 2.7 to 1, and HCC incidence rates are higher among African Americans than Caucasians (6.1 vs 2.8 per 100,000 in men). Hispanics, Asians, Pacific Islanders, and Native Americans have a much higher HCC frequency. Independent of HBV status, a family history of HCC in first-degree relatives carries a relative risk (RR) of 2.4 and overall risk (OR) of 2.9 (17). Familial aggregation and germline mutations of the APC (adenomatous polyposis coli) gene have been reported in hepatoblastoma (18).
ETIOLOGY AND RISK FACTORS
Hepatocellular carcinoma develops commonly, but not exclusively, in a setting of liver cell injury, which leads to inflammation, hepatocyte regeneration, liver matrix remodeling, fibrosis, and ultimately cirrhosis. The major etiologies of liver cirrhosis are diverse and include chronic HBV and HCV infection, alcohol consumption, certain medications or toxic exposures, and genetic metabolic diseases. The mechanisms by which these varied etiologies lead to HCC are not fully elucidated. The principal risk factors that have been associated with cirrhosis and HCC are listed in Table 22-3.
| Category | Specific Etiology | Comment |
|---|---|---|
| Infectious (77% of cases of HCC worldwide attributed to viral hepatitis) | Hepatitis B virus | Underlying etiology in a significant majority of HCC cases worldwide, primarily in Asia, sub-Saharan Africa |
| Hepatitis C virus | Principal underlying etiology in Japan, the United States, Western Europe, Mediterranean basin countries; may account for 20%-25% HCC cases worldwide | |
| Metabolic disorders deficiency | Hemachromatosis a1 antitrypsin | |
| Wilson disease | ||
| Porphyria cutanea tarda | ||
| Glycogen storage disease | ||
| Citrullinemia | ||
| Familial cholestatic cirrhosis | ||
| Other | Alcohol | Significant cause of liver cirrhosis; cofactor with HCV |
| Aflatoxin B | Cofactor with HBV that increases risk of developing HCC | |
| Relative risk varies from two- to fourfold in nonendemic regions | ||
| Androgenic steroids | Some association reported, primarily case reports and small series | |
| Oral contraceptives | ||
| Autoimmune hepatitis | ||
| Nonalcoholic steatohepatitis (NASH) | Increasing evidence for association with HCC with or without cirrhosis; incidence of NAFLD is rising in the United States | |
| Tobacco | Weak association suggested that it is independent of HBV infection, alcohol |
Chronic hepatitis B or C viral infection is the most important risk factor for developing HCC. Alone, HCV causes about 40% of HCCs in the United States. Chronic HBV or HCV carriers usually take 10 to 20 years to develop hepatic cirrhosis and 30 to 40 years to develop HCC. Hepatitis B virus is a DNA virus that commonly integrates into the host hepatocyte genome and may play a direct procarcinogenic role. Hepatitis C virus is an RNA virus with no insertional mutagenesis. Although HBV and HCV contain no known viral oncogene to immortalize hepatocytes, hepatitis Bx antigen may inactivate p53 protein and downregulate DNA repair ability (19,20). Some of the principal differences between HBV- and HCV-associated HCC are listed in Table 22-4.
| Factor | HBV | HCV |
|---|---|---|
| Mean age | 52-56 (20-80) | 62 |
| Highest incidence | 400 million carriers in Asia and Africa | 170 million infected worldwide; accounts for 50% of HCC cases in Japan, the United States, and Western Europe |
| Cirrhosis | 25%-50% | >75% |
| Morphology | Solitary lesions | Multifocal lesions, more severe inflammation |
| Rate of progression to HCC | 10-30 years | >30 years |
| Percentage likely to develop HCC | 4% per year | 1%-7% per year |
Excessive alcohol consumption can lead to hepatic cirrhosis and thus is a risk factor for HCC. The autopsies of patients with alcoholic cirrhosis have reported up to 10% undiagnosed HCC. In the United States, alcoholic cirrhosis is associated with about 15% of HCC and cholangiocarcinoma (21,22). In HCV carriers, alcohol increases circulating HCV viral titer and HCC risk. Other types of cirrhosis and parenchymal liver diseases—such as primary biliary cirrhosis, hemochromatosis, Wilson disease, alpha-1 antitrypsin deficiency, and glycogen storage disease—significantly increase HCC risks when alcohol is a cofactor.
Food contaminated with aflatoxin, a mycotoxin found in grains, can induce HCC in animals. There is also a strong association between aflatoxin exposure and HBV carrier status. Relative risks of HCC are 3-fold for aflatoxin, 9-fold for chronic HBV infection, and 59-fold for concurrent aflatoxin and chronic HBV infection. The underlying mechanism is polymorphism variants of glutathione-S-transferase M1 and epoxide hydrolase genes and G-to-T point mutation of the p53 gene (20).
The use of oral contraceptive pills (OCPs) significantly increases the incidence of benign hepatic adenomas. There is some evidence that OCPs also increase HCC risk. Multiple studies of tobacco smoking and HCC risk have yielded mixed conclusions. Occupational exposure to arsenic or vinyl chloride significantly increases the risk of liver angiosarcoma. Exposure to the x-ray contrast medium thorium dioxide from 1930 to 1955 is associated with an extremely high risk of hemangiosarcoma, cholangiocarcinoma, and HCC.
CLINICAL PRESENTATION
Most cases of HCC are identified incidentally or through screening programs of high-risk individuals. It is common for patients to be asymptomatic until their disease is very far advanced; fewer than 30% of patients are candidates for surgery or other liver-directed therapy at presentation. Many patients present with symptoms of advanced liver dysfunction from both cirrhosis and HCC. The most common initial symptom is right upper quadrant abdominal pain. Anorexia or early satiety with weight loss is the second most common symptom. Also, HCC may present with various paraneoplastic symptoms through the secretion of numerous hormones. Late-stage symptoms include jaundice, tumor fever, bone pain due to metastatic lesions, and complications from portal venous hypertension, such as esophagogastric varices, hypoalbuminemia, ascites, thrombocytopenia, and coagulopathy.
On physical examination, hepatomegaly is present in over 90% of patients. A hepatic arterial bruit or a friction rub, ascites, splenomegaly, and jaundice are found in up to 50% of patients. Muscle wasting, fever, and dilated abdominal veins are also common. The Budd-Chiari syndrome is caused by malignant invasion and occlusion of the hepatic veins. The HCC marker alpha-fetoprotein (AFP) is often elevated to above 400 ng/mL.
PATHOLOGY
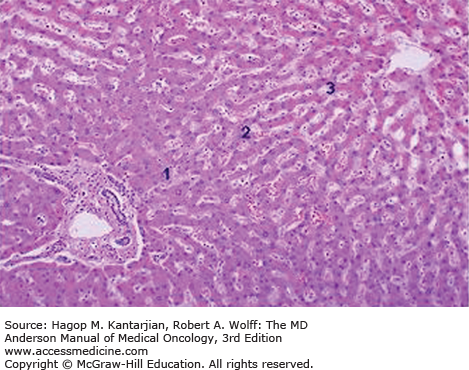
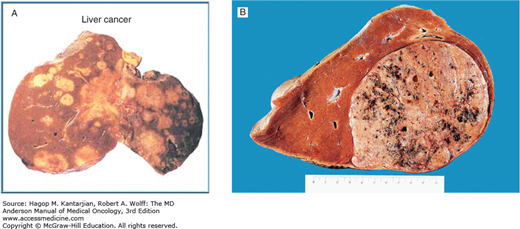
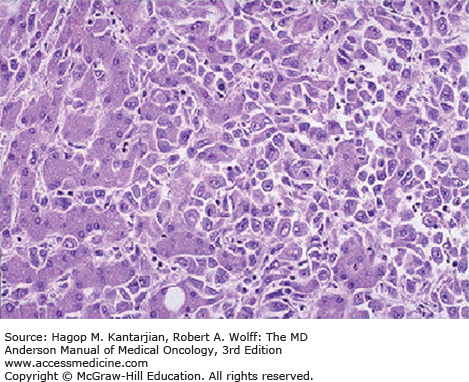
Based on the growth pattern, HCC may be classified into four major gross anatomic types: spreading, multifocal, encapsulated, and combined patterns (23). Normal liver parenchyma is shown in Fig. 22-1. The spreading type of HCC grows in nodular, pseudolobular, or invasive patterns with poorly defined margins, occurs in the setting of hepatic cirrhosis, and accounts for nearly 50% of cases in the United States. The multifocal type has numerous tumors of similar size that make it difficult to determine whether the lesions are intrahepatic metastases or second primary tumors (Figs. 22-2A and 22-2B). The encapsulated type of tumor grows by expanding, compressing, and distorting the surrounding liver tissue. Satellite or metastatic lesions are seen in late-stage disease. This type is most common in Asia and Africa but seen in only 13% of cases in the United States. The combined patterns of the three are seen in up to 25% of cases. Figure 22-3 shows an HCC histopathology specimen.
FIGURE 22-2
A. Gross pathologic specimens of multifocal hepatocellular carcinoma. B. Gross appearance of liver cell carcinoma. Courtesy of Dr RA Cooke, Brisbane, Australia. Reproduced with permission from Cooke RA, Stewart B: Colour Atlas of Anatomical Pathology, 3rd ed. Edinburgh, Churchill Livingstone, 2004.
Approximately 60% to 70% of Caucasian and 80% to 90% of Asian HCC cases show elevated AFP, which is the most useful marker for HCC. Originally, AFP is produced by the fetal liver and yolk sac but falls to below 10 ng/mL in adult serum. A transient elevation of AFP to 20 to 400 ng/mL may occur when there is hepatocyte regeneration, as in cirrhosis, active hepatitis, or partial hepatectomy. The HCC positive predictive value of an AFP level of 400 ng/mL is over 95%, and normal AFP levels may exist in patients with low tumor burden. The lectin-reactive isoenzyme of AFP (AFP-L3) has shown increased sensitivity. Other markers, such as gamma-glutamyl transferase isoenzymes, alkaline phosphatase, isoferritins, and monoclonal antibodies are not more useful than AFP (24). Currently, serum AFP level and ultrasonography are the “gold standard” for HCC screening in high-risk populations (24).
There are five HCC variants: HCC with biliary differentiation, clear cell HCC, cholangiocellular carcinoma (CCC), FLHCC, and focal nodular hyperplasia (FNH). Cholangiocellular carcinoma is a combination of cholangiocarcinoma and HCC. It occurs in the noncirrhotic liver and behaves like a cholangiocarcinoma; it has a predominance for men. The outcome of patients with CCC is uniformly fatal.
Fibrolamellar HCC is predominantly seen in the right hepatic lobe, accounts for 2% to 4% of HCC, and occurs equally in men and women. It typically occurs in adolescents and young adults; the etiology is unknown. It is characterized by fibrosis arranged in lamellar fashion around HCC cells. Fibrolamellar HCC consists of well-circumscribed, large, solitary lesions without hepatic cirrhosis or elevated AFP (25). The imaging studies often show a heterogeneous mass with a central scar that is similar to FNH. In comparison to classic HCC, FLHCC demonstrates a higher resection rate and better survival, with a 3-year survival of almost 100%.
Focal nodular hyperplasia occurs predominantly in young women. Liver function studies and the serum AFP level are normal. A technetium sulfur colloid radioisotope scan of the liver shows increased radioisotope uptake in FNH compared with hepatic adenomas or carcinomas. The prognosis is excellent.
Clear cell HCC has a distinguishing appearance and better prognosis. Hepatocellular carcinoma with biliary differentiation has a much poorer prognosis because of its rapid growth, decreased vascularity, and resistance to embolic therapy.
Rare primary liver neoplasms include hepatoblastoma, sarcoma, angiosarcoma, rhabdomyosarcoma, and epithelioid hemangioendothelioma. Patients may present with fatigue, anorexia, weight loss, and abdominal pain. Hemorrhagic ascites is common, and AFP level is normal. Angiography and contrast-enhanced computed tomography (CT) of the liver are the best diagnostic tools. Open or percutaneous liver biopsy is needed for diagnosis. Surgical resection is still the principal means of therapy if tumors are diagnosed at relatively early stages. They are often resistant to chemotherapy and radiotherapy (RT), and the overall prognosis is poor.
Hemangiomas are the most common benign tumors of the liver. Their size ranges from a few millimeters to 25 cm. They appear as calcified solitary lesions in up to 7% of the general population. Magnetic resonance imaging (MRI) is much better than CT in distinguishing HCC from hemangioma on heavily T2-weighted images. Surgical resection is used for symptomatic lesions or when malignancy cannot be excluded. Hepatic artery ligation is an alternative for large cavernous hemangiomas. Hepatic adenoma is another common benign solitary tumor seen in women who have used OCPs for more than 10 years. It is composed of sinusoids, central veins, and arteries without well-defined portal tracts or bile ducts. Hepatic angiography is the most valuable diagnostic tool. Small adenomas usually regress when OCPs are discontinued. Symptomatic lesions are treated with resection.
DIAGNOSTIC EVALUATION, STAGING, AND PROGNOSIS
In addition to performing a complete medical history and physical examination, the diagnostic workup for a patient suspected of having HCC should include serum for complete blood cell count, electrolytes, liver function tests (LFTs), albumin, prothrombin time, hepatitis B and C serologies, and tumor markers (AFP, CA19-9, CA125). The medical history should include a thorough review of potential HCC risk factors: transfusions, tattoos, intravenous drug abuse, high-risk sexual practices, familial syndromes, OCP or hormone replacement use, androgenic steroid use, and chemical exposures.
Several radiographic imaging modalities are useful in evaluating a patient with HCC. Ultrasound often serves as the initial screening modality, followed by triple-phase CT scan or MRI. Randomized studies have shown that hepatic ultrasonography has 78% sensitivity and 93% specificity to detect HCC in high-risk populations, especially for patients with normal AFP levels (26,27). Color-flow Doppler can assist preoperative assessment and planning.
Abdominal CT has relatively higher sensitivity and specificity than ultrasonography. With special arterial- and venous-phase scans, CT also makes it possible to evaluate the blood supply of the normal liver parenchyma (portal vein) and neoplastic lesions (hepatic artery). Magnetic resonance imaging is useful in distinguishing benign lesions from malignant tumors by the combination of T2-weighted phase contrast and spin echo sequences. Also, MRI can demonstrate fatty degeneration of tumor and vascular invasion (28).
Hepatic radionuclide imaging has low spatial resolution and is only about 70% sensitive in demonstrating neoplasms. Using the glucose metabolic difference between neoplastic and normal cells, positron emission tomography (PET) differentiates benign lesions from malignant tumors, detects extrahepatic metastasis, and evaluates response to therapy.
In summary, ultrasonography is the most cost-effective HCC screening test in high-risk populations. Abdominal CT with liver protocol is the most helpful in accurately staging patients prior to surgery. No single diagnostic modality has greater than 50% to 60% sensitivity in detecting lesions less than 1 cm in size. The combination of AFP level, ultrasonography, and CT provides the best hope of early diagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree