INTRODUCTION
Head and neck cancers are a diverse group of diseases, each with distinct epidemiologic, anatomic, and pathologic features. The natural history and treatment considerations may vary widely. In this chapter, our focus is on the primary management of squamous cell carcinomas (SCCs) of the head and neck (HNSCC). In recent years, we have observed significant advances in diagnosis and treatment and recognition of the human papillomavirus (HPV) as a significant causative agent and prognostic factor for cancers of the oropharynx. Tumor imaging is increasingly precise. Primary therapy eradicates disease in a majority of patients with early-stage HNSCC, and the long-term management of these patients currently involves an emphasis on general medical care, avoiding known carcinogens such as alcohol and tobacco, and participation in chemoprevention strategies to reduce the risk of second primary tumors. Therapy for patients with locally advanced disease is multimodal, and success has been achieved in improving local tumor control, disease remission, organ preservation, and overall survival. The integration of chemotherapy and novel “targeting” systemic treatment approaches with surgery and/or radiotherapy is under study and discussed.
EPIDEMIOLOGY
In the United States, HNSCC is estimated to represent approximately 3% (46,000) of new cancer cases and 2% (9,000) of cancer deaths in 2015 (1). However, the disease is more common in many developing countries, with a worldwide annual incidence of more than 500,000 (2).
The risk of developing head and neck cancer increases with age; most patients are older than age 50. There has been a clearly demonstrated association with tobacco and alcohol use. Molecular studies provide evidence that carcinogens found in these substances have a causal role. The prevalence and spectrum of p53 mutations are prominent in cancers of patients with a history of tobacco and alcohol use (3). Cancers of the oral cavity, larynx, and hypopharynx are uncommon in persons with no smoking history.
Human papillomavirus infection is now widely accepted as another etiologic factor for HNSCC. In the United States, more than 50% of cancers arising in the oropharynx, particularly in the palatine tonsils and tongue base, harbor oncogenic HPV (4). The incidence of oropharyngeal cancer in the United States is increasing, primarily due to HPV-associated cases in men (5). It appears that the HPV-positive oropharyngeal malignancy represents a distinct clinical and pathologic subgroup of HNSCC, with poorly differentiated basaloid histopathology (6) and marked tumor responsiveness to radiation and chemotherapy. Moreover, HNSCCs with transcriptionally active HPV-16 DNA are characterized by occasional chromosomal loss, whereas those lacking HPV DNA typically have gross deletions, involving chromosomal arms known to be abnormal in HNSCC (7). Thus, HPV-16 infection may be an early carcinogenic event. Patients with HPV DNA–positive tumors, particularly those associated with E6 and E7 proteins, have improved survival after chemoradiotherapy when compared to patients with HPV-negative tumors (8,9). A recent case-control study reported that HPV-16–positive HNSCCs were independently associated with several measures of sexual behavior and exposure to marijuana but not with cumulative measures of tobacco smoking, alcohol use, or poor oral hygiene (10). These findings indicate that HPV-16–positive HNSCC and HPV-16–negative HNSCC have different risk factor profiles. In addition to detection of oncogenic HPV DNA by in situ hybridization, tumor overexpression of p16 by immunohistochemistry serves as a surrogate for HPV association in oropharyngeal cancer with prognostic implications, particularly when coupled with patient smoking status (8). In addition, HNSCC patients with heavy tobacco and alcohol exposures are at high risk of developing multiple cancers, with “field cancerization” throughout the upper aerodigestive tract and bladder. The observation that treated head and neck cancer patients may have a high risk (estimated to be 3%-4% per year) of metachronous tumors has driven chemoprevention trials designed to reduce the risk of second primary tumors.
MOLECULAR PATHOGENESIS
The progression of HNSCC is thought to involve multiple stepwise alterations of molecular pathways in the squamous epithelium (11). Aberrations in the p53 and Rb tumor suppressor pathways are the most common molecular events, resulting in uncontrolled cell proliferation. Approximately 50% to 80% of HPV-negative tumor samples harbor a p53 mutation (12,13). The Rb pathway can be disrupted either through inactivation of the CDKN2A gene, which encodes p16, an inhibitor of cyclin-dependent kinase, or amplification or the CCND1 gene encoding cyclin D1 (14,15,16).
The epidermal growth factor receptor (EGFR) is overexpressed in invasive HNSCC in a majority of sample tumors (17). Binding to EGFR by its natural ligands, mainly epidermal growth factor or transforming growth factor α (TGF-α), prompts a conformational change in the receptor through dimerization, which results in subsequent autoactivation of the tyrosine kinase from the intracellular domain of the receptor. This process activates an intracellular signaling pathway, leading to the inhibition of apoptosis, activation of cell proliferation and angiogenesis, and an increase in metastatic spread potential (18).
In 2011, Stransky et al performed whole exome sequencing on 74 HNSCC tumor specimens (19). Not surprisingly, the mutational landscape of the disease is quite complex, and smoking-related HNSCC specimens had a mutation rate approximately twice that of HPV-positive HNSCC. Mutations in genes previously implicated in HNSCC were detected at a high rate, including TP53, CDKN2A, PTEN, PIK3CA, and HRAS, validating this approach. However, this unbiased approach also identified a high rate of mutations in numerous genes not previously linked to HNSCC. In approximately 30% of cases, mutations were identified in genes that regulate squamous differentiation, such as NOTCH1, IRF6, and TP63. Mutations in genes regulating apoptosis were also frequent events. These novel findings have provided the rationale for testing novel therapeutic targets such as NOTCH inhibitors.
There are numerous challenges to the development of molecularly targeted therapies in HNSCC, including frequent tumor suppressor loss and difficulty in identification of true driver mutations within a complex mutational landscape. However, an improved understanding of the molecular biology of this disease should facilitate discovery of new prognostic markers and therapeutic targets.
DIAGNOSIS AND STAGING
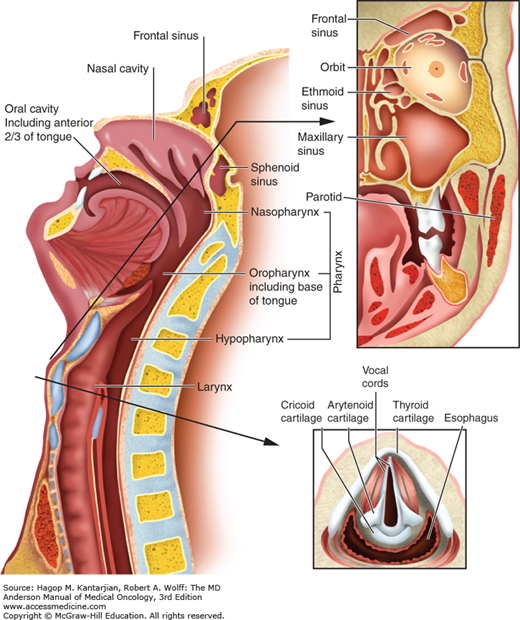
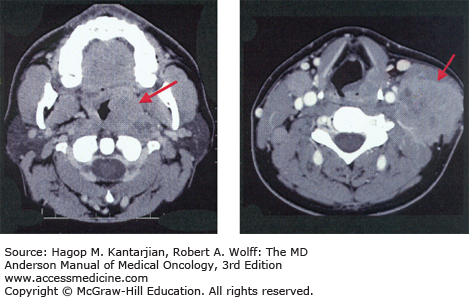
Optimal therapy and treatment outcomes depend on the precise identification of the primary tumor (Fig. 19-1) as well as the local, regional, and distant extent of disease.
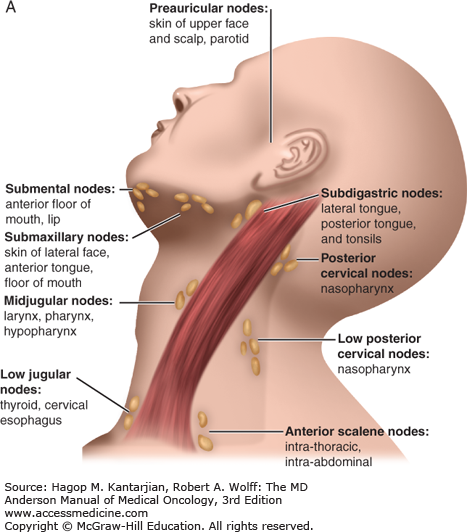
Patients with early-stage disease may present with vague symptoms and minimal physical findings, which is why a high index of suspicion for early diagnosis is needed, especially for tobacco users. A majority of patients will present with signs and symptoms of locally advanced disease, which vary according to the subsite in the head and neck. Sinusitis, unilateral nasal airway obstruction, and epistaxis may be early signs of cancers of the nasal cavity or paranasal sinuses. Otitis media that is recurrent or is refractory to antibiotics is an indication for a complete ear, nose, and throat evaluation to rule out a nasopharyngeal neoplasm. Chronic otalgia, dysphagia, odynophagia, and throat soreness lasting weeks may be the presenting symptoms of oropharyngeal or hypopharyngeal cancer. However, many patients with HPV-associated oropharyngeal cancer present with an otherwise asymptomatic neck mass and may have limited or no smoking history. Persistent hoarseness demands visualization of the larynx. Supraglottic laryngeal neoplasms do not usually present early, and a neck mass may be the presenting sign. Careful examination of lymph nodes in the facial, cervical, and supraclavicular regions is important because the anatomic patterns of lymphatic drainage may reflect the specific subsite of a head and neck primary tumor (Fig. 19-2) (20). Level 2/3 adenopathy, for example, suggests a primary cancer of the oral tongue or oropharynx, and posterior cervical adenopathy is frequently a result of regional spread of a nasopharyngeal tumor.
Physical examination should include careful inspection of the skin and oral/oropharyngeal mucosal surfaces; palpation of the tongue, floor of the mouth, and oropharynx; and systematic palpation of the neck. A complete examination also requires an indirect mirror examination of the oropharynx, hypopharynx, and larynx, complemented by fiberoptic endoscopy (21). Leukoplakia (white mucosal patches that cannot be removed by scraping) and higher risk erythroplakia (red or mixed red-white patches) are the most common premalignant lesions in the head and neck. Any suspicious surface in the oral mucosa should undergo biopsy.
Three-dimensional imaging with computed tomography (CT), magnetic resonance imaging (MRI), and/or ultrasonography is also needed to evaluate the extent of disease and to complete staging. Magnetic resonance imaging is the preferred local imaging modality for nasopharyngeal cancer. Because the lungs are the most common sites of distant metastases, a chest x-ray should be performed as well. Computed tomography scanning of the chest should be performed for symptomatic or high-risk patients. This would include patients with nasopharyngeal cancer or those with primary tumors of other sites presenting with N2b or greater nodal disease and low neck or supraclavicular metastases. Circulating tumor markers that would be reliable in early detection of HNSCC have not yet been identified.
Patients who present with a suspicious neck mass and no obvious primary mucosal lesion should undergo a systematic examination of the head and neck. Head and neck imaging may be helpful. If no obvious primary site is found, fine-needle aspiration of the mass may establish a diagnosis of cancer. Detection of HPV or Epstein-Barr virus DNA in a lymph node suggests a tumor of oropharyngeal or nasopharyngeal origin, respectively. If metastatic SCC is demonstrated, examination under anesthesia is often performed. Suspicious lesions are biopsied, and consideration is given to tonsillectomy and blind biopsies of the nasopharynx, base of the tongue, and hypopharynx, depending on the pattern of lymphadenopathy. Open biopsy of the neck mass may be performed if fine-needle aspiration biopsy and panendoscopy have failed to yield a diagnosis or in patients suspected of having an alternative process (eg, lymphoma). An experienced head and neck surgeon may be prepared to proceed with selective neck dissection if SCC of unknown head and neck primary origin is determined.
Staging criteria for head and neck cancers are based on the American Joint Committee on Cancer TNM staging system, which classifies tumors according to anatomic site and extent of disease (22). Head and neck primary tumor (T) staging is complex, varying with the primary subsite in the head and neck region. Classifications for lymph node (N) and distant metastases (M) are uniform for sites (Table 19-1) other than nasopharynx (22a).
| Primary Tumor (T) | |
|---|---|
| TX | Primary tumor cannot be assessed. |
| T0 | No evidence of primary tumor. |
| Tis | Carcinoma in situ. |
| T1 | Tumor ≤2 cm in greatest dimension. |
| T2 | Tumor >2 cm but not >4 cm in greatest dimension. |
| T3 | Tumor >4 cm in greatest dimension. |
| T4(lip) | Tumor invades through cortical bone, inferior alveolar nerve, floor of mouth, or skin of face, ie, chin or nose. |
| T4a | Tumor invades structures adjacent to the oral cavity (eg, through cortical bone, into deep [extrinsic] muscle of tongue [genioglossus, hyoglossus, palatoglossus, and styloglossus], maxillary sinus, skin of face). |
| T4b | Tumor invades masticator space, pterygoid plates, or skull base and/or encases internal carotid artery. |
| Note: Superficial erosion alone of bone/tooth socket by gingival primary tumor is not sufficient to classify a tumor as T4. | |
| Regional Lymph Nodes (N) | |
| NX | Regional lymph nodes cannot be assessed. |
| N0 | No regional node metastases. |
| N1 | Metastasis to a single ipsilateral lymph node ≤3 cm in greatest dimension. |
| N2 | Metastasis to a single ipsilateral lymph node >3 cm but not >6 cm in greatest dimension, or to multiple ipsilateral lymph nodes none >6 cm in greatest dimension, or to bilateral or contralateral lymph nodes none >6 cm in greatest dimension. |
| N2a | Metastasis to a single ipsilateral lymph node >3 cm but not >6 cm in greatest dimension. |
| N2b | Metastasis to multiple ipsilateral lymph nodes >3 cm but not >6 cm in greatest dimension. |
| N2c | Metastases to bilateral or contralateral lymph nodes none >6 cm in greatest dimension. |
| N3 | Metastasis in a lymph node >6 cm in greatest dimension. |
| Distant Metastasis (M) | |
| MX | Presence of distant metastasis cannot be assessed. |
| M0 | No evidence of distant metastasis. |
| M1 | Distant metastasis. |
NATURAL HISTORY AND IMPLICATIONS FOR THERAPY
Two-thirds of patients with HNSCC will present with stage III or IV disease. For patients with T1/2 disease (stage I/II), surgery or radiotherapy as a single modality is most often applicable and effective. Depending on the precise primary site and stage, a curative outcome will be achieved in 70% to 95% of cases. In patients with intermediate and locally advanced disease at diagnosis, combined treatment strategies have become the standard of care. Multimodal treatment plans are designed to balance competing goals of tumor eradication and organ preservation (11,23,24). Despite optimal local therapy, 30% to 50% of patients may develop local or regional recurrence, and nearly 20% to 30% are at risk for distant metastases depending on the primary site and staging.
Over 95% of endemic nasopharyngeal carcinomas (NPCs) are associated with EBV. Nasopharyngeal carcinoma tends to occur in younger persons and is not associated with tobacco use. Nasopharyngeal carcinoma is an aggressive neoplasm with cervical lymph node metastases present in 60% to 90% of patients at diagnosis. Because of unique anatomic, biologic, and clinical characteristics, therapy for NPC is distinctive. Radiotherapy is the mainstay of local treatment. The anatomy of the nasopharynx and tumor sensitivity to radiotherapy limit the role of surgery to obtaining the initial biopsy and, for selected patients, resection of residual lymphadenopathy after radiotherapy. Nasopharyngeal carcinomas are highly sensitive to chemoradiotherapy. Because of the proximity of the nasopharynx to normal critical structures of the central nervous system and given the propensity of NPC for skull base invasion, intensity-modulated radiation therapy (IMRT) is the usual radiation therapy technique for NPC, which improves tumor coverage, reduces xerostomia, and improves patient quality of life compared to traditional techniques (25,26). Locoregional tumor control following chemoradiotherapy approaches 90%.
For stages III and IV, concomitant chemotherapy and radiotherapy followed by adjuvant chemotherapy is the accepted standard of care based on the Head and Neck Intergroup NPC 0099 trial (27). Compared to radiation alone, chemoradiation with cisplatin (100 mg/m2 on days 1, 22, and 43), followed by adjuvant chemotherapy with cisplatin and 5-fluorouracil (5-FU) demonstrated significant improvement in 3-year survival (78% vs 47%). Chan et al (28) have also demonstrated the efficacy of concomitant radiation and weekly cisplatin 40 mg/m2. Phase III studies investigating the value of induction chemotherapy are under way. The NRG-HN001 study is an ongoing phase II/III study investigating the value of measuring plasma EBV DNA as a marker of the efficacy of concomitant chemoradiotherapy. With undetectable DNA, patients are randomized to observation or adjuvant chemotherapy. Patients with detectable DNA after chemoradiotherapy receive additional treatment, testing an alternative regimen consisting of paclitaxel and gemcitabine versus cisplatin and 5-FU.
The majority of oral cavity neoplasms occur in the anterior two-thirds of the tongue (oral tongue) and the floor of the mouth. Surgical resection, often with postoperative radiotherapy, is the most common and effective local treatment approach (11,24). Depending on site and tumor volume, early cancers should be resected but may be treated with radiotherapy. Local tumor control rates of patients with stage I and II tumors are 80% to 90% and 50% to 80%, respectively (29). For deeply invasive T1/2 disease, we favor surgical resection and neck dissection with postoperative concomitant chemoradiotherapy for selected patients with narrow margins or nodal metastases, particularly if there is extracapsular spread. Perineural invasion is also a significant negative prognostic sign. Forty percent of patients present with clinically evident lymph nodes, and bilateral nodal involvement is not uncommon. Although primary surgical approaches are preferred at our center, interstitial radiotherapy (brachytherapy) has been used in combination with external-beam therapy for selected cases to achieve higher control rates than external radiation alone.
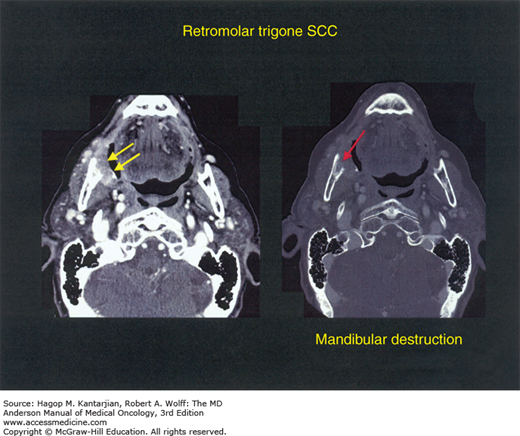
For patients with locally more advanced disease (Fig. 19-3 shows an example of a patient with a retromolar trigone primary tumor invading bone, T4), surgery followed by radiation therapy (or chemoradiotherapy) is the most widely accepted approach. At the University of Texas MD Anderson Cancer Center (MDACC), selective neck dissections are routinely performed for patients with stages II to IVa disease.
The most common cancers of the oropharynx are of the base of tongue and tonsils, and an increasing percentage of these are HPV associated, with an improved prognosis. In an unplanned, post hoc analysis of RTOG 0129, Ang et al classified oropharyngeal cancer patients treated with concurrent chemoradiotherapy in to three risk-of-death groups using recursive partition analysis. The “low-risk” group were those with p16 positive tumors and minimal smoking history (94% 3-year overall survival). Conversely, the “high-risk” group were those characterized mostly by p16 negative tumors and greater smoking intensity (42% 3-year overall survival) (8). Radiation therapy serves as the principal treatment modality for the majority of oropharyngeal malignancies and is used as a single modality for T1 and many T2 tumors. Local control is obtained in over 90% of patients (30). Regional lymph nodes are treated in all cases, and unilateral neck radiation is considered for well-lateralized earlier-stage tonsillar primaries, which reduces greatly the radiation dose to the contralateral parotid gland and key swallowing structures. Given recent technical advances and the popularity of minimally invasive transoral approaches, surgical resection for oropharyngeal cancer is being performed more frequently at many centers, and this approach is currently under study (Eastern Cooperative Oncology Group [ECOG] 3311). However, adjuvant radiation or chemoradiation may also be required, depending on the surgical pathology findings.
Concomitant chemoradiotherapy using IMRT is the current standard of care for patients with locally advanced disease. Under study, protons have unique physical properties compared with x-rays or photons due to the Bragg peak, where most of the proton dose is delivered at a finite depth, thus reducing dose to certain nontarget structures. Investigators at MDACC are currently conducting a phase II/III randomized trial of concomitant chemotherapy with intensity-modulated proton therapy vs IMRT for stage III/IV oropharyngeal cancer. Objectives are to compare tumor control and long-term toxicity.
With 75% of lesions occurring in the pyriform sinus, carcinoma of the hypopharynx is relatively uncommon but virulent (Fig. 19-4). Small-volume disease may be treated with surgery or radiation, but later-stage disease requires multimodal therapy. At presentation, more than 75% of patients have advanced disease (T3 or T4). The overall 5-year survival rate is lower than 30%. For many patients, surgical treatment also requires removal of the larynx. The European Organization for Research and Treatment of Cancer (EORTC), in a phase III trial, demonstrated that laryngeal preservation with sequential chemoradiotherapy is a feasible alternative to radical surgery for many patients with locally advanced disease (31). In a more recent trial of patients with resectable advanced SCC of the larynx or hypopharynx, Lefebvre et al (32) compared sequential treatment with two cycles of cisplatin and 5-FU followed by radiotherapy with an arm of four cycles of cisplatin and 5-FU administered during weeks 1, 4, 7, and 10, alternating with radiotherapy. Survival with a functional larynx was similar in both arms, as was overall survival (median, 4.4 vs 5.1 years, respectively). Please see the “Organ Preservation” section for further discussion.
Given the critical role of the larynx in communication, swallowing, respiration, and airway protection, organ preservation to maintain functional status and quality of life has been the focus of laryngeal cancer treatment since the 1970s. The most widely used treatment of T1 and T2 cancers of the larynx is radiotherapy, which has demonstrated control rates greater than 90% for T1 disease and approximately 70% to 80% for T2 tumors (33). For carefully selected patients with intermediate-stage disease, sequential chemotherapy followed by radiation and surgical salvage, if needed, showed equivalent survival outcomes compared with surgery in the Veterans Affairs (VA) laryngeal study (34). Although the rate of local failure is higher with organ preservation approaches, salvage surgery is effective, and this approach allows 60% of patients to preserve organ function (35). Larynx cancer treatment strategies are discussed in detail in the “Organ Preservation” section.
Tumors of the salivary glands are uncommon, with approximately 5,000 cases per year in the United States. Histologies are diverse, and risk factors are poorly defined, although radiotherapy may be causative. The age range of patients affected is broad. Many salivary neoplasms are benign, often involving the parotid gland, accounting for approximately 80% of parotid tumors, 50% of tumors arising in submandibular glands, and 25% of tumors arising in minor salivary glands.
Table 19-2 lists primary salivary malignancies.
Primary treatment depends on tumor extent and histology. Notably, parotid lymphadenopathy may reflect metastatic involvement by squamous cancers of the scalp or melanomas, and this must be borne in mind when evaluating these patients. Following a complete head and neck evaluation, consideration may be given to CT imaging of chest and a bone scan because these are common metastatic sites.
Surgical resection is the fundamental primary treatment for most patients, and the approach will be influenced by the primary histology (36). Adenoid cystic carcinoma (ACC) tends to track along nerves and may involve structures of the skull base, an important consideration in surgical and radiation therapy planning. C-kit is overexpressed in ACC (37). Lymph node metastases are uncommon. Low-grade mucoepidermoid carcinomas tend to be localized and are most often treated by surgery alone. High-grade mucoepidermoid carcinomas carry a much higher risk of lymph node and distant metastases. Salivary ductal carcinomas may be high grade and share biomarker characteristics, such as estrogen or progesterone receptor and HER2/neu overexpression, with breast cancer.
As a generalization, large tumors or those with close surgical margins will require postoperative radiotherapy. Postoperative concomitant chemoradiation is now under study (Radiation Therapy Oncology Group [RTOG] 1008) in a randomized trial of high-risk patients and is a consideration for patients with good performance status with locally advanced resectable disease.
For the palliative treatment of patients with recurrent disease not amenable to further local treatment or those with distant metastases, treatment with systemic chemotherapy, most often with a platinum-based combination, is an option (38). Cisplatin, 5-FU, cyclophosphamide, and doxorubicin are active compounds. The taxanes also have activity, although not demonstrated in patients with ACC. Combinations may be more effective, with response rates ranging from 20% to 30%. Salivary ductal cancers are much more sensitive to chemotherapy than ACC.
Treatment goals in the setting of distant metastatic disease are palliative because there has not been an overall survival advantage with chemotherapy. See Table 19-3 for a listing of tumor markers in salivary cancer that have prompted clinical trials. EGFR, KIT, HER2, and AR are prospective targets for systematic study. Fibroblast growth factor receptor (FGFR) expression (39) and MYB-NFIB fusion oncogene (40) have been identified in subsets of ACC. In a phase II trial, lapatinib, a dual inhibitor of EGFR and erbB2 tyrosine kinase activity, showed biologic activity in ACC (41).
| Histology | EGFR Expression | EGFR Mutation | HER2 Expression | HER2 Amplification | c-kit Expression | Androgen Receptor Expression |
|---|---|---|---|---|---|---|
| Adenoid cystic cancer | Yes | Rare | Rare | No | Yes | Rare |
| Mucoepidermoid cancer | Yes | No | Yes | Uncommon | Rare | Uncommon |
| Adenocarcinoma | Yes | — | Yes | Uncommon | Rare | Uncommon |
| Salivary duct cancer | Yes | — | Yes | Yes | Rare | Yes |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree