INTRODUCTION
SUMMARY
Normal pregnancy involves many changes in maternal physiology, including alterations in hematologic parameters. These changes include expansion in maternal plasma volume. The increase in plasma volume is relatively larger than the increase in red cell mass resulting in a decrease in hemoglobin concentration. An increase in the levels of some plasma proteins alters the balance of coagulation and fibrinolysis. Worldwide, the predominant cause of anemia in pregnancy is iron deficiency. Fetal requirements for iron are met despite maternal deficiency, but maternal iron deficiency has a number of adverse consequences including an increased frequency of preterm delivery and low-birth-weight infants. Bleeding disorders in pregnancy are a common reason for hematologic consultation and evoke concern for both the mother and child. Life-threatening bleeding caused by disseminated intravascular coagulation is seen with some complications unique to pregnancy, including placental abruption, retained dead fetus, and amniotic fluid embolism. von Willebrand disease is the commonest inherited bleeding disorder, but because of increases in factor VIII level and von Willebrand factor during pregnancy, excessive bleeding at delivery is rarely a problem in such patients. Factor levels fall rapidly postpartum, and serious hemorrhage can occur during this period. Carriers of hemophilia A and B should be monitored during pregnancy to determine if factor levels will be adequate for delivery at term. Caution should be exercised at delivery and during the first few days of life with offspring of hemophilia carriers until hemophilia testing is completed and the infant’s status is known. Acquired hemophilia as a result of factor VIII autoantibodies is rare, but can occur during pregnancy or the puerperium. Thrombocytopenia is not uncommon in pregnancy, and its causes include several conditions that are unique to pregnancy, such as preeclampsia. Idiopathic thrombocytopenic purpura (ITP) is common, it is often exacerbated in pregnancy, and is managed conservatively if possible; close followup of newborns of mothers with ITP is essential. HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome and TTP (thrombotic thrombocytopenic purpura)/hemolytic uremic syndrome are also seen in pregnancy and the puerperium. HELLP syndrome is managed by immediate delivery, if possible, whereas TTP, usually, can be managed with plasma exchange. Inherited and acquired prothrombotic conditions can be exacerbated by pregnancy and can result in adverse reproductive outcomes as well as maternal venous thromboembolism. The strongest evidence for an association between a thrombophilia and recurrent fetal loss exists for antiphospholipid antibody syndrome; however, evidence is mounting for a connection between inherited thrombophilias and the severity of some complications of pregnancy. These thrombophilias increase the risk of maternal venous thromboembolism in pregnancy and the puerperium. Treatment of hematologic malignancies in pregnancy can present a difficult dilemma both in terms of staging studies and management. In many cases of Hodgkin lymphoma, treatment can be delayed safely until after delivery. In contrast, in aggressive lymphomas and acute leukemias rapid initiation of chemotherapy is often necessary to save the life of the mother. In general, the teratogenic effects of chemotherapy are greatest in the first trimester; however, care must be taken in later trimesters to avoid cytopenias of both mother and fetus at delivery. Hemorrhagic and thrombotic complications associated with pregnancy in females with essential thrombocythemia and polycythemia vera present a unique challenge because of the lack of controlled trials in these situations.
Acronyms and Abbreviations:
DDAVP, desmopressin acetate, a synthetic analogue of the pituitary hormone vasopressin; DIC, disseminated intravascular coagulation; ESR, erythrocyte sedimentation rate; ET, essential thrombocythemia; HELLP, hemolysis, elevated liver enzymes, low platelets syndrome; ITP, idiopathic thrombocytopenic purpura; PNH, paroxysmal nocturnal hemoglobinuria; PT, prothrombin time; PTT, partial thromboplastin time; PV, polycythemia vera; TTP, thrombotic thrombocytopenic purpura; VTE, venous thromboembolism; VWD, von Willebrand disease; VWF, von Willebrand factor.
BLOOD VOLUME, ERYTHROPOIETIN LEVEL, AND HEMOGLOBIN CONCENTRATION
Maternal blood volume increases by an average of 40 to 50 percent above the nonpregnant level.1 Plasma volume begins to rise early in pregnancy, with most of the escalation taking place in the second trimester and prior to week 32 of gestation.2 Red cell mass increases significantly beginning in the second trimester and continues to expand throughout pregnancy, but to a lesser extent than plasma volume.2 Erythropoietin levels increase throughout pregnancy, reaching approximately 150 percent of their prepregnancy levels at term.3,4 The overall effect of these changes in most women is a slight drop in hemoglobin concentration, which is most pronounced at the end of the second trimester and slowly improves approaching term.
The effect of pregnancy on maternal platelet count is somewhat more controversial; some studies demonstrate a mild decline in platelet count over the course of gestation,5 whereas others do not.6 In general, white cell counts rise during pregnancy with the occasional appearance of myelocytes or metamyelocytes in the blood.7 During labor and the early puerperium, there is a rise in the leukocyte count. Leukocytosis appears to be linearly related to the duration of labor.8
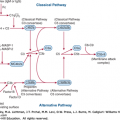
The levels of some plasma proteins also increase during pregnancy. In particular, C-reactive protein concentration is higher in pregnant women and rises even further during labor.9 Erythrocyte sedimentation rate (ESR) rises during pregnancy, and is affected by both hemoglobin concentration and gestational age.10 The rise in ESR during pregnancy, in large part a result of an increase in levels of plasma globulins and fibrinogen, makes its use as a marker of inflammation difficult. The levels of many of the procoagulant factors increase during pregnancy whereas activity of the fibrinolytic system diminishes in preparation for the hemostatic challenge of delivery. Plasma levels of von Willebrand factor (VWF), fibrinogen, and factors VII, VIII, and X all increase markedly, whereas factors II, V, IX, and XII are essentially unchanged and factor XIII declines.11 Levels of protein C and antithrombin remain stable throughout pregnancy whereas total and free protein S fall with increasing gestational age.12 Fibrinolysis is also impaired by increases in plasminogen activator inhibitors I and II, the latter a product of the placenta.13
ANEMIA IN PREGNANCY
Worldwide, the contribution of anemia to maternal and fetal morbidity and mortality is well recognized; in some parts of Africa, more than 75 percent of pregnant women are anemic, and there is a significant correlation between maternal mortality and anemia.14 It has been suggested that iron deficiency may protect against placental malaria, but epidemiologic studies have not been conducted to verify this supposition.15 In pregnant women, anemia is defined as a hemoglobin concentration of less than 11 g/dL in the first and third trimesters, and less than 10.5 g/dL in the second trimester.15 In both the industrialized and the developing world, iron-deficiency anemia (Chap. 43) is the commonest cause of anemia.16 On average approximately 1 g of iron is required during a normal pregnancy; 300 mg of iron are required by the fetus and the placenta, whereas expansion of the maternal red cell mass requires 500 mg, and 200 mg are lost via excretion.17 These requirements exceed the iron storage of most young women and in general cannot be met by the diet. Even in cases of maternal iron deficiency, the fetal requirements for iron are always met; thus there is no correlation between the hemoglobin of the fetus and that of the mother.18
Iron-deficiency anemia during the first two trimesters of pregnancy is associated with a twofold increased risk for preterm delivery and a threefold increased risk for delivery of a low-birth-weight infant.19 However, a large randomized trial comparing routine iron prophylaxis in pregnancy versus iron supplementation given only as needed demonstrated no significant differences in adverse maternal or fetal outcomes.20 As in nonpregnant individuals, iron-deficiency anemia can generally be diagnosed using laboratory values such as serum ferritin, and transferrin saturation levels (Chap. 43). Pica, the ingestion of nonnutritive substances, is said to be more common among iron-deficient pregnant women than among other populations with iron deficiency. Ice, clay or dirt, and starch are the most frequent substances ingested (Chap. 43); to some extent, however, the choice appears to be cultural and much more widespread than most practitioners realize.21
Apart from iron deficiency, folate deficiency is the next most frequent nutritional deficiency leading to anemia in pregnant women. In the United States, where foodstuffs are supplemented with folate and the level of awareness of the association between folate deficiency and neural tube defects in the embryo is high, folate deficiency is relatively unusual. Folate requirements in pregnancy are roughly twice those in the nonpregnant state (800 mcg/day vs. 400 mcg/day), and if diet is insufficient may exceed the body’s stores of folate (5–10 mg) relatively quickly.22 Anemia related to folate deficiency most often presents in the third trimester and responds to folate supplementation with reticulocytosis within 24 to 72 hours.16 Reports of severe pancytopenia and even states resembling the HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome as a result of folate deficiency in pregnancy have appeared in the literature.23,24 Despite these case reports, a review of 21 trials measuring the effect of folate supplementation on biochemical and hematologic parameters and pregnancy outcome (excluding neural tube defects) revealed improvement in low hemoglobin level in late pregnancy, but had no measurable effect on any substantive measures of pregnancy outcome (Chap. 41).25
Vitamin B12 (cobalamin) deficiency during pregnancy is rare, in part because deficiency of this vitamin leads to infertility. Serum cobalamin levels are known to fall during pregnancy.26 A shift from the serum to tissue stores is proposed to account for the drop in serum B12 levels. However, values less than 180 pmol/L usually are not observed in healthy women, and these low-normal levels are not accompanied by increased levels of methylmalonic acid, an indicator of cellular deficiency of cobalamin (Chap. 41).27
A rare cause of anemia in pregnancy is pure red cell aplasia (Chap. 36). In pure red cell aplasia, anemia tends to occur early in pregnancy and often resolves within weeks of delivery. The pathogenic mechanism leading to red cell aplasia does not appear to be transferred to the fetus, but does tend to recur in subsequent pregnancies.28,29 Conservative treatment, if feasible, is probably best until delivery; successful prenatal treatments with glucocorticoids and with intravenous immunoglobulin have been reported.30,31
BLEEDING DISORDERS AND CAUSES OF THROMBOCYTOPENIA
Bleeding disorders in pregnancy require consideration of maternal bleeding and hemorrhagic complications in the newborn. Data on the fetus are often lacking, and the practitioner must base decisions on past experience and the mother’s previous reproductive history.
Life-threatening bleeding is seen with some pregnancy-unique complications, resulting in disseminated intravascular coagulation (DIC). Because of the changes in coagulation factor levels, D-dimer, and platelet count during pregnancy, the normal range for tests routinely used to diagnose DIC in a nonpregnant state cannot be extrapolated directly to DIC in pregnancy. Serial measurement of the prothrombin time (PT), partial thromboplastin time (PTT), D-dimer, and fibrinogen are likely to be more helpful than measuring a single value.32 The DIC score developed by the International Society on Thrombosis and Hemostasis has been modified for pregnancy and this score may be more useful in identifying DIC.33
Complications of pregnancy that lead to DIC include placental abruption, a retained dead fetus, and amniotic fluid embolism (Chap. 129). Although amniotic fluid embolism is a significant cause of maternal death in developed countries, the mortality decreased from 86 percent in 1979 to less than 30 percent in 1994 and 1995, perhaps from a better supportive therapy.34 Amniotic fluid embolism is most likely to occur in older multiparous women whose pregnancies have gone beyond the 40th week and during tumultuous labor. Amniotic fluid enters the maternal circulation through tears in the chorioamniotic membranes, injury to the uterine veins, or uterine rupture. Its onset is heralded by maternal vascular collapse with dyspnea, hypotension, and cardiac arrhythmias followed by DIC that is manifested by oozing from intravenous lines, hematuria, hemoptysis, and excessive uterine bleeding. Atypical presentations have also been reported in which there is rapid deterioration of the fetus, followed by maternal respiratory and cardiovascular deterioration with development of DIC.35
In amniotic fluid embolism, DIC appears to involve an abnormal host response to exposure to various foreign antigens with the subsequent release of endogenous mediators which drive the clinical manifestations.36 Treatment is not significantly different than in other cases of DIC with bleeding (Chap. 129); however, there are some reports of successful management with uterine artery embolization.37
Placental abruption has also led to development of DIC, and the spectrum of hemostatic failure is broad and appears to be related to the degree of placental separation.38 Volume resuscitation, delivery of the fetus, and infusion of blood products to correct the maternal coagulation defect are indicated. Regional anesthesia is contraindicated because of the risk of bleeding in the epidural space and of the pooling of blood in the lower limb vascular bed, which could worsen hypovolemia.38 Fetal trophoblast cells have distinct properties which may activate coagulation including expression of tissue factor, suppression of fibrinolysis, and exposure of anionic phospholipids.39 Finally, intrauterine fetal death can also lead to DIC. Thromboplastic substances and specifically tissue factor released from dead fetal tissues into the maternal circulation are thought to trigger DIC; however, this is not usually detectable by laboratory tests until 3 or 4 weeks after fetal demise. Overt DIC is present in approximately 50 percent of women who retain a dead fetus for 5 weeks or longer.40
Although von Willebrand disease (VWD) is transmitted in an autosomal dominant fashion, women appear to be disproportionately affected with bleeding symptoms, primarily menorrhagia and postpartum hemorrhage (Chap. 126). In normal women and in types 1 and 2 (but not type 3) VWD patients, levels of factor VIII and VWF rise during pregnancy, with the most pronounced increase in the third trimester.39 As a result, prophylactic administration of VWF-containing factor concentrates at delivery is often unnecessary in type 1 and type 2 VWD patients; however, the risk of postpartum hemorrhage is significant (13–29 percent) as levels fall rapidly after birth.41 Thus in type 1 patients, factor VIII levels should be tested not only late in the third trimester, but also for 1 to 2 weeks postpartum. These patients should be monitored for increases in menstrual blood flow for at least 1 month. Risk of bleeding appears to be minimal when factor VIII levels are greater than 50 U/dL. There are several reports of severe thrombocytopenia developing late in pregnancy in patients with type 2B VWD,42,43 and at least one of these patients developed a pulmonary embolus while receiving cryoprecipitate for postpartum hemorrhage. Despite the possible risk of thrombosis, these patients may require treatment with plasma-derived VWF-containing concentrates at delivery or postpartum if there is abnormal bleeding, and with platelets if thrombocytopenic bleeding is not controlled with infusion of VWF concentrate. Type 3 VWD patients require infusion of a plasma-derived VWF-containing concentrate at delivery, typically 40 to 80 IU/kg, followed by doses of 20 to 40 IU/kg daily for a week then tapered over the next few weeks.44 Use of desmopressin acetate (DDAVP) antepartum is controversial because of the theoretical risk of vasoconstriction and placental insufficiency and the risk of maternal hyponatremia. Guidelines for management of VWD at delivery and during the puerperium have been published and are also reviewed in Chap. 126.45,46
Carriers of hemophilia A and B generally have factor levels approximately 50 percent of normal; however, a wide range of values have been reported as a result of random inactivation of the X chromosome (Chaps. 10 and 123).47,48 Ideally, carriers are identified before pregnancy when prenatal counseling can be offered. Baseline factor levels should be tested at the first visit during pregnancy and again in the third trimester, but it should be noted that factor IX levels generally do not rise during the course of the pregnancy.47 The sex of the fetus should be determined to guide the obstetrician at delivery. With the recognition that maternal serum contains cell free fetal DNA, genomic strategies have been developed to determine fetal gender as early as 7 weeks of gestation. Similarly, strategies to determine whether a male fetus is affected by hemophilia based on testing of maternal blood have now been developed and will doubtless enter the clinical arena in the near future.49 Cranial hemorrhage is the commonest site of bleeding in newborns with severe hemophilia, and has the highest potential for long-term serious sequelae. Risk factors for cranial hemorrhage include prolonged labor and use of instruments during delivery.48 To protect a potentially affected or known hemophiliac fetus, vacuum extraction should be avoided at delivery and forceps should be used only with caution. All intramuscular injections should be withheld from the newborn until hemophilia testing is completed. If an infant’s hemophilia status is not known, testing should be done on cord blood to avoid potential bleeding or bruising after a blood draw.48 The mother’s factor level should be followed for a few days after delivery and menstrual bleeding should be monitored to ensure adequate hemostasis.
There is also an association between pregnancy and acquired hemophilia caused by factor VIII autoantibodies (Chap. 127). This condition usually appears 1 to 4 months postpartum, but emerges during pregnancy in up to 14 percent of patients.50 In general, the Bethesda titer of the inhibitor is low and in most cases the inhibitor disappears spontaneously. Inhibitors can recur in subsequent pregnancies.51
Rarely, pregnant women with factor deficiencies other than factors VIII and IX may be identified. The most important of these to recognize is deficiency of factor XIII, which is associated with habitual hemorrhagic abortions and postpartum hemorrhage. In rare pregnancies reaching term, bleeding complications, including intracranial hemorrhage in the infant, have been observed.52,53 Treatment of this deficiency with fresh-frozen plasma, cryoprecipitate, or plasma-derived factor XIII concentrates (now available in the United States) prevents abortion in women, although there are no controlled studies.54 Most authorities recommend more frequent prophylactic therapy during pregnancy (every 3 weeks vs. every 5–6 weeks) with booster doses during labor or before cesarean section to ensure a level of 5 percent or greater.55 Although rare, congenital afibrinogenemia, hypofibrinogenemia, and dysfibrinogenemia (Chap. 125) can cause hemorrhagic and thrombotic pregnancy complications. Most experts recommend fibrinogen replacement (using cryoprecipitate or fibrinogen concentrate) to maintain a level of 60 to 100 mg/dL during pregnancy and for 6 weeks postpartum.56
Thrombocytopenia in pregnancy is relatively common, with up to 5 percent of all pregnant women exhibiting asymptomatic thrombocytopenia.57 Many causes of thrombocytopenia in pregnancy are identical to those seen in the nonpregnant state, with some predisposing to bleeding whereas others predispose to clotting. However, there are several conditions leading to thrombocytopenia that are unique to pregnancy, including gestational thrombocytopenia, preeclampsia/HELLP syndrome/eclampsia, and acute fatty liver of pregnancy.
Gestational thrombocytopenia and idiopathic thrombocytopenic purpura (ITP) are best discussed together as they can be difficult to differentiate and, in fact, may be two extremes of a spectrum of disease. In general, gestational thrombocytopenia is asymptomatic and is said to occur later in pregnancy and be less severe than ITP. Most sources suggest that gestational thrombocytopenia occurs in the second and third trimesters, with platelet counts rarely falling below 70,000/μL.58 Gestational thrombocytopenia can sometimes be diagnosed with certainty only after delivery; usually there is no past history of low platelets, except perhaps with previous pregnancies, the platelet count returns to normal after delivery, and there is no association with fetal thrombocytopenia. It is not clear whether or not gestational thrombocytopenia is a variant of immune-mediated platelet destruction (Chap. 117).58
In contrast to gestational thrombocytopenia, ITP can occur at any point in pregnancy and the fall in platelet count can be severe. Diagnosis is essentially the same as it would be in any patient in that alternative causes of thrombocytopenia must be ruled out. As in other cases, treatment of ITP in pregnancy must take into account the severity of the thrombocytopenia and the presence or absence of symptoms. In general, platelet counts less than 10,000/μL require treatment regardless of the trimester; platelet counts of 30,000 to 50,000/μL without bleeding require no treatment, and platelet counts of 10,000 to 30,000/μL in later trimesters or in the presence of bleeding require treatment. Although glucocorticoid and intravenous immunoglobulin are safe in pregnancy, they may have no effect on fetal counts and should only be used to treat the mother.59 Splenectomy for ITP in pregnancy is best done in the second trimester if platelet counts are extremely low and unresponsive to treatment.58 One small study evaluated the safety of anti-D antibodies during pregnancy; all 10 of the women studied achieved a platelet count greater than 30,000/μL, but larger studies are needed before this intervention can be recommended.60 Similarly, there are case reports of rituximab administration for treatment of refractory ITP in pregnancy; at least one report demonstrated transient inhibition of neonatal B-lymphocyte development.61 There are no adequate and well-controlled studies of either eltrombopag or romiplostim in pregnant women and both are considered pregnancy category C drugs. In animal studies, both drugs crossed the placental and fetal effects included thrombocytosis, postimplantation loss, increase in fetal mortality, but no major structural malformations were reported.62 Case reports describing the use of romiplostim in pregnancy have appeared and in one report the newborn had severe thrombocytopenia at birth complicated by intracranial hemorrhage.63 Maternal platelet counts of greater than 50,000/μL usually are safe for both vaginal and cesarean delivery. In most cases, spinal anesthesia should not be used if the platelet count is less than 75,000/μL.64 Less than 5 percent of babies born to mothers with ITP have platelet counts less than 20,000/μL, although there does seem to be some correlation between very severely depressed maternal platelet count and severe thrombocytopenia in the newborn.65 No clear recommendations can be given for measuring fetal platelet count prior to or at delivery as measurements are fraught with error; however, if the fetal platelet count is known to be less than 20,000/μL, cesarean section is probably reasonable. Newborns of mothers with ITP should be monitored for 5 to 7 days after delivery to ensure that the platelet count does not drop (Chap. 117).
The spectrum of hypertensive disorders of pregnancy ranging from preeclampsia to severe preeclampsia and HELLP syndrome to eclampsia (see Chap. 51) may also result in thrombocytopenia, although thrombosis is more of an issue than is bleeding. There is some debate in the literature as to whether thrombocytopenia can be diagnosed in preeclampsia without HELLP syndrome; however, data from one large study57 indicated that approximately 15 percent of cases of preeclampsia are complicated by thrombocytopenia. In general, the symptoms of preeclampsia, including hematologic manifestations, resolve with delivery; however, in a small proportion of cases they persist, worsen, or even develop immediately postpartum. When symptoms persist postpartum, the differentiation from thrombotic thrombocytopenic purpura (TTP)/hemolytic uremic syndrome becomes more difficult. Some data suggest that maternal recovery from the HELLP syndrome is accelerated by administration of intravenous dexamethasone66; however, a meta-analysis demonstrated no clear advantage to the use of glucocorticoids to decrease maternal or perinatal morbidity or mortality.67 A collaborative randomized controlled trial of glucocorticoids in HELLP syndrome (COHELLP) is underway to determine the effectiveness of dexamethasone to accelerate the postpartum recovery of patients with class I HELLP syndrome.68 Observation or treatment of HELLP with glucocorticoids alone postpartum should probably not persist beyond the third postpartum day. If the patient is not clearly improving, plasma exchange should be initiated as one would do for thrombotic thrombocytopenic purpura (TTP).69,70 Although not associated with hypertension, acute fatty liver of pregnancy is another rare disorder that can present in the third trimester with severe liver dysfunction, but thrombocytopenia, if present, is generally mild and does not require treatment (Chaps. 51, 117, and 129).
THROMBOPHILIA
Pregnancy is a prothrombotic state. Inherited prothrombotic conditions contribute to 50 percent of the cases of venous thromboembolism and pulmonary embolism, as well as to stroke in pregnancy and the puerperium. Hereditary thrombophilias (Chap. 130) may also predispose to fetal loss through placental vascular disorders. The best evidence for an association between a thrombophilia, albeit acquired, and recurrent fetal loss exists for antiphospholipid antibody syndrome in which the association between the antibodies and pregnancy loss has been recognized for more than 20 years.71 As many as 20 percent of women with recurrent fetal loss have antiphospholipid antibodies,72 and studies show that without treatment up to 90 percent will experience fetal loss.73 One study suggests that poor pregnancy outcomes occur more frequently in primary antiphospholipid syndrome when patients have more than one positive laboratory test (lupus anticoagulant, immunoglobulin [Ig] G/IgM anticardiolipin, IgG/IgM antihuman β2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree