(1)
State University of New York at Buffalo Kaleida Health, Buffalo, NY, USA
Metastatic involvement of the liver occurs usually from adenocarcinomas in parts of the gastrointestinal tract such as the pancreas, stomach, or large bowel. Adenocarcinoma of the head of the pancreas metastatic to the liver is usually inoperable because of the multiplicity of sites involved and because the disease is aggressive; even when a liver resection for limited disease is technically feasible, it is very unlikely to significantly prolong life because of the presence of undetected microscopic disease in other sites. The same is true for adenocarcinoma of the stomach, although occasionally low-grade carcinomas of the stomach will exhibit a long disease-free interval between the resection of the primary tumor and the appearance of resectable hepatic metastases, which may be resected with an expectation of reasonable palliation. For synchronous involvement of the liver with adenocarcinoma of the colon, it is a matter of surgical judgment as to whether the patient can tolerate resection of the primary tumor and simultaneous liver resection. The presence of extrahepatic disease is a relative contraindication to resection of colorectal liver metastases, whereas the inability to achieve complete resection is an absolute contraindication. The number of liver metastases is no longer considered a factor affecting the advisability of their resection. With curative resection (negative margins), 5-year survival rates of 40–58 % have been reported. The resectability rate can be increased by preserving or increasing hepatic reserve (e.g., two-stage hepatectomy, portal vein embolization), by combining resection with ablation, and by decreasing the tumor size with preoperative chemotherapy [1]. Hepatic resection for primary tumor of the liver or metastatic disease has been applied more extensively than in the past since the demonstration of the subdivision of the liver into lobes and segments according to the distribution of the hepatic duct, portal vein, and hepatic artery branches in the hepatic parenchyma.
Right Hemihepatectomy
The incision for liver resection may be a midline incision from the xiphoid to 3–4 cm below the umbilicus, going through the linea alba and peritoneum. For thin patients, this is an adequate incision and provides good exposure. Otherwise, a right subcostal incision may be used, which may extend to the left side partly with an extension in the midline from the apex of the joined subcostal incisions, to the xiphoid (Mercedes incision). The round ligament is ligated and divided, and the falciform ligament is also divided at the beginning of the operation. For large tumor masses in the liver, possibly involving the diaphragm, a thoracic extension of the abdominal incision may be performed in order to provide better access to the inferior vena cava posteriorly, as well as better control of the hepatic veins draining to the inferior vena cava. When this extension is needed, one can decide which intercostal space would best be divided by looking at the exposed tumor mass of the liver, which could serve as a guideline to the space that would provide the best exposure. The diaphragm is opened in a direction towards the hepatic veins.
After the decision to pursue a hemihepatectomy has been made, the structures in the porta hepatis are first explored. The imaginary line separating the right lobe from the left lobe of the liver, which extends from the gallbladder bed to the confluence of the hepatic veins at the inferior vena cava, is visualized and marked with the cautery on Glisson’s capsule. One makes certain that the disease is confined to the true right lobe of the liver. The hepatogastric ligament is incised to allow entry in the lesser sac and the medial opening of the foramen of Winslow. The hepatoduodenal ligament is incised anteriorly by incising the peritoneum covering the structures of the porta hepatis, and the common bile duct is exposed (Fig. 27.1). To further expose the porta hepatis, the cystic duct is ligated and divided close to its junction with the choledochus and the cystic artery close to the gallbladder, which is more safely removed by dissecting it first from its fossa in the liver (Fig. 27.2). The gallbladder is removed. The choledochus is followed into the common hepatic duct, and the confluence of the right and left hepatic ducts is exposed. On the left side of the hepatoduodenal ligament, the hepatic artery is readily palpable by using the index finger and the thumb, with the left index finger in the foramen of Winslow. This foramen is anteriorly defined by the peritoneum covering the posterior aspect of the structures in the porta hepatis and posteriorly defined by the peritoneum covering the inferior vena cava.
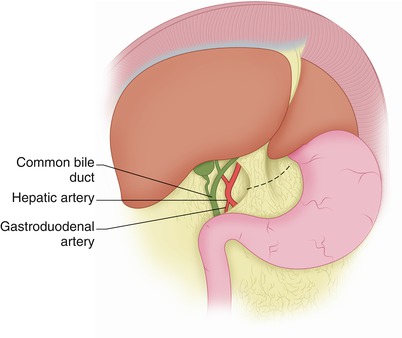
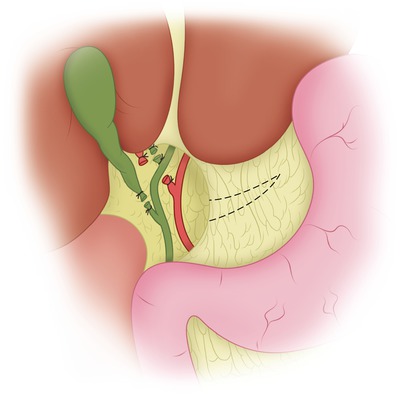

Fig. 27.1
The hepatoduodenal ligament is incised anteriorly, exposing the common bile duct and hepatic artery. The gastrohepatic ligament is incised on its right side sufficiently to permit visualization of the medial opening of the foramen of Winslow

Fig. 27.2
The cystic duct and cystic artery have been ligated and divided. The gallbladder is usually removed at this point, and the right hepatic duct and right hepatic artery are exposed, ligated, and divided
It is important to expose circumferentially the hepatoduodenal ligament so that if bleeding occurs from the transected liver parenchyma, one can temporarily apply a vascular clamp on the hepatoduodenal ligament to decrease the bleeding so that the bleeding points can be more quickly and safely ligated and divided. An alternate method for the so-called Pringle maneuver is the passing of an umbilical tape around the hepatoduodenal ligament, the two ends of the tape passing through a short piece of 18 Fr rubber catheter, and a clamp applied to them, thus making a tourniquet that can be tightened in the event of bleeding by pulling the two ends of the tape through the catheter and applying the clamp on the tapes at their exit point through the catheter. The tourniquet can be applied for up to 15 min at a time, alternating with at least 10 min of release. For a more effective application of the tourniquet, the peritoneum covering the structures of the porta hepatis is incised, at least anteriorly.
After ligation and division of the right gastric artery, the common hepatic duct and the confluence of the right and left hepatic ducts are identified (Fig. 27.2). In 64 % of patients, the right hepatic artery crosses behind the common hepatic duct, occupying a position to the right of the common hepatic and right hepatic bile ducts, but in 24 % it crosses in front of the common hepatic duct, and in 12 % it runs parallel and to the right of the choledochus–common hepatic duct arising from the superior mesenteric artery. The bifurcations of the hepatic artery and common hepatic duct are clearly exposed, and the right hepatic duct and right hepatic artery are ligated and divided (in the sequence favored by the local anatomy), to expose the right branch of the portal vein. With careful dissection, a right ankle clamp is passed around the right branch of the portal vein, followed by a vessel loop. By holding the vessel loop up, further attachments of the peritoneum over the portal vein and its continuation into the right portal branch are exposed and divided. The right portal branch has a short and wide trunk. Two vascular clamps are placed and clamped tightly. Before dividing the right portal branch of the portal vein, two vascular 4-0 or 5-0 Prolene double-armed sutures are placed on each side of each stump that is about to be formed. The right portal branch is divided between vascular clamps. The vascular suture is placed under the vascular clamp in a sewing-machine fashion. Following completion of this suture from one side of the stump to the other, one proceeds with the other end of the vascular suture to apply it at the stump over and over, the vascular clamp is removed, and the Prolene sutures are tied. This is done for both the distal stump and the proximal stump. The right portal branch is short, so the vascular sutures in each side of the right portal branch are placed on the outer side of each vascular clamp so that when the right portal branch is divided (with the help of a #15 blade), there is room to suture beneath each clamp (sewing machine) and starting over and over, with the other vascular stitch loosely applied; this stitch is tightened as the clamp is removed and is tied to the other stitch at the edge of the stump. Thus, there is no risk of losing the stump by retraction from the jaws of the vascular clamp, because even if this should occur, the vascular sutures should help to bring it to vision and allow suturing.
If the proximal stump slips out of the vascular clamp and retracts behind the hepatoduodenal ligament before the placement of vascular sutures at the edges of the stump, there is not enough space to place a vascular clamp on the hepatoduodenal ligament close to the duodenum in order to occlude the slipped proximal stump. In this case, the index finger is placed behind the hepatoduodenal ligament at the appropriate level to apply mild pressure and stop the bleeding. While the pressure on the proximal segment of the stump is maintained, the finger is retracted slightly to the right edge of the hepatoduodenal ligament and the stump is sutured with a running Prolene suture at the edge of the hepatoduodenal ligament, even though some of these sutures may go through the common bile duct. At this point, it is important to stop the bleeding effectively, and after the hepatectomy has been completed, the common bile duct can be opened and a Bakes dilator passed up into the left hepatic duct to ensure that the suture previously placed does not compromise the lumen of the common hepatic duct or the choledochus. A T-tube then can be placed in the common bile duct. This type of intraoperative complication has happened only once in our experience, apparently either because a vascular clamp was defective or because its application was not sufficiently tight to hold the proximal stump of the right branch of the portal vein in place. There are now vascular stapling devices, which facilitate safe stapling and division of large venous trunks.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree