Since the early 1980s T-cell depletion has allowed haploidentical bone marrow transplantation to be performed in patients with primary immunodeficiency for whom a matched sibling donor was not available, without causing severe graft versus host disease (GVHD). This review article presents the available data in the literature on survival, GVHD, and immune reconstitution in different categories of patients, with special emphasis on the impact of different T-cell depletion methods.
Primary immunodeficiency (PID) describes a group of inherited disorders characterized by impairment of innate or adaptive immunity. Although rare, PID commonly leads to lethal complications mainly as a result of severe and recurrent infections. In the last 50 years there has been enormous progress in understanding and identifying the genetic variability causing PID, with more than 150 different primary immune deficiency syndromes known today. Despite this variability in etiology, especially for severe cases of immune deficiency, hematopoietic stem cell transplantation (HSCT) remains the major curative treatment to correct the immunodeficiency and reverse the predicted poor prognosis. The first HSCT for treatment of severe combined immunodeficiency was performed in 1968 using a bone marrow donation from an human leukocyte antigen (HLA) identical sister to correct the immune function of an infant with severe combined immunodeficiencies (SCID). However, because of the lethal complication of graft versus host disease (GVHD), the procedure could be employed only in patients who had an HLA identical donor. It was 12 years later that successful stem cell selection, using differential agglutination with soybean agglutinin (SBA) and subsequent T cell depletion (TCD) with sheep red blood cells (SRBC), made it possible to perform lifesaving stem cell transplantations using a haploidentical stem cell donor, without causing lethal GVHD.
T cell depleted stem cell transplantation
Stem cell transplantation offers a curative treatment for many patients with a severe form of immune deficiency, as well as malignant and nonmalignant hematologic disorders. As an identical, related, stem cell donor is available only in a small minority of cases (15%–20%), alternative donors are needed. The 2 alternative options are allogeneic stem cell transplantation from an unrelated identical donor and haploidentical related donor (in which the donor and recipient share only 1 of 2 possible HLA haplotypes). For the first option, despite the world registry network, the odds of finding a matched unrelated donor in the registries varies with the patient’s race and ranges from approximately 60% to 80% for whites to less than 10% for ethnic minorities. Another major disadvantage is the time required to identify a donor from a potential panel; the severely immune compromised patient may succumb to severe infectious complications during this time. Furthermore, with the development of molecular analysis, close matching has become more accurate in an attempt to reduce the risk of GVHD but, at the same time, the chance of finding a suitable matched donor reduces even more. For these reasons, allogeneic identical stem cell transplantation is not available for most treatment candidates. On the other hand, virtually all patients have a readily available haploidentical family member. Using a full haplotype mismatched related donor offers several significant advantages:
- 1.
Immediate donor availability
- 2.
Immediate access to donor-derived cellular therapies if required after transplantation, including repeated transplantation from the same donor or from the other haploidentical parental donor in case of graft failure
- 3.
Ability to select the donor of choice out of several available relatives by their clinical status and natural killer (NK) alloreactivity.
However, the use of haploidentical donors has presented a major challenge in the past 4 decades, because of life-threatening GVHD. Following a lead originally attributed to Delta Uphoff, the possibility that fetal liver at the appropriate time in development, lacking post-thymic immunocompetent cells, could be used as a source of stem cells without producing GVHD was studied. Later, similar results were achieved using splenocytes from neonatally thymectomized mice to protect lethally irradiated mice without causing GVHD.
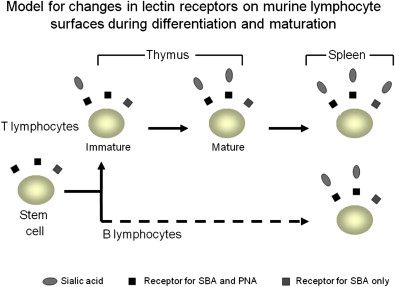
Further work in the late 1970s using specific anti-T cell antibodies in mice or antilymphocyte antibodies in rats demonstrated that TCD can effectively enable radioprotection without GVHD. Furthermore, as many as 0.3% of mature T cells in the donor graft can cause a high incidence of lethal GVHD. In parallel with this work, we have demonstrated that the lectin SBA can effectively separate hemapoietic stem cells from T cells, to enable successful bone marrow transplantation in lethally irradiated mice from fully disparate donors. This procedure was established based on the earlier observation that the peanut agglutinin (PNA) and SBA, which are exposed on hematopoietic stem cells (HSCs), are masked by sialic acid in the maturation process in the thymus medulla ( Fig. 1 ).
It was then clear that the key to successful haploidentical stem cell transplantation is dependent on a highly T cell depleted stem cell graft.
Based on the initial work on rodents with SBA we further tested our ability to purify bone marrow stem cells from mature T cells in primates. Although a modification of the separation protocol was needed, a successful TCD was achieved and an allogeneic T cell depleted stem cell transplantation was fully engrafted without causing GVHD.
In 1980, following further adaptation to human bone marrow to attain more than 3 log TCD, this approach finally enabled us to perform an allogeneic haploidentical bone marrow transplantation on a 10-month-old girl with acute leukemia. Eight weeks after transplantation of a haploidentical paternal graft, the girl showed complete recovery of the stem cell graft with T cells and other marrow cells of the donor type, without any evidence of GVHD in the absence of GVHD prophylaxis. In parallel, this method was used successfully in 1980 for the treatment of the first SCID patient with his mother’s bone marrow.
The era of T cell depleted stem cell transplantations had begun.
Methods of TCD
Three major TCD methods have been used successfully for the treatment of PID patients.
Stem Cells Fractionation by Lectins
Lectins are sugar-binding proteins that are highly specific for their carbohydrate moieties. Stem cell fractionation using lectins is based on the principle that cell subpopulations expressing different lectin receptors can be separated by differential agglutination. Of the different lectins used for cell separation and identification, plant lectin SBA was shown to be useful in the separation of bone marrow cells and depletion of mature T cells. After several modifications, an efficient and rapid technique was developed for the large-scale removal of T lymphocytes from human bone marrow. The method achieved a highly purified cell population rich in blast cells and all myeloid precursors but depleted of T lymphocytes. Briefly, there are 4 main steps in this process : (1) selective removal of red blood cells (RBCs) by gravity sedimentation; (2) agglutination with SBA and differential sedimentation of the agglutinated (SBA + ) cells; (3) removal of E-rosette forming T cells from the SBA − cell fraction by centrifugation over Ficoll (RBCs form around human T cells on incubation); (4) repetition of the E-rosetting step using neuraminidase-treated sheep RBCs to eliminate residual T cells in the SBA − E − fraction. The cells collected at the end of the process (SBA − E − E N − ) were used for transplantation.
Following the successful transplantation of a haploidentical stem cell graft in a patient with acute leukemia as described earlier, this method was used in the treatment of patients with SCID using a haploidentical stem cell parental graft.
Although the initial reports were limited because of the small number of recipients and short duration of follow-up, several significant issues were demonstrated. First, although more than 1 stem cell donation was needed in some of the patients, durable engraftment was achieved. Each of the patients developed mixed chimerism with T cells that were exclusively of donor type, and non-T populations that were either of mixed or host origin. Second, full reconstitution of cell-mediated immunity and partial reconstitution of humoral immunity was achieved. Despite the capability of engrafted paternal lymphocytes for a strong alloreactive response, reactivity against host cells in vitro was diminished. Third, no significant GVHD developed.
A modification of the method described is the use of E-rosetting with neuraminidase-treated SRBCs without prior lectin separation. Although the degree of TCD was less than that described earlier, requiring posttransplant immunosuppressive treatment to prevent GVHD, it was hoped that the presence of more donor T cells in the graft might enable better engraftment and faster immune reconstitution.
TCD Using Anti-T Cells Monoclonal Antibodies
Shortly after the development of lectin-based cell separation, another method using monoclonal antibodies was examined. The first antibody used was the anti-CD3 monoclonal antibody OKT3. Although engraftment was achieved, significant acute GVHD (aGVHD) could not be prevented. This could be explained by insufficient ex vivo TCD and by the inability of this murine antibody to activate human complement and continuously inactivate donor T cells after transplantation.
In 1983 a new monoclonal antibody was presented by Hale and colleagues. The antibody, a rat monoclonal anti-CD52 designated CAMPATH 1(alemtuzumab in the humanized form), has been proved to fix human complement and efficiently eliminate lymphocytes (>99% depletion) when incubated with complement-containing autologous plasma, while sparing the colony-forming cells in the bone marrow. Comparison between the 2 methods for TCD showed a similar degree of reduction in bone marrow T cells (99.8% for SBA vs 99.4% for Campath 1). Soon after, Campath 1 became widely used for T cell depletion in allogeneic bone marrow transplantation for the treatment of malignant diseases and a significant reduction in the occurrence and degree of GVHD relative to untreated bone marrow was reported, although most of these preliminary studies described patients who received transplants from HLA-matched donors and with posttransplant immune suppression. Since then, anti-CD52 antibodies has been used for prevention of graft rejection in organ transplantation and as an antineoplastic drug.
Positive Selection of Hematopoietic CD34+ Stem Cells
In the late 1990s, following a successful positive selection of CD34+ stem cells, this approach afforded a third option for TCD. The most commonly used methods, Isolex or the Milteney CliniMACS system, based on magnetic beads attached to an anti-CD34 antibody, enable marked purification of CD34+ stem cells. This procedure can be performed with or without additional negative depletion of CD3+ cells. In particular, in this method peripheral blood can be used as a source of stem cells affording up to 4 log TCD.
The following paragraphs summarize the efficacy of TCD HSCT in relation to overall survival, GVHD prevention, and recovery after transplantation and immune reconstitution. As most of the larger retrospective studies regarding HSCT in PID compare TCD haploidentical HSCT to unmanipulated identical HSCT (either related or unrelated), without distinguishing between the method used for T cell depletion, most of the data presented focuses on the differences between TCD HSCT and unfractionated HSCT. Data regarding the effect of the method used for TCD on posttransplantation outcome is presented where available.
Methods of TCD
Three major TCD methods have been used successfully for the treatment of PID patients.
Stem Cells Fractionation by Lectins
Lectins are sugar-binding proteins that are highly specific for their carbohydrate moieties. Stem cell fractionation using lectins is based on the principle that cell subpopulations expressing different lectin receptors can be separated by differential agglutination. Of the different lectins used for cell separation and identification, plant lectin SBA was shown to be useful in the separation of bone marrow cells and depletion of mature T cells. After several modifications, an efficient and rapid technique was developed for the large-scale removal of T lymphocytes from human bone marrow. The method achieved a highly purified cell population rich in blast cells and all myeloid precursors but depleted of T lymphocytes. Briefly, there are 4 main steps in this process : (1) selective removal of red blood cells (RBCs) by gravity sedimentation; (2) agglutination with SBA and differential sedimentation of the agglutinated (SBA + ) cells; (3) removal of E-rosette forming T cells from the SBA − cell fraction by centrifugation over Ficoll (RBCs form around human T cells on incubation); (4) repetition of the E-rosetting step using neuraminidase-treated sheep RBCs to eliminate residual T cells in the SBA − E − fraction. The cells collected at the end of the process (SBA − E − E N − ) were used for transplantation.
Following the successful transplantation of a haploidentical stem cell graft in a patient with acute leukemia as described earlier, this method was used in the treatment of patients with SCID using a haploidentical stem cell parental graft.
Although the initial reports were limited because of the small number of recipients and short duration of follow-up, several significant issues were demonstrated. First, although more than 1 stem cell donation was needed in some of the patients, durable engraftment was achieved. Each of the patients developed mixed chimerism with T cells that were exclusively of donor type, and non-T populations that were either of mixed or host origin. Second, full reconstitution of cell-mediated immunity and partial reconstitution of humoral immunity was achieved. Despite the capability of engrafted paternal lymphocytes for a strong alloreactive response, reactivity against host cells in vitro was diminished. Third, no significant GVHD developed.
A modification of the method described is the use of E-rosetting with neuraminidase-treated SRBCs without prior lectin separation. Although the degree of TCD was less than that described earlier, requiring posttransplant immunosuppressive treatment to prevent GVHD, it was hoped that the presence of more donor T cells in the graft might enable better engraftment and faster immune reconstitution.
TCD Using Anti-T Cells Monoclonal Antibodies
Shortly after the development of lectin-based cell separation, another method using monoclonal antibodies was examined. The first antibody used was the anti-CD3 monoclonal antibody OKT3. Although engraftment was achieved, significant acute GVHD (aGVHD) could not be prevented. This could be explained by insufficient ex vivo TCD and by the inability of this murine antibody to activate human complement and continuously inactivate donor T cells after transplantation.
In 1983 a new monoclonal antibody was presented by Hale and colleagues. The antibody, a rat monoclonal anti-CD52 designated CAMPATH 1(alemtuzumab in the humanized form), has been proved to fix human complement and efficiently eliminate lymphocytes (>99% depletion) when incubated with complement-containing autologous plasma, while sparing the colony-forming cells in the bone marrow. Comparison between the 2 methods for TCD showed a similar degree of reduction in bone marrow T cells (99.8% for SBA vs 99.4% for Campath 1). Soon after, Campath 1 became widely used for T cell depletion in allogeneic bone marrow transplantation for the treatment of malignant diseases and a significant reduction in the occurrence and degree of GVHD relative to untreated bone marrow was reported, although most of these preliminary studies described patients who received transplants from HLA-matched donors and with posttransplant immune suppression. Since then, anti-CD52 antibodies has been used for prevention of graft rejection in organ transplantation and as an antineoplastic drug.
Positive Selection of Hematopoietic CD34+ Stem Cells
In the late 1990s, following a successful positive selection of CD34+ stem cells, this approach afforded a third option for TCD. The most commonly used methods, Isolex or the Milteney CliniMACS system, based on magnetic beads attached to an anti-CD34 antibody, enable marked purification of CD34+ stem cells. This procedure can be performed with or without additional negative depletion of CD3+ cells. In particular, in this method peripheral blood can be used as a source of stem cells affording up to 4 log TCD.
The following paragraphs summarize the efficacy of TCD HSCT in relation to overall survival, GVHD prevention, and recovery after transplantation and immune reconstitution. As most of the larger retrospective studies regarding HSCT in PID compare TCD haploidentical HSCT to unmanipulated identical HSCT (either related or unrelated), without distinguishing between the method used for T cell depletion, most of the data presented focuses on the differences between TCD HSCT and unfractionated HSCT. Data regarding the effect of the method used for TCD on posttransplantation outcome is presented where available.
Long-term survival after TCD HSCT
A review of the literature regarding the long-term results of T cell depleted stem cell transplantation reveals an unexpected variability in the outcome and survival of patients treated. Some of the disparity could result from the nature of the primary disease but other variables should be considered, such as supportive care, the degree of TCD, pretreatment conditioning regimen (CR) and posttransplantation prophylaxis.
Survival in SCID Patients
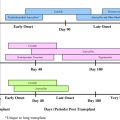
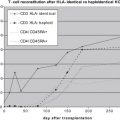
When considering the information on the overall survival of TCD HSCT in SCID patients, a careful analysis of the data should be made. Although the overall survival in the European Group for Blood and Marrow Transplantation multicenter study, which was published in 1998, showed a poor prognosis of 52% long-term survival, almost at the same time 2 other studies published by Buckley and colleagues and Small and colleagues in North America reported a long-term survival rate of near 80% ( Fig. 2 A). However, there are some significant differences between the groups. The method used for TCD in the North American studies was SBA and E-rosetting; the depletion in the European study comprised several methods including E-rosetting ± albumin gradient separation, SBA and E-rosetting or monoclonal antibodies with complement lysis. The difference in the degree of TCD might be associated with the disparity presented. In addition, the CR and the posttransplantation prophylactic treatment were different between the groups. In consideration of the marked sensitivity of SCID patients even to mild conditioning, in the early studies during the 1980s the North American groups preferred to avoid using conditioning and largely resorted to repeated transplantation. Moreover, no GVHD prophylaxis was used. On the other hand, in the European study conditioning and posttransplantation prophylaxis were given in 73% and 33% of the cases, respectively. Two other areas of uncertainty are the phenotypical composition of the SCID population involved (as discussed later) and the quality of supportive care used. The quality of supportive care is supported by a more recent analysis from the European group (based on data gathered in the SCETIDE (Stem Cell Transplantation for Immunodeficiencies) registry and includes the largest cohort of patients described) demonstrating significant improvement in long-term survival with time ( Fig. 2 B) (reaching near 80% in transplantation performed after 1996, in accordance with the data presented by the North American groups). In 1996, CD34+ positive selection began to be used as a method for TCD. Better TCD and improvement in the diagnosis and treatment of infectious complications can explain improved survival.
There is no doubt that related HLA identical stem cell transplantations result in the highest survival with more than a 90% long-term survival rate. This survival rate can be further improved, even with haploidentical TCD HSCT, when performed in the neonatal period. However, for most patients, the haploidentical stem cell graft is more than a reasonable alternative in the absence of an HLA identical family member, even compared with unmanipulated, matched, unrelated donor graft with ∼80% survival rate.
Despite the favorable results of TCD HSCT, there is much diversity in the outcome of SCID patients based on the disease phenotype and genetic cause. To simplify matters, based on the existence of B lymphocytes, SCID can be separated into 2 main groups: the first is the autosomal recessive (AR) inherited, characterized by the absence of T and B lymphocytes but by the presence of normal NK cells (T−B−NK+ SCID); the second is either X-linked or AR, characterized by the absence of T cells and NK cells but by the presence of nonfunctional B cells (T−B+NK− SCID). In general, the results of haploidentical TCD HSCT are significantly better for patients with T−B+NK− SCID (60% survival) than for T−B−NK+ SCID (35%). Although with time, and younger age at transplantation and the use of conditioning treatment, these results improved, the outcome of B− SCID transplantations are still less favorable with overall survival near 50%. Similar disparity was not found with an identical related stem cell donor. It is traditionally believed that existing NK cells are responsible for graft rejection, causing a low rate of engraftment and poor prognosis.
ADA SCID represents another group of patients in which the efficacy of TCD HSCT is difficult to evaluate. In a retrospective study, data from 87 patients demonstrated a 1-year survival of 88%, 67%, and 29% to 43% with related identical donor, unrelated identical donor, and haploidentical donor, respectively. However, when evaluating patients who received a highly T cell depleted haploidentical stem cell graft, without any pretransplantation treatment, the long-term survival rate approximates 75%, almost the same as in other B+ SCID.
Survival in Non-SCID PID Patients
Despite supportive management, premature mortality mostly as a result of infectious complications, has led to the use of HSCT as a curative treatment. Although a heterogeneous group of diseases, combined analysis of the available data predicts an unfavorable outcome for TCD haploidentical HSCT compared with matched related HSCT (71% vs 42% 3-year survival), without evidence for improvement over time.
Of the non-SCID PIDs treated with HSCT, patients with Wiskott-Aldrich syndrome (WAS) are the largest group. A recent analysis of 96 patients treated with HSCT for WAS showed inferior results for haploidentical HSCT relative to mostly unmanipulated identical HSCT, either related or unrelated (55%, 88% and 71%, respectively).
The data suggest that when considering the treatment of choice for non-SCID PID, as the threat of imminent death does not exist, a search for a fully matched stem cell donor should be conducted, as long as the procedure is performed at a young age.
Method of T Cell Depletion and Survival
Because of the lack of data comparing the efficacy of the different methods for TCD, it is almost impossible to conclude which is the preferred method for T cell depletion. One study has demonstrated reduced engraftment and survival in T−B−NK+ SCID patients when monoclonal antibodies were used for T cell depletion. Similarly, although not analyzed for statistical significance, a slightly reduced survival was implicated when comparing the long-term sequelae of anti-CD52 depletion and CD34+ positive selection (19/27 vs 19/22). As these studies reflect separation procedures which, in part, were performed more than 10 years ago, it is more than reasonable to assume that improvement in separation techniques will render this difference insignificant.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree