INTRODUCTION
SUMMARY
Over the past 60 years, the field of hematopoietic cell transplantation (HCT) has evolved from experimental animal models of marrow transplantation to curative therapy for tens of thousands of people yearly who are affected by a wide variety of marrow failure states, myeloid and lymphoid malignancies, immune deficiencies, and inborn errors of metabolism. Advances in transplantation immune biology combined with improvements in supportive care have made this evolution possible and have ushered in the modern era of HCT. This chapter discusses the biologic principles and clinical applications of HCT along with its future applications. Selected results demonstrating important principles are highlighted.
Acronyms and Abbreviations:
ALL, acute lymphoblastic leukemia; ALK+, anaplastic lymphoma kinase–positive; AML, acute myeloid leukemia; APC, antigen-presenting cells; ASBMT, American Society for Blood & Marrow Transplantation; ATG, antithymocyte globulin; BCNU, 1,3-bis(2-choloroethyl)-1-nitrosurea; BEAM, BCNU, etoposide, cytarabine, and melphalan; BMT-CTN, Blood & Marrow Transplant Clinical Trials Network; BU, busulfan; CIBMTR, Center for International Blood and Marrow Transplant Research; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; CMV, cytomegalovirus; CR, complete remission; CR1, first complete remission; CT, computed tomography; CXCL12, extracellular-matrix-bound stromal cell–derived factor-1; CXCR4, chemokine-related receptor 4; CY, cyclophosphamide; DAH, diffuse alveolar hemorrhage; DLI, donor lymphocyte infusion; EBMT, European Society for Blood and Marrow Transplantation; ECP, extracorporeal photopheresis; FDG, 18-fluorodeoxyglucose; FLU, fludarabine; G-CSF, granulocyte colony-stimulating factor; GI, gastrointestinal; GM-CSF, granulocyte-monocyte colony-stimulating factor; GVHD, graft-versus-host disease; GVT, graft-versus-tumor; HCT, hematopoietic cell transplantation; HCT-CI, HCT-specific Comorbidity Index; HL, Hodgkin lymphoma; HLA, human leukocyte antigen; HSC, hematopoietic stem cell; HSV, herpes simplex virus; IFN, interferon; Ig, immunoglobulin; IL, interleukin; IPS, idiopathic pneumonia syndrome; KTLS, c-kit+, Thy-1.1lo, lineage marker–/lo, and Sca-1+; MDS, myelodysplastic syndrome; MHC, major histocompatibility complex; MMF, mycophenolate mofetil; MSC, mesenchymal stromal cell; MTX, methotrexate; NHL, non-Hodgkin lymphoma; NIH, National Institutes of Health; NK, natural killer; NMDP, National Marrow Donor Program; PAM, Pretransplant Assessment of Mortality; PBPC, peripheral blood progenitor cell; PCA, patient-controlled anesthesia; PCR, polymerase chain reaction; PET, positron emission tomography; Ph, Philadelphia chromosome; PSGL-1, P-selectin glycoprotein ligand-1; PTLD, posttransplantation lymphoproliferative disorder; RIC, reduced-intensity conditioning; SCID, severe combined immunodeficiency; SOS, sinusoidal obstruction syndrome; SRL, sirolimus; TAC, tacrolimus; TBI, total-body irradiation; Th, T-cell helper; TKI, tyrosine kinase inhibitor; TLI, total lymphoid irradiation; TNF, tumor necrosis factor; TPN, total parenteral nutrition; Treg, regulatory T cell; TRM, transplant-related mortality; UCB, umbilical cord blood; VCAM, vascular cell adhesion molecule; VOD, venoocclusive disease; VZV, varicella-zoster virus.
HISTORY
The successful clinical application of hematopoietic cell transplantation (HCT) required a century of key developmental discoveries (Table 23–1). Between 1868 and 1906, European and American investigators established that marrow cells were the source of blood cell production. In 1939, the first documented human marrow transplant was performed in a patient with gold-induced marrow aplasia.1 The patient was infused intravenously with marrow from a brother with an identical ABO blood type. The transplant was not successful, and the patient died 5 days after the marrow infusion.
| Years | Event |
|---|---|
| 1868–1906 | Discovery that marrow was the source of the various blood cell types |
| 1896–1900 | Discovery of ABO system making blood transfusions possible |
| 1939 | First documented clinical marrow transplant |
| 1949–1954 | Development of preclinical models of marrow and organ transplantation |
| 1956–1959 | Early efforts of marrow grafting to treat human diseases |
| 1960–1965 | Development of the hierarchical stem/progenitor cell model of hematopoiesis |
| 1960s | Period of pessimism for the clinical application of marrow grafting for the treatment of human diseases |
| 1968–1969 | First successful allogeneic HCT in patients with SCID |
| 1975 | First successful series of allogeneic HCT for leukemia |
| 1978 | First successful series of autologous HCT for leukemia |
| 1988 | Isolation of the murine HSC |
| 1990 | Nobel Prize in Physiology or Medicine awarded to Dr. E.D. Thomas |
| 2008 | More than 700,000 patients worldwide transplanted, more than 125,000 have survived 5 years or beyond after transplantation |
In 1922, a Danish investigator modified radiation injury in guinea pigs by shielding their femora against radiation, preventing the typical radiation-induced thrombocytopenia and hemorrhage.2 This work went essentially unnoticed for more than 2 decades. The period of 1949 to 1954 was marked by a political climate concerned with the threat of continued atomic warfare, which stimulated support for experiments studying the effects of irradiation and led to the development of the field of organ and marrow transplantation. Jacobson and colleagues found that mice could survive an otherwise lethal irradiation exposure if the spleen (a hematopoietic organ in the mouse) was protected by lead foil.3 Soon afterward, Lorenz and colleagues showed that lethally irradiated mice and guinea pigs were protected by the administration of syngeneic marrow after irradiation, thereby demonstrating the therapeutic efficacy of allogeneic and xenogeneic marrow suspensions.4 These investigators and others considered that chemicals and/or components from the shielded spleen or infused marrow stimulated endogenous hematopoietic cell recovery after total-body irradiation (TBI).5,6,7 In 1954, Barnes and Loutit showed that if mice were immunized against marrow cells from mice of another strain and then lethally irradiated, no protection was observed by the injection of marrow cells from the strain to which they were immunized. However, if nonimmunized mice were lethally irradiated and injected with the same marrow cells, normal protection was seen and all mice survived more than 60 days. This experiment supported the cellular hypothesis of hematopoiesis and was the first to consider that hematopoietic recovery resulted from cellular repopulation and not from humoral factors.8
In 1956, Barnes and associates described the treatment of murine leukemia by supralethal irradiation and marrow grafting.9 Researchers pointed out that irradiation alone would not kill all leukemia cells, but that residual leukemia cells might be eliminated by transplanted cells through immunologic mechanisms, and the term adoptive immune therapy was coined. Their publication stimulated tremendous interest, and the period from 1956 to 1959 was characterized by an increasing appreciation of the potential application of marrow grafting to treat individuals exposed to lethal irradiation and to treat human leukemia. Thomas and colleagues began clinical studies in patients with terminal leukemias, and in 1957 described six patients treated with irradiation and intravenous infusion of marrow from healthy donors.10 Only two patients developed transient detectable donor hematopoietic engraftment, and none of the six survived beyond 100 days from the cell infusion. In 1959, Thomas and associates described a patient with terminal leukemia who received total body irradiation (TBI) and an intravenous infusion of marrow from an identical twin.11 The patient showed prompt hematopoietic recovery and disappearance of the leukemia for 4 months, confirming for the first time that lethal irradiation followed by compatible marrow could have an antileukemic effect and restore normal marrow function. In the same year, Mathé and associates reported the infusion of marrow into six patients exposed to potentially lethal irradiation in a reactor accident in Belgrade, Serbia.12 Five patients survived, yet there was no clear evidence of donor hematopoietic engraftment and thus no firm agreement over the contribution of marrow transplantation to patient recovery.
The first attempts at autologous marrow transplantation appeared during this time as well. In 1958, Kurnick and colleagues described two patients with metastatic cancer whose marrow was collected and stored by freezing.13 Following intensive radiation therapy, the marrow was thawed and infused intravenously. One patient died from transplantation complications, while the other showed hematopoietic recovery after a prolonged period of pancytopenia. In Philadelphia, an autologous marrow transplant was carried out after high-dose nitrogen mustard conditioning in a patient with malignant lymphoma who lived for more than 30 years after transplantation, the majority of that time in complete remission.14
Despite the many successful preclinical models of marrow transplantation and the predictive value of in vitro histocompatibility testing, the period of 1960 to 1967 was marked by increasing pessimism about allogeneic marrow grafting in humans. In a published compendium of 203 human allogeneic marrow grafts carried out throughout the 1950s and 1960s, none were considered successful.15 The first positive results came from studies of children with severe combined immunodeficiency (SCID). In 1968, Gatti and colleagues performed the first successful allogeneic marrow HCT in a child with SCID.16 The lymphoid elements of the donor graft corrected the immunodeficiency, and two similar cases were reported shortly thereafter.17,18 These patients remained alive and well 25 years later.19
These successes stimulated a resurgence of enthusiasm for marrow transplantation, and by 1975 strikingly improved results were published by the Seattle team.20 These investigators reported the outcomes of 37 patients with aplastic anemia and 73 patients with leukemia who had reached an advanced stage of their disease before transplantation. This study stressed the importance of histocompatibility and proper preparation of the patient before transplantation, detailed the technique of marrow transplantation, emphasized the role of posttransplant immunosuppression and supportive care, and raised the possibility of using unrelated donors. This report ushered in the modern era of allogeneic HCT. In 1977 and 1980, the first successful HCT procedures from unrelated marrow donors were reported.21,22 At the end of 1978, the first series of successful autologous HCT for lymphoma were reported.23,24 In 1990, the Nobel Prize in Physiology or Medicine was awarded to E. Donnall Thomas in recognition of his pioneering work in the field of marrow transplantation. By 2013, more than 700,000 patients worldwide had undergone transplantation during the previous 3 decades and more than 19,000 transplants were being performed annually.25
STEM CELL MODEL OF HEMATOPOIESIS
At the single-cell level, stem cells self-renew and give rise to progeny that differentiate into functional cells carrying out specific functions (Chap. 18).26 Progenitor cells can be multipotent, oligopotent, or unipotent, but lack self-renewal capabilities. Hematopoietic stem cells (HSCs) are cells that give rise to more HSCs and form all elements of the blood. HSCs are entirely responsible for the development, maintenance, and regeneration of blood-forming tissues for life, and are the most important, if not the only, cells required for successful engraftment in hematopoietic transplantations.26 In the adult mouse marrow, all HSC activity is contained in a population marked by the composite phenotype of c-kit+, Thy-1.1lo, lineage marker–/lo, and Sca-1+ (designated KTLS).27,28 When transplanted at the single-cell level into irradiated mice, KTLS HSCs gave rise to lifelong hematopoiesis, including a steady state of thousands of HSCs with more than 109 blood cells produced daily in the mouse.27,28,29 In humans, the combination of positive selection for CD34, Thy-1, and negative selection for lineage markers identified a homologous HSC population.30
Following the success in rodent models, purified populations of human HSCs were tested in three separate clinical trials of patients with myeloma, non-Hodgkin lymphoma (NHL), and metastatic breast cancer.31,32,33 The goal of these trials was to purify HSC and thereby reduce the risk of occult malignant cells contaminating the autografts. These trials presented technical challenges primarily because of the rarity of HSC in marrow and granulocyte colony-stimulating factor (G-CSF)–mobilized blood. However, adequate numbers of HSC could be isolated that were tumor-free in the majority of patients. The times to neutrophil and platelet recovery following purified HSC infusion were comparable to those seen with unmanipulated marrow, but T-cell recovery (especially that of CD4+ T cells) was delayed by up to 6 months in almost all patients. A number of patients developed unusual infections (e.g., severe cases of influenza, respiratory syncytial virus, cytomegalovirus [CMV] and Pneumocystis pneumonia), thus raising concern over “pure” HSCs as the sole source of hematopoietic reconstitution in clinical transplantation. Although these studies were not powered to detect an impact of purified HSCs on relapse or overall survival, these outcomes appeared favorable compared to historical controls.34 Cotransplantation of antigen-specific mature T cells is being investigated as an approach to address the T-cell–specific immunodeficiency seen in patients receiving purified HSC autografts.35
TRAFFICKING AND HOMING OF HEMATOPOIETIC STEM CELLS
The ability of HSCs to migrate from marrow to blood and back has been conserved throughout evolution. Although the biologic role and physiologic significance of constitutive HSC circulation remains unclear, this capacity to traffic leads to hematopoietic cell reconstitution and is essential for the success of HCT in the treatment of hematologic and nonhematologic diseases.
The restoration of adequate hematopoiesis after transplantation requires a series of balanced interactions between the infused HSCs and the complex supporting marrow microenvironment (Chap. 5). Initially, infused HSCs must adhere to the marrow endothelium with sufficient strength to overcome the shear forces of blood flow.36 Adhesion and arrest of HSCs are mediated primarily by the selectin ligand P-selectin glycoprotein ligand-1 (PSGL-1) and by the hematopoietic cell L- and E-selectin ligands, which interact principally with endothelial E-selectin.37,38 Other HSC surface adhesion molecules that mediate adherence to the marrow endothelium include members of the integrin superfamily, principally very late antigen-4, integrin α4β7 and lymphocyte function antigen-1, that interact with endothelial immunoglobulin (Ig) superfamily receptors (e.g., vascular cell adhesion molecule [VCAM]-1), and the hyaluronate receptor CD44.39,40 HSCs that are null for the β integrins cannot migrate to their marrow niche even though they proliferate and differentiate in the fetal liver.41 Following firm adherence, the transendothelial movement and intraparenchymal homing to hematopoietic niches within the inner endosteal surface of the bone are predominantly regulated by a gradient of extracellular-matrix-bound stromal cell–derived factor-1 (also known as CXCL12).12 Mice deficient in CXCR4 develop fetal liver hematopoiesis but die prenatally as a consequence of the lack of marrow hematopoiesis.42 The requirement for CXCR4 expression on HSCs for homing and engraftment is well-documented,43 and has led to the development of CXCR4 antagonists such as plerixafor, which help mobilize marrow stem cells for clinical use.44
Following successful homing, the initial adhesion of HSC within the hematopoietic niche appears to be regulated at least in part by annexin II.45 The marrow niche is a complex biologic unit that includes potentially self-renewing mesenchymal stromal cells (MSCs), regulatory T cells (Treg), and cells with the defined phenotype of parathyroid-hormone-receptor-bearing osteoblasts.46,47 MSCs promote engraftment when cotransplanted with HSCs.48 Osteoblasts, possibly in conjunction with sinusoidal endothelial cells, also appear to play a pivotal role in the regulation of HSC engraftment by producing a number of molecules, such as annexin II, VCAM-1, intercellular adhesion molecule-1, CD44, CD164, and osteopontin, which promote engraftment.49,50 Stimulation of osteoblasts with parathyroid hormone results in expansion and mobilization of the HSC pool in animals,51 although a clinical trial in human cord-blood recipients did not demonstrate a benefit.52 In addition to the regulators of HSC adhesion and homing, the function of HSCs is further regulated by intrinsic genetic programs for quiescence, self-renewal, proliferation, differentiation, and apoptosis that are dependent on communication with a network of interacting cells in the marrow microenvironment, including various T-cell subpopulations, adipocytes, and fibroblasts. Given the complexity of HSC trafficking and control, it is surprising that clinical HCT has a relatively low rate of graft failure.
SOURCES OF HEMATOPOIETIC CELLS
In humans, HSCs for transplantation can be collected from several sources, including directly from the marrow; from the blood after mobilization; and from umbilical cord blood (UCB) obtained at the time of delivery.
Marrow has historically been the traditional source of HSCs for allogeneic and autologous transplantation. Marrow for transplantation is typically aspirated by repeated placement of large-bore needles into both sides of the posterior iliac crest, generally involving 50 to 100 aspirations per side, with the patient under regional or general anesthesia. The lowest cell dose which ensures stable long-term engraftment has not been defined with certainty; typical collections contain at least 2 × 108 total nucleated marrow cells per kg of recipient body weight. Current guidelines indicate that collection of up to 20 mL/kg of donor body weight is considered safe.
Marrow harvesting is considered a very safe procedure, and serious side effects are rare. A review of almost 10,000 healthy adult volunteer unrelated donors by the National Marrow Donor Program (NMDP) found that the risk of serious adverse events was 2.38 percent, most of which were mechanical or anesthesia-related and self-limited. Unexpected, life-threatening, or chronic complications occurred in 0.99 percent of donors.53 Evaluation of pediatric marrow donor safety is more limited, but a safety review of 453 pediatric donors by the European Group for Blood & Marrow Transplantation (EBMT) found no serious adverse events; pain was the most common complaint but lasted a median of only 1 day after donation.54 A survey of pediatric transplantation hematologists confirmed that 90 percent of centers were willing to perform a marrow harvest on children, even on those younger than 6 months old.55
HSCs are normally present in the blood at very low levels. However, a number of different stimuli, including chemotherapy, hematopoietic growth factors, and inhibitors of certain chemokine receptors, result in the mobilization of HSC from marrow to blood. Once mobilized, HSC can be collected by apheresis; this product has been termed “peripheral blood progenitor cells (PBPCs)” to differentiate it from “blood stem cells,” a term that should be reserved for instances where the HSC population itself has been isolated. Agents used to mobilize HSCs include G-CSF, granulocyte-monocyte colony-stimulating factor (GM-CSF), interleukin (IL)-3, thrombopoietin, and the CXCR4 antagonist plerixafor.44,56,57
Autologous PBPCs are most commonly mobilized with G-CSF, with or without additional chemotherapy. In contrast, PBPCs for allogeneic HCT are typically mobilized with G-CSF alone, so as to avoid exposing healthy donors to chemotherapy. PBPC mobilization and collection is very safe; a review of nearly 7000 healthy unrelated PBPC donors performed by the NMDP found that the rate of serious adverse events was 0.56 percent, making PBPC donation significantly safer than marrow donation.53 The most common side effect of PBPC mobilization and collection is bone pain as a result of G-CSF administration. More serious side effects, such as splenic rupture or intracranial hemorrhage, have been described in case reports but are extremely rare.58,59
Theoretical concern exists about the potential of short-term growth factor therapy to increase the risk of leukemia in normal donors. However, long-term followup of healthy adult PBPC donors has shown no late effects of G-CSF administration or donation—in particular, no increased risk of cancer, autoimmune disease, or stroke.53 Detailed white cell subset analysis by fluorescent-activated cell sorting of healthy donors at 1 year after donation shows no changes in B, T, and natural killer (NK) cells or monocytes and neutrophils compared with analysis before G-CSF administration.60
The adequacy of PBPC products is generally measured through the absolute number of CD34+ cells per kg of recipient body weight. Most laboratories measure CD34+ cell content by fluorescent-activated cell sorting. A threshold of greater than 2 × 106 CD34+ cells/kg is often considered the minimum acceptable dose for PBPC products, although successful engraftment can occur with lower doses.61 Platelet recovery appears most impacted by low PBPC CD34+ cell dose. Higher CD34+ cell doses are associated with more rapid engraftment, and thus a dose of equal to or greater than 4 × 106 CD34+ cells/kg is considered optimal.61 The impact of very high CD34+ cells doses in allogeneic HCT is somewhat unclear; some studies have associated doses greater than 8 × 106 CD34+ cells/kg with a higher risk of extensive chronic graft-versus-host disease (GVHD) in matched-related-donor HCT, although this association was not confirmed in unrelated-donor allogeneic HCT. Because there is no evidence of benefit for CD34+ cell doses greater than 8 × 106/kg in allogeneic HCT, this threshold is sometimes, although not universally, used as a maximum.61
Although inadequate mobilization of healthy donors is rare, patients with malignancies undergoing mobilization for autologous HCT often have difficulty collecting adequate numbers of CD34+ cells because of marrow damage from previous chemotherapy or radiation therapy. Approximately 10 to 20 percent of patients preparing for autologous HCT do not mobilize sufficient numbers of CD34+ cells using G-CSF alone or in combination with chemotherapy. Unfortunately, it has proven difficult to identify these individuals prospectively. For patients with NHL, Hodgkin lymphoma (HL), and myeloma who fail mobilization with G-CSF alone, the majority proceed to collect a transplantable dose of CD34+ cells (>2 × 106 cells/kg) when remobilized with plerixafor plus G-CSF.62 Studies of allogeneic HCT using plerixafor-mobilized grafts have also confirmed prompt and stable donor cell engraftment.63 Animal models suggest that plerixafor-mobilized PBPCs have a different phenotype and cytokine profile than G-CSF–mobilized PBPCs and may be associated with a higher risk of acute GVHD; however, the relevance of these findings to human allogeneic HCT is unclear.64 For patients who are thought to be at high risk of poor mobilization, strategies include the upfront use of plerixafor and/or chemotherapy to supplement G-CSF mobilization, as well as large-volume leukapheresis.61 In 2014, the American Society for Blood & Marrow Transplantation (ASBMT) published guidelines on the mobilization and collection of PBPCs for autologous and allogeneic HCT.61
There is some evidence that circadian activity in the hypothalamus regulates the cyclic release of HSCs by altering the expression of CXCL12 in the marrow microenvironment, with the peak time for HSC release in humans in the evening.65 Preliminary clinical data suggest that CD34+ yield is higher in donors collected in the later afternoon compared to the morning, and more abundant PBPC collections were reported from healthy donors when apheresis was performed at 8:00 PM as opposed to 8:00 AM.66 Efforts to exploit this circadian rhythm dependence to increase HSC yield in PBPC products have thus far been inconclusive.67
In the setting of autologous HCT, the superiority of PBPCs over marrow as a stem-cell source is clear. Randomized trials have shown that PBPC autografts in this setting are associated with more rapid engraftment, better quality of life, and lower costs compared to marrow autografts.68,69,70 On the basis of these and other results, most transplantation centers use mobilized PBPCs for autologous HCT and have adopted a minimum CD34+ cell of 2 × 106 CD34+ cells/kg.61
In the allogeneic setting, the situation is considerably more complex. PBPC grafts contain approximately 10-fold more T cells compared to marrow grafts, leading to concern over a potentially increased incidence and severity of GVHD. At the same time, G-CSF can induce functional immune tolerance in healthy individuals, and T cells from G-CSF–mobilized PBPC grafts show a predominantly immune-tolerant profile with upregulation of genes related to T-cell helper type 2 (Th2) and Treg cells, and downregulation of genes associated with Th1 cells, cytotoxicity, antigen presentation, and GVHD.71
A number of randomized clinical trials have compared PBPC and marrow grafts in the setting of allogeneic HCT.72,73,74,75 These studies have consistently reported similar or better overall and disease-free survival with PBPCs compared to marrow allografts. Most, although not all, of these randomized trials found a higher risk of chronic GVHD with PBPCs compared to marrow allografts, and one reported a longer duration of immunosuppression in patients receiving a PBPC graft.73 PBPC allografts were also associated with faster engraftment and a lower risk of graft failure. Systematic reviews and meta-analyses have similarly reached varying conclusions.76,77,78 A 2014 systematic review performed by the Cochrane Library found that overall survival was similar with PBPC and marrow allografts, and that PBPC allografts were associated with faster engraftment but also a higher incidence of chronic GVHD. The effects of stem cell source on relapse and on acute GVHD were unclear.76
Currently, the choice between PBPCs and marrow allografts is generally individualized and depends on patient, donor, and institutional considerations. Patients with advanced or high-risk hematologic malignancies may preferentially be given PBPC grafts to reduce their risk of relapse, a strategy with some support in the literature.79 Conversely, patients with standard-risk malignancies are often given marrow allografts to reduce their risk of chronic GVHD. Likewise, patients transplanted for nonmalignant diseases such as aplastic anemia are typically given marrow allografts to reduce their risk of chronic GVHD, as they derive no benefit from T-cell–mediated graft-versus-malignancy effects. In settings where the risk of GVHD is particularly high—for example, with human leukocyte antigen (HLA)-mismatched unrelated donors—many institutions prefer to use marrow allografts to mitigate this risk. For patients receiving reduced-intensity conditioning (RIC), most centers use PBPC allografts exclusively, as engraftment in this setting is largely dependent on the presence of donor T cells in the allograft. Donor factors also play a role in the choice of allograft product, as some donors may specifically decline either marrow donation or PBPC mobilization and collection. Finally, with the recent predominance of PBPC as a graft source, institutional resources for and expertise in marrow collection have decreased, sometimes limiting the availability of marrow allograft products.
One of the most significant advances in allogeneic HCT in the past 10 years is the increasing experience with alternative donors for patients who lack HLA-identical siblings or suitably HLA-matched unrelated donors. Each full sibling has a 25 percent chance of being HLA-identical with another, so the likelihood of finding an HLA-identical sibling donor is proportionate to the number of siblings available. HLA-matched unrelated donors can be identified for approximately 75 percent of patients of northern European ancestry, but the odds of finding a suitable unrelated donor are much lower for patients who belong to ethnic groups that are underrepresented in donor registries, those of mixed ethnicity, and those with uncommon HLA haplotypes.80 As a result, a substantial fraction of patients who would benefit from allogeneic HCT lack “conventional” related or unrelated donors. For such patients, there are two widely used alternative sources of HSC for allogeneic HCT: UCB and HLA-haploidentical family members.
UCB, collected from the umbilical vessels in the placenta at the time of delivery, is a rich source of HSCs. Because these cells are immunologically naïve, it is feasible to cross major histocompatibility barriers, thus extending the donor pool to individuals for whom finding suitably HLA-matched adult donors can be difficult or impossible.81 The first successful allogeneic HCT using UCB, in a child with Fanconi anemia, was reported by Gluckman and colleagues in 1989.82 Since then, hundreds of thousands of UCB units have been collected and stored; searchable registries have been established to facilitate identification of suitable UCB units for transplantation; and more than 20,000 allogeneic HCTs have been performed using UCB.83 Cord blood units are fully HLA-typed before cryopreservation, and thus suitable units can be rapidly identified (as compared to unrelated-donor searches, which can take 2 to 6 months to complete).
Most UCB units are HLA-typed as HLA-A and HLA-B using low-resolution (serologic) methods, and as HLA-DRB1 using high-resolution (molecular) methods. UCB units matched at equal to or greater than 4/6 HLA loci are generally considered suitable for use, although UCB units matched at 5/6 or 6/6 HLA loci should be used if available because they are associated with lower transplant-related mortality.84,85 The role of HLA matching between UCB units in double UCB transplantation remains somewhat unclear, and unit-to-unit HLA matching does not appear to impact long-term engraftment rates or GVHD incidence.85,86
A major limitation has been the relatively small number of HSC available in cord blood units relative to the size of the average adult recipient.87 As a result, most UCB transplants in adults use two UCB units rather than one. The minimum acceptable cell doses for single-unit UCB transplantation are generally set at equal to or greater than 2.5 × 107 total nucleated cells or equal to or greater than 2 × 105 CD34+ cells/kg of recipient weight.84,88 It is difficult to locate units meeting these criteria for average-sized adult recipients, and thus most adult UCB transplants are performed using two UCB units. With double UCB transplantation, transient mixed donor chimerism from the two units is often observed, but ultimately one UCB unit dominates, eradicates the other unit, and is responsible for establishment of long-term hematopoiesis.86 Despite extensive investigation, the factors determining which unit becomes dominant remain somewhat unclear,89 although CD8+ T-cell responses against the nonengrafting unit have been implicated.90 For recipients with a single suitably sized UCB unit, single-unit UCB transplantation is preferable to double UCB transplantation, because of better platelet recovery and a lower risk of GVHD.88 For the majority of adult recipients, who lack a suitable single UCB unit, double UCB transplantation is typically used and produces equivalent overall survival.89 For children, a single UCB unit appears superior to double UCB transplantation.88 A major area of active research in UCB transplantation is the expansion of HSC or other hematopoietic progenitor cells in UCB products, with the goal of improving engraftment rates, shortening the period of preengraftment neutropenia, and reducing the need for double-unit UCB transplantation. Several approaches have been described in the literature, including Notch-mediated or prostaglandin-mediated expansion of progenitor cells and ex vivo mesenchymal cell coculture.91,92,93
Recipients of UCB allografts have a higher risk of opportunistic infections—particularly viral infection or reactivation—compared to recipients of PBPC or marrow allografts. Presumably the immunologic naïveté of UCB, which allows its use across HLA barriers, also contributes to impaired antiviral immunity and immune reconstitution after allogeneic HCT.94 CD8+ T-cell recovery is significantly delayed after UCB compared to marrow allotransplantation (median time to reach >0.25 × 109 CD8+ T cells/L, 7.7 months vs. 2.8 months, respectively), whereas CD4+ T cell and NK cell recovery is similar with these two graft sources.95 A novel syndrome of cord colitis has been described in UCB recipients and tentatively linked to Bradyrhizobium enterica, a newly identified bacterium,96,97 although other groups have questioned the existence of a distinct cord colitis syndrome and instead attributed the findings in question to conventional GVHD.98,99
Virtually all patients have HLA-haploidentical family members—including any parent, any child, and some siblings—available as donors. Haploidentical related donor HCT has been evaluated for more than 2 decades as an alternative source of HSCs. However, as a result of the substantial HLA disparity involved, early attempts at haploidentical HCT were associated with severe GVHD in T-cell-replete transplants and with graft rejection in T-cell–depleted transplants.100,101 Extensive ex vivo depletion of CD3+ and CD19+ lymphocytes, coupled with megadose CD34+ cells and antithymocyte globulin, can successfully overcome the barriers to engraftment.102 The extensive T-cell depletion used in these protocols to prevent GVHD would also be expected to result in weak or no graft-versus-malignancy effects. Yet despite the lack of T-cell–mediated alloreactivity and the unfavorable prognostic features at the time of transplantation, relapse rates with this approach remained at 18 to 30 percent in patients with acute leukemia transplanted in first complete remission (CR1).103 The low rate of relapse was attributed to a strong antitumor effect mediated by donor NK cell alloreactivity. Transplantation from NK-cell-alloreactive donors was associated with a significantly lower leukemia relapse rate and improved overall survival, leading some authorities to recommend selection of haploidentical donors based on NK cell alloreactivity.104 However, this approach to haploidentical HCT remains hampered by prolonged immune reconstitution and a high risk of serious infection.105
More recently, the group at Johns Hopkins has pioneered the use of posttransplantation cyclophosphamide (CY) as GVHD prophylaxis in T-cell-replete haploidentical HCT.106 In this approach, unmanipulated marrow from an HLA-haploidentical donor is infused after nonmyeloablative conditioning. A period of 48 to 72 hours elapses after infusion, during which alloreactive donor T-cell clones become activated and proliferate. CY is then administered on days +3 and +4 after allogeneic HCT, preferentially eradicating the activated alloreactive donor T-cell clones while leaving other, nonalloreactive clones relatively untouched.107 This approach has been remarkably well-tolerated and results in very low rates of GVHD and transplant-related mortality (TRM).108 Additionally, because this approach avoids indiscriminate T-cell depletion, immune reconstitution is relatively robust, and the typical complications of T-cell-depleted allotransplantation (such as posttransplantation lymphoproliferative disorder [PTLD]) are not seen.109 The major limitation of this approach is a relatively high rate of relapse, perhaps driven by the eradication of the alloreactive donor T-cell clones which would mediate graft-versus-tumor (GVT) effects along with those mediating GVHD.108
Retrospective studies have examined the question of selecting the optimal HLA-haploidentical donor when several such donors are available. Early evidence suggested that lower TRM was seen with HLA-haploidentical sibling donors compared to parental donors, while maternal donors were associated with less chronic GVHD and better overall survival than paternal donors.110,111 In contrast, a 2014 paper reporting outcomes of HLA-haploidentical HCT in China found less GVHD and better overall survival with paternal compared to maternal donors.112 As with previous studies, haploidentical siblings (particularly those disparate for noninherited maternal antigens) were associated with the best outcomes. There is some preclinical evidence that exposure to noninherited maternal antigens through breastfeeding may reduce the risk of GVHD in maternal-donor haploidentical HCT,113 and thus the disparate clinical results with maternal donors may be explained in part by variations in demographic patterns of breastfeeding. In view of the conflicting state of the existing literature, there is no universal standard approach to selecting an HLA-haploidentical donor; the decision is often guided by donor availability and health status, although the literature arguably contains weak support to prefer HLA-haploidentical siblings over parents, and mothers over fathers, as donors.
Although both UCB and HLA-haploidentical HCT have been established as feasible and effective options for patients lacking conventional donors, the optimal alternative-donor source remains unclear. Given the lack of robust comparative data, the choice between UCB and haploidentical HCT is often guided by institutional experience, comfort level, and research priorities. To address the relative merits of these approaches, the United States Blood & Marrow Transplant Clinical Trials Network (BMT-CTN) conducted parallel multicenter phase II clinical trials of RIC followed by either UCB or haploidentical HCT (the latter using T-cell-replete marrow as a graft source and posttransplantation CY as GVHD prophylaxis). These trials reported similar overall and disease-free survival at 1 year with the two approaches.108 UCB transplantation was associated with a higher risk of GVHD and nonrelapse mortality, while haploidentical HCT was associated with a higher risk of relapse. Based on these results, the BMT-CTN is currently conducting a national, multicenter phase III clinical trial randomizing participants between UCB and haploidentical HCT. In addition to clinical outcomes, this trial is designed to measure quality-of-life and cost differences between the two alternative-donor approaches in a comprehensive effort to clarify their relative advantages and disadvantages.
CONCEPTS OF CURATIVE THERAPY
The relationship between the dose of chemoradiotherapy administered and the number of tumor cells killed has been extensively studied in vitro and in preclinical animal models, and forms the rationale for myeloablative autologous HCT. For chemosensitive tumors (including most hematologic malignancies), a steeply rising dose–response curve is observed. The curative potential of autologous HCT is, therefore, derived from high-dose chemotherapy or chemoradiotherapy administered as transplant conditioning to enhance tumor cell kill and overcome drug resistance. This level of dose escalation is possible in autologous HCT because dose-limiting toxicities to the hematopoietic system are circumvented by the infusion of autologous HSCs, which rebuild hematopoiesis after high-dose conditioning.
Autologous HCT is associated with relatively low TRM. The use of mobilized PBPCs rather than marrow as a graft source has decreased the duration of neutropenia, and when combined with improved supportive care and patient selection has reduced the TRM associated with autologous HCT from approximately 8 to 10 percent in historical studies to 1 to 3 percent at most centers. In addition, the reduced risks and faster engraftment times seen with PBPC have enabled many centers to pursue outpatient autologous HCT, further easing the logistical burden on patients as well as treatment costs.
A consistent concern in autologous HCT is the possibility that residual tumor cells may contaminate the HSC product and contribute to relapse. The relative contributions of autograft contamination and residual disease in the patient are difficult, if not impossible, to distinguish. Several investigators have approached this question by marking the HSC product at the time of harvest and then assaying for the marker gene in malignant cells at the time of subsequent relapse. Studies using this approach have been performed in patients with leukemia, lymphoma, and myeloma, and have reached conflicting conclusions about the contribution of autograft contamination to relapse.114,115
A number of ex vivo and in vivo purging strategies have been investigated in autologous HCT. These include administration of rituximab before autologous HSC collection in patients with CD20+ malignancies; ex vivo positive selection for CD34+ cells; chemotherapy-based purging using CY derivatives; and purging via oncolytic viruses.116,117,118,119,120 Single-arm or uncontrolled studies have suggested a possible benefit in some of these instances, but there is little or no evidence from randomized clinical trials at present to support the efficacy of autograft purging, and its use remains investigational. One of the few randomized clinical trials in the field concluded that in vivo purging with rituximab administered before autologous HSC collection was as effective, and likely safer, than ex vivo CD34+ selection.121 Another randomized clinical trial found that CD34+ selection significantly reduced autograft contamination with myeloma cells, but did not improve clinical outcomes.122 Purging strategies carry some potential for harm, as they deplete the autograft of mature T cells and increase the risk of infectious complications, particularly CMV or other viral reactivation, after autologous HCT.123,124 At present, given the lack of convincing evidence of clinical benefit, purging strategies are not widely used and most centers collect and infuse unmanipulated autologous HSC (although these cells are often mobilized with a regimen containing chemoimmunotherapy, which arguably represents a form of in vivo purging). Because relapse remains a major concern after autologous HCT, ongoing research is focused on identifying more efficient and clinically effective means of autograft purging.
Allogeneic HCT is a considerably more complicated procedure than autologous HCT. It involves more pretransplantation preparation, poses a greater risk of complications to the patient, is associated with a significantly higher rate of TRM, and requires at least temporary postgrafting immunosuppression to enable engraftment and prevent GVHD. The decision to pursue allogeneic HCT is based on diagnosis, prognosis, and remission status, as well as the availability of an appropriate donor and the psychosocial resources of the patient to cope with the demands of the process. Decisions about eligibility for allogeneic HCT are typically made on a center-by-center and case-by-case basis, with inherent elements of subjectivity and patient and physician judgment.
A major obstacle to successful allogeneic HCT is the immune competence of the recipient. The potential to reject infused donor cells is mediated predominantly through regimen-resistant host T and NK cells. Strategies to reduce host immunity and promote donor hematopoietic cell engraftment include pretransplantation conditioning (most often chemotherapy and/or radiation) and postgrafting immunosuppressive medications. Donor T cells in the allograft play a key role in hematopoietic engraftment, and depletion of T lymphocytes from the allograft before transplantation is associated with a substantial increase in the occurrence of graft rejection.125,126 With modern conditioning regimens, rates of allograft rejection are low (typically <5 percent) for patients who have received previous chemotherapy, which weakens their immune response to the allograft. In contrast, graft rejection in seen more often in patients who have not received cytotoxic chemotherapy before allogeneic HCT (for instance, some patients with myeloproliferative neoplasms) or those with nonmalignant diseases such as aplastic anemia, thalassemia, or sickle cell disease, who are often highly sensitized against donor antigens by virtue of being heavily transfused before allogeneic HCT.
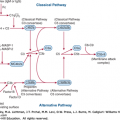
The dominant mechanism of cancer eradication following allogeneic HCT is the immunologic recognition and destruction of residual host tumor cells by donor-derived immune cells. This phenomenon, termed the GVT effect, has been conclusively demonstrated and represents one of the most significant biologic findings of the past half-century, with implications well beyond the transplantation setting. The existence of GVT effects is supported by the following lines of evidence:
Tumor relapse is lower after allogeneic than after syngeneic HCT: The appreciation of alloreactive GVT effects stemmed from the observation that recipients of genotypically identical (syngeneic) grafts had significantly higher rates of disease relapse than did patients who received grafts from HLA-identical siblings.127,128
Tumor relapse is higher in recipients of T-cell–depleted grafts: Further support for the allogeneic GVT effect came from studies of T-cell depletion of the graft.129 T-cell depletion was performed in the expectation that it would reduce the risk of GVHD. However, the later observation that these patients had a much higher risk of disease recurrence as well as graft rejection was unanticipated. These results linked GVHD with GVT effects, and supported the concept that patients who developed some degree of alloreactivity, as manifested by clinically apparent GVHD, had a reduced risk of disease relapse.
Donor lymphocyte infusions can induce remission: Perhaps the most definitive evidence for the existence of GVT effects came from the application of donor lymphocyte infusions (DLIs). In the early 1990s, Kolb and others demonstrated that patients with relapsed malignancies after allogeneic HCT could, in some cases, be returned to complete remission by the simple infusion of donor-derived lymphocytes.130,131,132 With increasing experience, it became clear that some diseases (such as chronic myelogenous leukemia [CML]) respond very well to DLI whereas others (for instance, acute lymphoblastic leukemia [ALL]) are much less responsive. Long-term followup of patients successfully treated with DLIs revealed that responders had remarkably durable remissions and excellent outcomes.132 These observations definitively established the GVT effect as a biologic entity capable of controlling an otherwise lethal condition such as leukemia.
The biology of the GVT effect remains incompletely understood. A number of immunologic targets recognized by donor immune effector cells have been proposed, including alloantigens (such as major or minor histocompatibility antigens depending upon donor–recipient genetic differences), lineage-specific antigens, and malignancy-specific antigens such as products of chromosomal translocations. Donor T cells clearly play a key role in GVT, and there is emerging evidence that NK cells are also responsible for tumor cell control, especially in the setting of T-cell-depleted HLA-haploidentical HCT.133 Humoral immunity has also been implicated as playing a role in the GVT effect.134 Based on preclinical models, the common effector cells that could potentially mediate a clinical GVT effect include (1) CD8+ cytotoxic T lymphocytes that recognize tumor-associated antigens in context of class I major histocompatibility complex (MHC) antigens; (2) CD4+ T cells that recognize tumor-associated antigens in context of class II MHC antigens and mediate their effects via Th1 cytokines such as interferon (IFN)-γ and IL-2, upregulating expression of class I MHC antigens and promoting expansion and activation of CD8+ cytotoxic T lymphocytes; and (3) NK cells that recognize stress ligands and cells lacking MHC expression.135,136,137,138,139,140,141 The impact of NK cells seems especially pronounced in HLA-haploidentical or mismatched allotransplantation.104,142
A major question continues to be whether the subset of T cells that induce GVHD is the same population of T cells responsible for the GVT effect. One of the central aims of research in allogeneic HCT is to separate the beneficial GVT effects from deleterious GVHD. Clinical evidence suggests that, in principle, the two should be separable, as some patients experience an apparent GVT effect in the absence of apparent GVHD.143 Although numerous approaches have succeeded in separating GVT effects from GVHD in preclinical and animal models, none of these approaches have yet been translated successfully to widespread clinical use. One approach supported by preclinical models involves the use of Treg,144 and recent studies in the HLA-haploidentical setting are supportive of this concept.145,146
TRANSPLANT PREPARATIVE REGIMENS
The transplant preparative regimens used in HCT must accomplish two goals. Because the majority of autologous and allogeneic HCT are performed in individuals with cancer, these regimens were designed, at least initially, to maximize tumor cytoreduction and disease eradication. In the case of allogeneic HCT, the regimen must be sufficiently immunosuppressive to overcome host rejection of the graft. In autologous HCT, where efficacy depends on exploiting the dose–response curve, high-dose conditioning regimens are universally used. In contrast, in allogeneic HCT much or all of the clinical benefit derives from donor alloimmunity, enabling the use of RIC designed to facilitate donor engraftment with minimal toxicity.
TBI has been a primary component of many autologous and allogeneic HCT preparative regimens since the inception of the field. TBI has excellent activity against a variety of hematolymphoid malignancies, has pronounced immunosuppressive properties, and is able to treat sanctuary sites like the testicles and the central nervous system. Aside from one very early study of high-dose TBI alone, most preparative regimens combine TBI with cytotoxic agents such as CY. Dose-finding studies suggest that higher TBI doses are associated with a lower risk of relapse with dose escalation as high as 15.75 Gy, but doses above 12 Gy are associated with higher risks of GVHD and TRM, which offset the reduced risk of relapse.147 Currently, most high-dose TBI-based conditioning regimens use a dose between 12 and 13.2 Gy. Long-term concerns with TBI-based regimens include the development of cataracts and hypothyroidism, impairment of growth and development in children, and secondary malignancies.148,149,150
Hyperfractionated TBI, in which relatively small dose fractions are given two to three times a day over a few days, minimizes leukemia regrowth and reduces lung and gastrointestinal toxicity, allowing higher TBI doses to be administered safely. Several clinical studies confirm decreased overall lung toxicity with fractionation.151,152 Excellent results are also reported with the combination of fractionated TBI and VP-16 (etoposide), particularly promising results in patients with ALL.153,154
An alternate form of irradiation is radioimmunotherapy, which involves the use of antibodies to deliver locally acting radionucleotides to targeted sites. In theory, this strategy could provide excellent targeted antitumor effects without increased systemic toxicity. Clinical trials incorporating anti-CD45 monoclonal antibodies conjugated to radioactive iodine (131I) or yttrium (90Y) show promising early results in both the autologous and the allogeneic setting.155,156,157,158 One approach to further improving the targeting of radiation is the use of α-particle emitters. Alpha particles have a very short effective range but carry immense kinetic energy, making them an attractive option to maximize malignant cell killing while minimizing bystander damage. Preclinical studies using bismuth-213 (213Bi) and astatine-211 (211At) have demonstrated efficacy,159,160,161,162 and this approach is currently being translated into clinical trials.
Patients with NHL or HL have often received prior intensive local radiotherapy, often to the mediastinum, which results in a high incidence of fatal interstitial pneumonitis following TBI.163 Therefore, non-TBI-containing conditioning regimens were developed. The choices of drugs include agents that can be significantly dose-escalated and have improved tumor cell killing at higher doses, yet have nonoverlapping toxicities; for example, a common preparative regimen for autologous HCT in lymphoma includes 1,3-bis(2-choloroethyl)-1-nitrosurea (carmustine or bis-chloroethylnitrosourea [BCNU]), VP-16, and CY.164 The dose-limiting non-hematologic toxicity of BCNU affects the lungs; VP-16 affects the liver; and CY, the heart. Consequently, using these drugs below the maximally tolerated doses results in relatively low regimen-related toxicity but maximizes tumor cell kill to overcome drug resistance. Many other chemotherapy-only regimens have been developed for autologous HCT according to similar principles, including BCNU, etoposide, cytarabine, and melphalan (BEAM) (for lymphomas) and high-dose melphalan (for myeloma).
A variety of chemotherapy-only conditioning regimens have been developed for allogeneic HCT. The most widely used combines oral busulfan (BU), at a total dose of 16 mg/kg given over 4 days, with CY at a total dose of 120 mg/kg given intravenously over 2 days (referred to as BU/CY).165,166 A randomized comparison between fractionated TBI plus CY (TBI/CY) and BU/CY in patients transplanted for CML demonstrated that the BU/CY regimen was better tolerated, but there was no significant difference in overall or event-free survival, TRM, or GVHD incidence.167 The development of intravenous BU further improved the availability and tolerability of this regimen, although steady-state plasma concentrations remain variable after intravenous BU and therapeutic drug monitoring arguably remains necessary.168,169 Interestingly, pretreatment with BU may deplete hepatic glutathione and thus potentiate the toxicity of CY.170,171 Reversing the sequence of conditioning agents (from BU/CY to CY/BU) has been studied as a means of reducing regimen-related toxicity, and this alteration to the regimen has been associated with reduced exposure to toxic CY metabolites and a lower risk of hepatotoxicity.172
Other high-dose chemotherapy-only conditioning regimens remain in use on an institution-, disease-, and patient-specific basis. The most common modification to BU/CY is the substitution of fludarabine (FLU) for CY (BU/FLU). This regimen has been proposed to avoid the hepatotoxicity of CY and to reduce regimen-related toxicity compared to BU/CY. However, two recent randomized clinical trials comparing BU/CY and BU/FLU have found increased risks of graft rejection and pneumonitis, as well as decreased overall and disease-free survival, with BU/FLU, raising the concern that this regimen is inferior to BU/CY.173,174
The demonstration that immune-mediated mechanisms are critical in eradicating malignancy after allotransplant challenged the rationale for relatively toxic full-dose conditioning. A number of reduced-intensity regimens have been developed which have lower regimen-related toxicity but are sufficiently immunosuppressive to allow full donor engraftment, shifting the responsibility for tumor eradication largely or entirely to immunologic GVT effects. The development of RIC is one of the most transformative advances in the field of allogeneic HCT in the past several decades, as it has allowed the expansion of eligibility for allogeneic HCT to older and less medically fit patients who would otherwise be ineligible for high-dose conditioning. Additionally, patients with relatively indolent disease may not require the immediate cytoreductive capacity of an aggressive preparative regimen and may be particularly suitable candidates for transplantation using RIC. These regimens are also useful in patients with nonmalignant diseases where the goal is strictly the establishment of donor hematopoiesis; examples include genetic disorders, autoimmune diseases, and the induction of tolerance in combined solid-organ and same-donor marrow transplantation.
A variety of RIC regimens with differing dose intensities have been developed. One significant regimen came from detailed studies in a canine model using a backbone of low-dose 2 Gy TBI followed by postgrafting immunosuppression with mycophenolate mofetil (MMF) and cyclosporine (CSP).175 This work was translated to patients with a variety of malignancies and resulted in reliable donor engraftment with the addition of intravenous FLU 90 mg/m2.176 This reduced-intensity regimen has been used successfully in more than 1000 patients with a wide variety of malignancies, especially in older patients and those with more indolent diseases such as follicular NHL or chronic lymphocytic leukemia (CLL).177,178,179 Another commonly used RIC regimen consists of intravenous FLU (between 90 and 150 mg/m2) and CY (between 900 and 2,000 mg/m2).180 This regimen, combined with rituximab or 90Y ibritumomab tiuxetan, has produced excellent long-term disease-free survival in patients with indolent lymphoma.181 A third common reduced-intensity regimen was developed in a rodent model using fractionated low-dose total lymphoid irradiation (TLI) combined with depletive T-cell antibodies (antithymocyte serum), which showed that recipients were protected from GVHD induction by donor-derived T cells.182 Rodents conditioned with this approach did not develop lethal acute GVHD despite the infusion of megadoses of donor T lymphocytes. TLI and antithymocyte serum altered residual host T-cell subsets to favor regulatory NK/T cells which suppress GVHD by polarizing the infused donor T cells toward secretion of noninflammatory cytokines such as IL-4, and by promoting expansion of donor CD4+CD25+FoxP3+ Tregs.183 This approach was successfully translated to clinical transplantation; patients conditioned with TLI and antithymocyte globulin (ATG) developed sustained donor-derived hematopoiesis with very low incidences of acute GVHD and TRM.184,185 The relative merits of RIC versus high-dose conditioning continue to be studied; a national multicenter prospective randomized trial comparing high-dose to RIC in patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) is ongoing under the auspices of the BMT-CTN.
A feature common to all RIC protocols is the incomplete eradication, at least initially, of host hematopoietic elements. As a consequence, a significant percentage of patients have mixed donor–recipient chimerism for months after transplantation before fully converting, if ever, to complete donor type. Most reports suggest that persistent mixed chimerism is a significant risk factor for disease relapse.185,186 Interventions such as withdrawal of immunosuppressive medications, CD34-selected donor cell boost, and DLI have been used to convert mixed chimerism to complete donor type, although these interventions are not without risk and can precipitate GVHD. A significant percentage of patients who received alemtuzumab-containing conditioning regimens required DLI to promote donor engraftment and protect against immune-mediated graft rejection, but the risk of developing post-DLI GVHD was significant.187 Mixed chimerism is not unique to RIC; prior to the addition of ATG, almost 60 percent of patients who received high-dose conditioning and allogeneic HCT for severe aplastic anemia had mixed chimerism, of whom two-thirds eventually converted to complete donor hematopoiesis while the remainder experienced late graft failure.188
Persistent mixed chimerism may have a role in allogeneic transplantation for noncancer patients. In organ transplantation, tolerance, defined as immunosuppressive drug withdrawal without graft rejection, was achieved in kidney transplant recipients who received the same donor marrow when sustained mixed chimerism was established, and not in recipients who experienced donor hematopoietic graft loss.189
EVALUATION AND SELECTION OF CANDIDATES FOR TRANSPLANTATION
Most transplantation candidates are referred by hematologists or oncologists to the tertiary center where HCT will be performed. Patients considered for transplantation require in-depth evaluation and counseling by experienced transplantation physicians, nurses, and social workers. A detailed review of initial diagnostic studies, previous drug and radiation treatments, and responses to these interventions, as well as a psychosocial assessment of the patient and their caregivers, are of the utmost importance. Table 23–2 highlights the issues and topics that should be addressed during counseling meetings with transplantation candidates and their caregivers.190 Important factors which consistently impact outcomes following HCT include, but are not limited to, disease status at transplantation, type and compatibility of donor, recipient age, and comorbid medical conditions. Early referral for transplantation consultation is critical, particularly if allogeneic HCT is under consideration, because of the time required to identify a suitable donor.
|
Disease status at the time of transplantation is perhaps the most powerful predictor of long-term disease-free survival following allogeneic and autologous HCT. Early studies of allogeneic HCT were performed using predominantly patients with refractory and progressive disease.191 Although a small percentage of these patients were salvaged, transplantation was unsuccessful in the majority of patients due to progressive malignancy. Patients with acute leukemias transplanted in complete remission (CR) have substantially better outcomes compared to those transplanted with active disease.192 Even very minimal amounts of residual disease are associated with significantly higher relapse risk in patients undergoing allogeneic HCT for AML, regardless of conditioning intensity,193,194,195 underscoring the importance of disease status at transplant as a prognostic factor. Likewise, disease status as determined by positron emission tomography (PET) prior to transplant is an important predictor of progression-free survival for patients with diffuse large B-cell lymphoma and HL undergoing autologous HCT.196,197,198,199 These scenarios highlight a truism: patients who have advanced poorly controlled disease at the start of transplant conditioning have significantly inferior outcomes compared to patients transplanted earlier in the course of their disease and to those who have achieved good, albeit temporary, control of their disease. On the other hand, attempts to salvage patients with advanced disease who have failed multiple therapies are rarely successful, and transplantation is often best considered early in the course of therapy. These discussions are complex, as earlier transplantation, especially allogeneic, carries significant risks to the patient. A number of other disease-specific considerations are important in determining the appropriate timing for transplantation, including the presence and/or persistence of cytogenetic and molecular abnormalities, the immune phenotype, and evidence of extramedullary or extranodal disease. Advanced genetic characterization of leukemia and lymphoma may provide improved insight into cohorts of patients for which HCT should be performed earlier in the course of disease.
Historically, older age was a significant barrier to allogeneic HCT, since older patients suffered severe and often prohibitive toxicity after high-dose conditioning regimens.200 However, the impact of older age is mitigated by the increasing use of RIC for allogeneic HCT.201 High-dose conditioning and allogeneic HCT is generally reserved for patients 60 years of age or younger, whereas allotransplantation with RIC has been performed successfully in patients into their eighth decade of life. Most centers in the United States do not have a stringent age cutoff for allogeneic HCT, although careful screening for comorbid medical conditions, such as heart, lung, kidney, and liver disease, is particularly important in older patients, and allotransplantation in patients 70 years of age and older remains somewhat controversial. Existing data suggest that allogeneic HCT can be safely performed in selected patients age 60 to 75 years, with a 5-year overall survival of 35 percent.202,203 Some investigators have proposed that age is a poor and imprecise prognostic marker, and instead advocate the use of comorbidity assessment and scoring to determine eligibility for allogeneic HCT.204 However, caution is warranted since this approach has not been prospectively validated; retrospective cohort studies necessarily suffer severely from patient selection bias, as they include only those older patients who were deemed appropriate candidates to proceed to allogeneic HCT. Outcomes for this selected group of older patients cannot be generalized to the population of older adults as a whole.
In contrast to allogeneic HCT, autologous HCT relies on high-dose conditioning for its antitumor efficacy. Thus, there is no way to reduce conditioning intensity without sacrificing some degree of efficacy. As a result, age limitations are often stricter for autologous HCT than for allogeneic HCT, as candidates for the former must be able to tolerate intensive, high-dose chemotherapy. It is unusual for autologous HCT to be offered to patients older than 75 years of age.205,206
Comorbid medical conditions have a significant impact on transplantation outcomes. Routine screening of heart and lung function to detect occult abnormalities is of critical importance, especially in older patients. Evaluation of liver and kidney function, as well as exposure to potential pathogens such as CMV, hepatitides B and C, herpes viruses, and HIV are routine and should be performed in all patients. Another major factor is the nutritional status of the patient, as extremes such as cachexia or obesity require special considerations and adversely impact TRM.207,208
Several scoring instruments have been devised to allow quantification and comparison of pretransplantation comorbidities. The most widely used of these are the EBMT Risk Score,209 the Pretransplant Assessment of Mortality (PAM) score,210 and the HCT-specific Comorbidity Index (HCT-CI; later modified to incorporate age).204,211 A number of efforts have been made to validate, revise, or combine these scores in diverse populations, with variable success.212,213,214,215,216 Several caveats are important when considering quantitative scoring of pretransplantation comorbidities. First, because these scoring instruments were derived from retrospective cohorts of patients, they suffer from an inescapable selection bias, as only patients who were deemed fit for transplantation were included in their derivation. This selection bias limits the ability to generalize their use to unselected patient populations. Second, transplantation outcomes have not remained stable over time; instead, TRM has steadily decreased over time with the availability of better supportive care and other refinements.217,218 Thus, it is conceivable that the relative impacts of specific comorbidities on transplantation outcomes are not fixed, but may vary over time. Efforts are currently underway to prospectively validate these comorbidity scoring instruments.
Every effort should be made to encourage potential patients to maintain good health practices, including discontinuation of alcohol use, tobacco smoking, and illicit drug use (if applicable). Centers vary in their approach to abstinence, but it is common to require that patients cease all tobacco use permanently as a condition of proceeding to autologous or, especially, allogeneic HCT. The risk of pulmonary complications from chemotherapy (e.g., BCNU) or from chronic GVHD involving the lung is potentiated by smoking, as is the risk of secondary cancers of the lung and other organs. A special situation deserving consideration is the use of marijuana, which is increasingly legal, available, and widely used by cancer patients to combat chemotherapy-related nausea and anorexia. Anecdotally, an increasing number of patients referred for transplantation consultation are actively using marijuana to control these symptoms during their pretransplantation chemotherapy. Cases of severe or fatal pulmonary aspergillosis from inhaled spores have been reported in immunosuppressed patients using marijuana.219,220 Transplant center policies regarding medical marijuana use and abstinence have lagged behind the rapidly changing legal status of marijuana in the United States, creating a challenge in assessing and counseling patients.
DISEASES TREATED WITH TRANSPLANTATION
Numerous malignant and nonmalignant hematologic disorders, as well as selected solid tumors, may be treated with HCT. The results obtained with transplantation are reviewed in detail in the disease-specific chapters of this book, and are discussed only briefly here.
In general terms, autologous HCT is recommended for patients whose malignancy exhibits chemosensitivity to conventional dose therapy and does not extensively involve the marrow; included are most lymphomas, germ cell tumors, and other selected pediatric tumors. In these instances, tumor eradication is a result of dose escalation of cytotoxic therapy in the conditioning regimen, and the autograft serves as hematopoietic cell rescue. In contrast, allogeneic transplantation is generally pursued for hematologic malignancies and disorders that primarily originate in the marrow, such as acute and chronic leukemias, aplastic anemia, MDSs, and myeloproliferative neoplasms. For some diseases with extensive marrow involvement, such as the low-grade lymphomas and myeloma, the decision to pursue autologous versus allogeneic HCT is more complex. In these settings, allogeneic transplantation has generally been more successful in controlling disease recurrence and reducing relapse risk. However, the associated risks, including GVHD and prolonged immunosuppression, result in a higher TRM compared to autologous HCT. Thus, the decision to pursue an allogeneic or autologous HCT for patients with these diseases depends on the combination of patient characteristics such as comorbidities and age, availability of a suitable donor, disease-specific characteristics, and often patient preference. For some hematologic conditions, such as MDSs, myeloproliferative neoplasms, and aplastic anemia, only allogeneic transplants are generally appropriate.
In addition, patients with selected solid tumors, such as testicular cancer, neuroblastoma, and other pediatric tumors, have had successful outcomes with autologous HCT.221,222,223,224 Extensive studies in women with breast and ovarian carcinoma, and more limited studies in patients with renal cell carcinoma and small cell lung cancer, have failed to demonstrate a role for HCT.225,226 Outside of the investigational setting, there are no currently accepted indications for allogeneic HCT to treat nonhematologic solid tumors.
A variety of congenital and acquired nonmalignant disorders can be successfully treated with HCT. The most well-established nonmalignant indication is for allogeneic HCT in patients with severe aplastic anemia, where outstanding results have been achieved, particularly for younger patients with HLA-matched sibling donors, where long-term disease-free survival rates of 88 to 100 percent have been reported.227,228 Hematopoietic cell transplantation for patients with clinically significant hemoglobin disorders, such as thalassemia major, has been very successful, especially in patients without significant liver disease.229,230 Likewise, allogeneic HCT is considered a treatment option for young patients with severe forms of sickle cell disease.231,232 Guidelines for patient selection and management of patients with thalassemia or sickle cell disease were published in 2014 by EBMT.233 In patients with hemoglobin disorders, transplantation serves as a form of gene therapy, using allogeneic hematopoietic cells as vectors for genes essential for normal hematopoiesis. Eventually the vector may well be autologous stem cells transformed by the insertion of normal genes.234
For patients with SCID syndrome and other congenital immunodeficiencies, allogeneic HCT remains the treatment of choice.235,236 The role of allogeneic HCT for patients with storage diseases, a diverse group of disorders that typically involve a single gene defect in a lysosomal hydrolytic enzyme or peroxisomal function, is evolving and it appears that subsets of mucopolysaccharidoses derive the most benefit.237
A comprehensive discussion of transplantation outcomes is beyond the scope of this chapter; please refer to other disease-focused chapters of this text for more complete information. A brief overview of transplantation-related outcomes in a number of diseases is presented here.
Allogeneic HCT has a major role in the management of patients with AML. For patients in CR1, the decision to proceed to allogeneic HCT as opposed to chemotherapy-based consolidation is predicated on prognostic markers and donor availability, as well as patient preference. For patients beyond CR1, allogeneic HCT often offers the only potentially curative treatment option.
A question of significant practical importance is how best to treat a younger AML patient who achieves a CR1 following induction chemotherapy. Several large prospective trials have been conducted using so-called genetic randomization: that is, patients in CR1 with an HLA-identical sibling received allogeneic HCT, while those without an HLA-identical sibling received chemotherapy-based consolidation or autologous HCT.238,239 Two meta-analyses have focused on the comparative outcomes of allogeneic HCT versus chemotherapy, both of which found that allogeneic HCT in CR1 yielded better overall and disease-free survival for patients with intermediate- and poor-risk cytogenetics, but not for those with favorable-risk cytogenetics.240,241 For patients with poor-risk cytogenetics, there is little dispute that allogeneic HCT is the preferred postremission therapy for medically fit patients with available donors. Conversely, patients younger than 60 years of age with favorable-risk cytogenetics who promptly achieve CR1 with induction chemotherapy are generally not considered for allogeneic HCT, and instead should receive chemotherapy-based consolidation. For younger patients with intermediate-risk cytogenetics, allogeneic HCT in CR1 from an HLA-identical sibling donor is the best available postremission therapy, but its advantage over other approaches is not as substantial as in the setting of poor-risk cytogenetics. One caveat is that the studies demonstrating the superiority of allogeneic HCT were performed using genetic randomization, and thus the results are, strictly speaking, only applicable to patients with HLA-identical sibling donors. That said, single-center and registry data suggest that outcomes with HLA-matched unrelated donor allotransplantation for AML in CR1 are similar to those with HLA-identical sibling donors,242,243 so it is reasonable to extrapolate the results of the genetic-randomization studies to patients with HLA-matched unrelated donors.
Patients older than 60 years of age typically have substantially higher relapse rates with chemotherapy alone (60 to 80+ percent)244,245 and have poorer outcomes than younger patients with equivalent cytogenetics, suggesting that older adults may benefit from allogeneic HCT in CR1. There are no prospective, genetically randomized trials in older adults with AML comparing allogeneic HCT to chemotherapy in CR1, but retrospective comparisons suggest that allogeneic HCT can reduce relapse risk and improve outcomes in this demographic.244,245 The decision to proceed to allogeneic HCT in older adults is often based less on disease risk factors and more on medical comorbidities and the estimated risk of TRM, which may be prohibitive.
In the genomic era of AML risk stratification, patients with intermediate-risk disease are often categorized based on the results of molecular testing for mutations of FLT3, NPM1, and CEBPA. Patients with intermediate-risk cytogenetics and biallelic CEBPA mutations or NPM1 mutations in the absence of FLT3 mutations have good-risk disease and are often not considered for allogeneic HCT in CR1.246 In contrast, patients with internal tandem duplications in FLT3 have poorer prognoses and are often considered for allogeneic HCT in CR1.
Detailed guidelines have been published by the European LeukemiaNet AML Working Party to guide the use of allogeneic HCT in AML patients in CR.247 Based on existing data, many experts would recommend allogeneic HCT for all medically fit patients younger than 60 years old with AML in CR1 except for those with favorable-risk disease (including those with intermediate-risk cytogenetics and favorable molecular markers) who achieve CR1 with their first cycle of induction and have no evidence of minimal residual disease. For all other younger CR1 patients—including those with intermediate-risk cytogenetics and negative molecular markers, and those in any cytogenetic risk group who do not promptly achieve CR with induction or who have minimal residual disease—allogeneic HCT should be strongly considered as the best postremission option.248 For older adults with AML in CR1, allogeneic HCT should be considered for all medically fit patients except those with favorable-risk disease, although there are fewer data to support this recommendation and medical comorbidities play an increasingly important role in decision making as patients age.
The role of consolidation chemotherapy before allotransplantation in patients in CR1 is unclear. Many transplant physicians recommend at least 1 to 2 cycles of consolidation, especially for patients slated to receive RIC, in order to maximize pretransplantation cytoreduction. However, two recent retrospective studies have questioned the benefit of consolidation chemotherapy, and instead favored moving forward to allogeneic HCT as soon as a suitable donor is available.249,250 Consolidation chemotherapy is to maintain remission during prolonged donor searches, but its benefit is less clear in patients who have a donor identified.
For patients with AML who relapse after attaining a CR1, allogeneic HCT is the treatment of choice. While no prospective randomized trials have compared allogeneic HCT to salvage chemotherapy alone in this setting, retrospective data strongly support the use of allogeneic HCT.251 Autologous HCT has been used historically in patients who lacked suitable allogeneic donors,252 but with the advent of improved alternative-donor options the use of autologous HCT for AML has become rare except in developing countries without the capability to perform allogeneic HCT. Patients with AML beyond CR1 who lack suitably HLA-matched donors are candidates for HLA-haploidentical or UCB allotransplantation, as retrospective studies have associated these approaches with equivalent overall and disease-free survival compared to allotransplantation from conventional donor sources.253,254
The likelihood of long-term survival is very low in AML patients whose disease fails to achieve CR1 following induction therapy (primary induction failure) and in those whose disease is chemorefractory at relapse. Allogeneic HCT with myeloablative conditioning has been reported to cure approximately 19 to 30 percent of such patients in retrospective analyses.192,255 These retrospective reports undoubtedly suffer from substantial selection bias; presumably patients with relapsed or refractory AML were not transplanted indiscriminately, but only if their physicians felt they had an above-average chance of responding. These success rates are therefore very unlikely to pertain to the overall population of adults with relapsed or refractory AML. Attempts have been made to construct prognostic tools to identify the subset of patients most likely to benefit from allogeneic HCT,192 and some authorities have argued that allogeneic HCT is underused in this population.248 Currently there is no single standard of care for treatment of patients with AML in refractory relapse or primary induction failure; reasonable options include additional salvage chemotherapy to induce remission; enrollment on a clinical trial; allogeneic HCT (in carefully selected patients); and palliative care, and the optimal choice must be individualized based on patient characteristics and donor availability.
Allogeneic HCT is widely used in adult patients with ALL, especially those patients with high-risk features (most often defined as white blood cell count at diagnosis >30,000 [or >100,000 in T-cell ALL], adverse cytogenetics, progenitor B-cell immunophenotype, age >60 years, or failure to achieve CR within 4 weeks of induction chemotherapy).256 Patients with none of these features are considered to have standard-risk disease; there is no clear “favorable-risk” population in adult ALL. While the advisability of allogeneic HCT is uncontroversial as postremission therapy for adults with high-risk ALL, it is also the treatment of choice for eligible adults with standard-risk ALL in CR1. Several large genetically randomized prospective trials have demonstrated a survival benefit for allogeneic HCT in CR1 for adults with standard-risk ALL.257,258 A 2013 meta-analysis confirmed the benefit of upfront allogeneic HCT for adults 35 years of age or younger; for older adults, the benefit was more difficult to demonstrate owing to increasing TRM.259
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree