Abstract
This chapter describes the evolution of breast cancer ideology, detection, and treatment, which resulted in advancement from the radical mastectomy to modern day breast conservation therapy. Radical mastectomy is necessary for the patient with locally advanced disease involving the pectoralis muscles, and the technique is thoroughly described.
Keywords
Halsted, radical mastectomy
Historical Aspects for Development of Radical Mastectomy
In 1894 the modern era of breast cancer therapy was transformed when William Stewart Halsted reported the seminal “results of the cure of cancer of the breast.” Ten days later the concurrent reports by Willy Meyer of New York indicated that the radical mastectomy followed the evolution of anatomic principles and the advancement of cancer biology of the 19th century. These prior achievements by renowned anatomists, physiologists, and surgeons were the genesis of what were considered “modern” biological and surgical therapies for cancer of the breast. The Halsted radical mastectomy, introduced in 1882 by Halsted at the Roosevelt Hospital in New York City, was popularized and scientifically embraced at the Johns Hopkins Hospital in Baltimore (est. 1889). The operation embodied the concept of routine complete en bloc resection of the breast with the pectoralis major and minor muscles and the regional lymphatics. The halstedian approach was largely directed at preventing local or regional recurrences for an oncologic disease considered principally only of locoregional concern. Halsted’s synthesis of the techniques of his predecessors in surgery and pathology allowed him to achieve unprecedented local and regional recurrent rates of 6% and 22%, respectively. The en bloc technique described by Halsted, although published simultaneously by Meyer, allowed a reduction in the local recurrence rate to 6% from rates of 51% to 82% for renowned European surgeons of the era. Table 30.1 compares the operations available to European surgeons during the era with the accompanying 3-year estimated “cure” rates for breast carcinoma at Johns Hopkins.
| Type of Operation | Study | Year | No. of Cases | 3-Year Cures (%) |
|---|---|---|---|---|
| Simple mastectomy | Winiwarter (Billroth) a | 1867–1875 | 4.7 | |
| Average | 4.7 | |||
| Complete mastectomy and axillary dissection in most cases | Oldekop b | 1850–1878 | 229 | 11.7 |
| Dietrich (Lucke) c | 1872–1890 | 148 | 16.2 | |
| Horner d | 1881–1893 | 144 | 19.4 | |
| Poulsen e | 1870–1888 | 110 | 20 | |
| Banks f | 1877 | 46 | 20 | |
| Schmid (Kuster) g | 1871–1885 | 21.5 | ||
| Average | 18.1 | |||
| Complete (total) mastectomy, axillary dissection, removal of pectoral fascia and greater or lesser amounts of pectoral muscle | Sprengel (Volkmann) h | 1874–1878 | 200 | 11 |
| Schmidt i | 1877–1886 | 112 | 18.8 | |
| Rotter j | 30 | 20 | ||
| Mahler k | 1887–1897 | 150 | 21 | |
| Joerss l | 1885–1893 | 98 | 28.5 | |
| Average | 19.9 | |||
| Modern radical mastectomy | Halsted m | 1889–1894 | 76 | 45 |
| Halsted n | 1907 | 232 | 38.3 | |
| Hutchison o | 1910–1933 | 39.4 | ||
| Average | 40.9 |
a Data from Winiwarter V (Billroth). Beiträge zur statistik d. carcinome. Stuttgart: Gedruckt bei L. Schumacher; 1878.
c Data from Dietrich G (Lucke). Beitrag zur Statistik des Mammacarcinom. Duch Z F Chir. 1892;33:471.
d Data from Horner F. Ueber die Endresultate von 172 operierten Fällen maligner Tumoren der weiblichen Brust. Beitr Z Klin Chir. 1894;12:619.
e Data from Poulsen K. Die Geschwülste der Mamma. Arch F Klin Chir. 1891;42:593.
f Data from Banks M. A plea for the more free removal of cancerous growths. Liverpool Manchester Surg Rep. 1878;192.
g Data from Schmid H (Kuster). Zur statistik der mammacarcinome und deren heilung. Dtsch Z F Chir. 1887;26:139.
h Data from Sprengel O (Volkmann). 131 Fälle von Brust-Carcinom. Arch F Klin Chir. 1882;27:805.
i Data from Schmidt GB. Die Geschwülste der Brustdrüse. Beitr Z Klin Chir. 1889;4:40.
j Data from Rotter J. Günstigere Dauererfolge durch eine verbesserte operative Behandlung der Mammakarzinome. Berl Klin Wochenschr. 1896;33:69.
k Data from Mahler F. Ueber die in der Heidelberger Klinik 1887–1897 behandelten Fälle von Carcinoma Mammae. Beitr Z Klin Chir. 1900;26:681.
o Data from Hutchison RG. Radiation therapy in carcinoma of the breast. Surg Gynecol Obstet. 1936;62:653. (Collected figures.)
Halsted was not the first surgeon to resect the pectoralis major muscle in the course of a radical mastectomy. Wolff documents that in 1570, Barthélemy Cabrol of Montpellier, France, reported the cure of a mammary carcinoma in a 35-year-old woman in whom the pectoralis major muscle was excised and the wound was sprinkled with vitriols. The patient survived 12 years, only to die of cancer of the lower lip. Eminent European surgeons such as Petit, Billroth, Volkmann, and others of the period not infrequently removed portions of the pectoral muscles in resection of various malignancies of the breast. This therapeutic approach violated the principles of en bloc resection of the neoplasm, a dictum espoused by Halsted and Meyer. Joerss attributed the modern operation to Heidenhain. Nonetheless, it was Halsted who advocated routine resection of both pectoralis muscles en bloc with breast tissues and all levels (I–III) of axillary nodes. Halsted provided acknowledgments that substantiated the contributions of other renowned surgeons of this era in formulation and adoption of this procedure.
In its final procedural form, the technique of the radical mastectomy espoused by Halsted embodied the following principles:
- •
Wide excision of the skin, covering the defect with Thiersch grafts
- •
Routine removal of both pectoral muscles
- •
Routine axillary dissection (levels I–III)
- •
Removal of all tissues en bloc, with wide resection as possible on all sides of the growth
- •
Routine sacrifice of the long thoracic nerve and the thoracodorsal artery, nerve, and vein
The evolution of the modern radical mastectomy, which began with Cabrol in 1570 and pinnacled with Halsted in 1890, is one of discordant retrogressions. The wide acceptance of the incurability of breast carcinoma, the consequences of sepsis, and the necessity for anesthesia played prominent roles in the delay of the development for surgery of the breast. To attribute the development of the modern operation to a single individual would discredit the remarkable contributions of Halsted’s predecessors. The early surgery and pathology pioneers extended the operation because of the clinical observations for the natural history of breast cancer. These leaders of medical science lacked the salient background in biology, pathology, anatomy, pharmacology, and statistics that enabled Halsted to complete the evolution of the radical mastectomy in the late 19th century. Again, the raison d’être of the procedure was local-regional control of disease.
Breast Cancer Treatment in the United States
Trends and Patterns of Care, 1971 to 1984
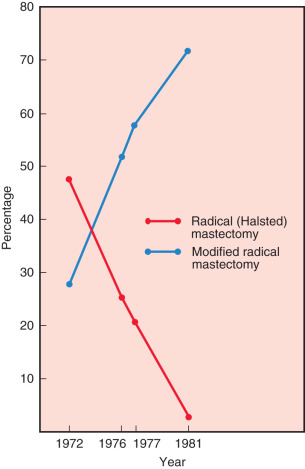
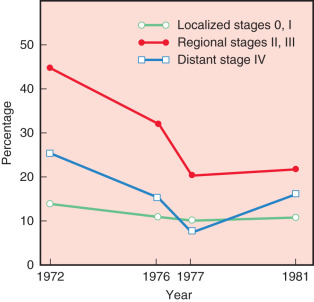
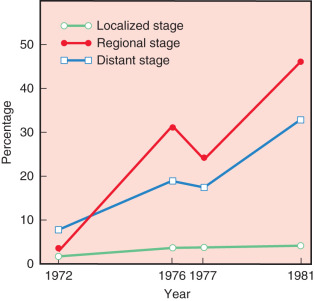
Fig. 30.1 confirms that most surgeons used the modified radical mastectomy on patients reported in 1971, 1976, 1977, and 1981. In 1972, 48% of patients were reported having had a Halsted-type radical mastectomy, whereas only 3% of patients underwent this procedure in 1981. Trends in the use of radiation therapy and chemotherapy also showed dramatic alterations in application from 1972 through 1981. Trends for the use of radiation therapy in these years are depicted in Fig. 30.2 . The proportion of patients at all stages reported to have received irradiation decreased substantially as the sole adjuvant therapy in distant disease, from 33% in 1972 to 18% in 1981. However, this trend is most apparent for regional stage disease (45%–21%), in the era. In contrast, the introduction of effective adjuvant and systemic chemotherapy and the emerging principles of pharmacotherapeutics initiated a dramatic increase in the application of chemotherapy. Chemotherapy increased from only 7% of patients treated in 1972 to 22.7% in 1981. This dramatic application of this effective therapy is depicted in Fig. 30.3 ; this exponential increase in the use of chemotherapy was limited to patients with regional and distant stages of disease. Currently, the use of adjuvant chemotherapy for treatment of localized disease (stages 0 and I) has experienced a renaissance. A discussion of the application of adjuvant chemotherapy and hormonal therapy for breast cancer is provided in Chapter 54 , Chapter 55 , Chapter 56 , Chapter 57 .
The 1982 National Survey by the American College of Surgeons (ACS) evaluated 5-year survival rates by stage, type of treatment, and type of adjuvant therapy. Table 30.2 depicts survival rates for patients initially treated in 1976 by type of operation, with or without adjuvant irradiation, and chemotherapy. Similar survival rates for patients with localized disease were observed for treatment by partial mastectomy alone and by partial mastectomy plus irradiation to the breast, the axilla, or both. In addition, 5-year survival rates for patients treated by the modified radical mastectomy technique were similar to 5-year survival rates for those treated by the Halsted radical mastectomy. Wilson and colleagues noted that survival rates were also similar for those who received additional irradiation therapy or chemotherapy with one of the two surgical procedures.
| Type of Operation | TYPE OF ADJUVANT THERAPY BY STAGE | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LOCALIZED | REGIONAL | DISTANT | |||||||
| None | RT | CT | None | RT | CT | None | RT | CT | |
| Partial Mastectomy | |||||||||
| 5-year survival (%) | 82.6 | 83.9 | 100 | 64.4 | 56.9 | 81 | 29 | 9.5 | 20.4 |
| No. of patients | 301 | 81 | 7 | 54 | 45 | 8 | 32 | 18 | 37 |
| Total Mastectomy Only | |||||||||
| 5-year survival (%) | 86.8 | 81 | 80.1 | 60 | 51.3 | 55.9 | 30.2 | 23.4 | 19.7 |
| No. of patients | 1034 | 154 | 37 | 183 | 116 | 32 | 72 | 44 | 43 |
| Total Mastectomy With Low Axillary Dissection | |||||||||
| 5-year survival (%) | 92.6 | 88 | 82.7 | 75 | 64.7 | 74 | 24.8 | 19 | 36.4 |
| No. of patients | 540 | 55 | 24 | 242 | 159 | 77 | 15 | 17 | 13 |
| Modified Radical Mastectomy | |||||||||
| 5-year survival (%) | 92.4 | 89.2 | 84.3 | 80.2 | 72.3 | 71.6 | 46.1 | 29.4 | 32.4 |
| No. of patients | 6537 | 630 | 280 | 2131 | 1292 | 1553 | 85 | 55 | 85 |
| Radical (Halsted) Mastectomy | |||||||||
| 5-year survival (%) | 92.8 | 89.4 | 88.7 | 78 | 73.1 | 71.1 | 52.5 | 51.4 | 51.5 |
| No. of patients | 3058 | 335 | 104 | 1190 | 795 | 640 | 50 | 29 | 37 |
Because the aforementioned publications of short- and long-term surveys are not prospective trials, this bias in treatment selection cannot be eliminated. In addition, follow-up data were limited, and these data represent only the trends in therapeutic approaches. Nonetheless, there has been an important transition in curative surgical procedures used by US surgeons from the Halsted radical to the modified radical mastectomy techniques. This transition was apparent at the time of the 1977 survey reported by Nemoto and associates. The 1981 survey confirmed that most patients receiving treatment underwent a modified radical mastectomy rather than a radical mastectomy (77% vs. 3%). This departure from a radical to a less deforming procedure is credited to Sir David Patey and W.H. Dyson of the Middlesex Hospital in London. Patey concluded from scientific study that excision of the pectoralis major muscle was not routinely justified on anatomic or pathologic observations; further, he was able to confirm that complete anatomic nodal clearance of the axilla remained possible, with excision only of the minor muscle. This allowed Patey to coin the term modified radical mastectomy after description of the procedure.
Thereafter, results of the short-term survey also confirmed an increase in the proportion of patients being treated with partial mastectomy, largely attributed to the seminal contribution of clinical trials conducted by Fisher and coworkers in association with the National Surgical Adjuvant Breast and Bowel Project (NSABP). Survival rates of patients treated by the various procedures are not comparable in the absence of more detailed data because of confounding biological and patient-related factors that may affect prognosis.
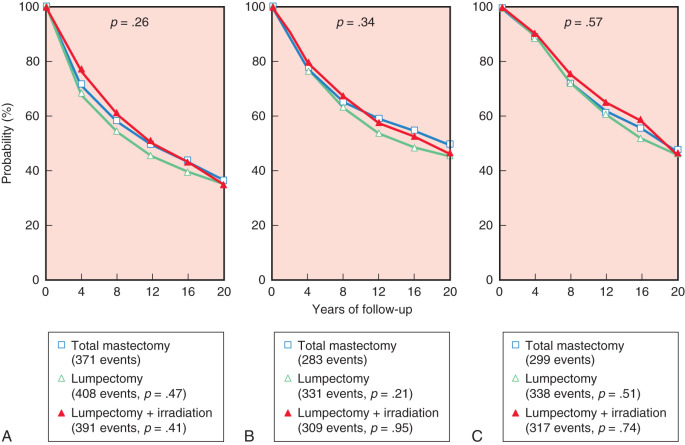
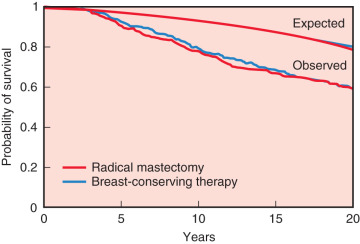
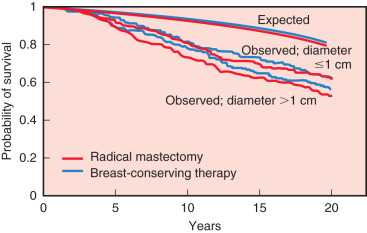
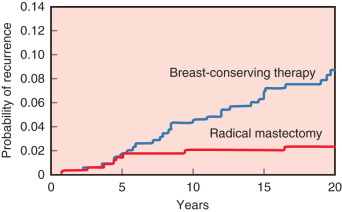
The 20-year update by Fisher and colleagues (2002) confirmed the value of lumpectomy followed by breast irradiation as an appropriate therapy for breast carcinoma—provided that resection margins are clear of neoplasm. Importantly, breast conservation and total mastectomy (which implies radical mastectomy as an overtreatment) have equivalent disease-free, distant disease-free, and overall survival rates ( Fig. 30.4 ). The 20-year analysis (2002) by Veronesi and associates validated the findings of Fisher and the NSABP—long-term survival is equivalent for women undergoing breast conservation compared with radical mastectomy ( Fig. 30.5 ) and tumor size (<1 cm vs. >2 cm) of the primary lesions are similar ( Fig. 30.6 ). This European study further confirmed the crude cumulative incidence of local recurrence after radical mastectomy and recurrence after breast conservation therapy. Fig. 30.7 indicates that the cumulative incidence of local recurrence was 8.8% with breast conservation versus 2.3% for radical mastectomy at 20 years. No differences were evident between the two groups relative to rates of contralateral disease, second primary cancers, or distant metastases.
The ACS conducted two surveys for the treatment of breast carcinoma in the United States: a long-term survey in 1976 and a short-term survey in 1981. Table 30.3 shows that in the short-term survey, more than twice the percentage of patients were treated by partial mastectomy (7.2%) compared with the long-term survey, which reported that 2.8% of surgeons used this technique in the primary therapy of breast cancer ( p < .0001). The most significant change in this survey was in the type of operations used for treatment of operable breast cancer. There is an increased use of modified radical mastectomy (55.6% in the long-term survey compared with 78.2% in the short-term survey) and a marked decline in the reported use of the Halsted radical mastectomy (27.5% of patients in the long-term survey compared with 3.4% in the 1981 data; see Fig. 30.1 ). For each stage of disease, there was a greater reported use of the modified radical procedure in the more recent short-term survey data compared with that for patients treated 5 years earlier. Interestingly, no significant changes were observed in the other types of surgical procedures between 1976 and 1981.
| Operation | CLINICAL STAGE REGIONAL TO: | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LOCALIZED | AXILLARY NODES WITH OR WITHOUT ADJACENT TISSUE | ADJACENT TISSUE ONLY | DISTANT | TOTAL | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| Long-Term Survey (1976) | ||||||||||
| Partial mastectomy | 408 | 3 | 74 | 0.8 | 58 | 8.1 | 153 | 14.3 | 693 | 2.8 |
| Total mastectomy only | 1261 | 9.3 | 203 | 2.2 | 178 | 24.7 | 278 | 26 | 1920 | 7.8 |
| Total mastectomy with low axillary dissection | 632 | 4.6 | 509 | 5.5 | 35 | 4.9 | 98 | 9.2 | 1274 | 5.2 |
| Modified radical mastectomy | 7584 | 55.7 | 5481 | 59.1 | 295 | 41 | 361 | 33.7 | 13,721 | 55.6 |
| Radical (Halsted) mastectomy | 3571 | 26.2 | 2909 | 31.4 | 144 | 20 | 169 | 15.8 | 6793 | 27.5 |
| Extended radical mastectomy | 152 | 1.1 | 99 | 1.1 | 9 | 1.2 | 11 | 1 | 271 | 1.1 |
| T otal | 13,608 | 100 | 9275 | 100 | 719 | 100 | 1070 | 100 | 24,672 | 100 |
| Short-Term Survey (1981) | ||||||||||
| Partial mastectomy | 819 | 8.5 | 236 | 3.4 | 58 | 12.1 | 170 | 22.1 | 1283 | 7.2 |
| Total mastectomy only | 530 | 5.5 | 129 | 1.9 | 73 | 15.2 | 184 | 23.9 | 916 | 5.2 |
| Total mastectomy with low axillary dissection | 511 | 5.3 | 340 | 5 | 26 | 5.4 | 83 | 10.8 | 960 | 5.4 |
| Modified radical mastectomy | 7436 | 77.1 | 5777 | 85 | 302 | 62.9 | 312 | 40.6 | 13,827 | 78.2 |
| Radical (Halsted) mastectomy | 295 | 3 | 272 | 4 | 17 | 3.5 | 19 | 2.5 | 603 | 3.4 |
| Extended radical mastectomy | 57 | 0.6 | 41 | 0.6 | 4 | 0.8 | 1 | 0.1 | 103 | 0.6 |
| T otal | 9648 | 100 | 6795 | 100 | 480 | 100 | 769 | 100 | 17,692 | 100 |
When treatment modalities were evaluated according to the clinical stage in the ACS survey, 95% of patients were treated in the long- and short-term surveys by surgical treatment alone or in combination with other modalities. Table 30.4 confirms that in the 1976 long-term survey, 60.6% of patients were treated by operation alone, compared with 58.8% in the 1981 short-term analysis. In addition, the use of surgical therapy plus irradiation (with or without chemotherapy) decreased from 19.8% to 16.6%, and the use of chemotherapy (with or without irradiation) with operation increased from 16.4% to 22.7% during this 5-year period. The change was limited to patients with regional and distant disease, and there was no significant change in the use of other treatment modalities between the long- and short-term surveys. For both analyses, 82% to 84% of patients in the localized disease stage were treated with surgery alone. For both surveys, the use of operative therapy as a sole modality decreased with advancing stage of disease, and the proportion of patients treated with surgery alone was similar in both surveys (see Table 30.4 ). Furthermore, operation plus irradiation was used more often than operation plus chemotherapy in the treatment of patients with cancer diagnosed as localized or regional to adjacent tissue. For the long-term study, operation plus irradiation and operation plus chemotherapy were used equally (24.2% vs. 24.1%) for treatment of patients with axillary node involvement. The short-term study confirmed that operation plus chemotherapy was 3.5 times more likely to be used than operation plus radiation (35% vs. 10.4%) for treatment of patients with positive axillary nodes. Wilson and colleagues noted that in both surveys, similar proportions of patients with regional disease underwent operation followed by radiation and chemotherapy. For patients with disease in the advanced stage, irradiation, chemotherapy, or hormone therapy, alone or in combination, was used more often (42.1% long term and 38.7% short term).
| Operation | CLINICAL STAGE REGIONAL TO: | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LOCALIZED | AXILLARY NODES WITH OR WITHOUT ADJACENT TISSUE | ADJACENT TISSUE ONLY | DISTANT | TOTAL | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| Long-Term Survey (1976) | ||||||||||
| Surgical treatment only | 11,592 | 84.4 | 3395 | 35.8 | 454 | 53.9 | 261 | 14.1 | 15,702 | 60.6 |
| Surgical treatment and radiation | 1275 | 9.3 | 2289 | 24.2 | 148 | 17.6 | 165 | 8.9 | 3877 | 15 |
| Surgical treatment and chemotherapy | 460 | 3.4 | 2284 | 24.1 | 57 | 6.8 | 215 | 11.6 | 3016 | 11.6 |
| Surgical treatment and hormone therapy | 75 | 0.5 | 132 | 1.4 | 12 | 1.4 | 103 | 5.6 | 322 | 1.2 |
| Surgical treatment, radiation, and chemotherapy | 154 | 1.1 | 911 | 9.6 | 34 | 4 | 142 | 7.7 | 1241 | 4.8 |
| Surgical treatment and others a | 52 | 0.4 | 264 | 2.8 | 14 | 1.7 | 184 | 10 | 514 | 2 |
| Others a | 120 | 0.9 | 198 | 2.1 | 123 | 14.6 | 778 | 42.1 | 1219 | 4.7 |
| T otal | 13,728 | 100 | 9473 | 100 | 842 | 100 | 1848 | 100 | 25,891 | 100 |
| Short-Term Survey (1981) | ||||||||||
| Surgical treatment only | 8065 | 82.2 | 2444 | 35 | 285 | 53.5 | 140 | 11.2 | 10,934 | 58.8 |
| Surgical treatment and radiation | 1035 | 10.6 | 731 | 10.4 | 94 | 17.6 | 56 | 4.5 | 1916 | 10.3 |
| Surgical treatment and chemotherapy | 355 | 3.6 | 2444 | 35 | 44 | 8.3 | 209 | 16.7 | 3052 | 16.4 |
| Surgical treatment and hormone therapy | 78 | 0.8 | 179 | 2.6 | 8 | 1.5 | 96 | 7.7 | 361 | 1.9 |
| Surgical treatment, radiation, and chemotherapy | 88 | 0.9 | 857 | 12.3 | 37 | 6.9 | 181 | 14.4 | 1163 | 6.3 |
| Surgical treatment and others a | 27 | 0.3 | 140 | 2 | 12 | 2.3 | 87 | 6.9 | 266 | 1.4 |
| Others a | 162 | 1.6 | 192 | 2.7 | 53 | 9.9 | 485 | 38.7 | 892 | 4.8 |
| T otal | 9810 | 100 | 6987 | 100 | 533 | 100 | 1254 | 100 | 18,584 | 100 |
a Radiation, chemotherapy, or both, and radiation, hormone therapy, or both.
Trends and Patterns of Care, 1985 to 2002 National Cancer Database—American College of Surgeons Commission on Cancer
The evolution of less radical approaches for the therapy of breast cancer followed the convincing reports of the past two decades for the efficacy and equivalency for locoregional control with conservative approaches (see Chapter 32 ). Since 1985, the National Cancer Database (NCDB) of the Commission on Cancer of the ACS tracked therapy trends for breast cancer. These data, illustrated in Tables 30.5 and 30.6 , identify the high usage of breast conservation for early-stage disease (T 1 , T 2 ; stage 0) by US Census Region in 1990. The trends and patterns of care by board-certified fellows of the ACS reflect decreasing usage of radical mastectomy in 1985, 1988, and 1990 (1.9%, 1.5%, and 0.6%, respectively). A similar pattern was observed for modified radical mastectomy with 63.2%, 64.8%, and 59.7% of surgeons using the procedure in 1985, 1988, and 1990, respectively (see Table 30.6 ). The explanation for decreasing use of the radical procedure is found in the shift to the segmental (partial) mastectomy, which represented 28.4% of mastectomies in 1990.
| Region | 1985 | 1988 | 1990 |
|---|---|---|---|
| New England | 39.9 | 44 | 52.6 |
| Mid-Atlantic | 16.4 | 33.6 | 48.9 |
| South Atlantic | 27.8 | 27.2 | 32 |
| East North Central | 19.6 | 25.9 | 40.7 |
| East South Central | 28.3 | 16.3 | 17.5 |
| West North Central | 14.1 | 20.1 | 24.2 |
| West South Central | 25.8 | 20.6 | 28.8 |
| Mountain | 14.5 | 25 | 40.1 |
| Pacific | 34.1 | 36 | 41.3 |
| All regions | 25.8 | 29.8 | 38.1 |
| No. of cases | 5592 | 12,420 | 18,641 |
| Surgery | 1985 | 1988 | 1990 |
|---|---|---|---|
| Radical mastectomy | 1.9 | 1.5 | 0.6 |
| Modified radical mastectomy | 63.2 | 64.8 | 59.7 |
| Total mastectomy | 3.4 | 3.8 | 2.8 |
| Partial (segmental) mastectomy | 18.4 | 22.2 | 28.4 |
| Subcutaneous mastectomy | 0.5 | 0.5 | 0.5 |
| Surgery type unknown | 3.5 | 1 | 1.1 |
| No surgery | 7 | 5.4 | 4.5 |
| Unknown if surgery done | 2.1 | 0.8 | 2.4 |
| T otal | 100 | 100 | 100 |
| No. of cases | 14,509 | 26,465 | 39,869 |
Surgical therapy with breast conservation is being used with increasing frequency; partial (segmental) mastectomy use was 3.4%, 7.2%, and 25.4% in 1972, 1981, and 1990, respectively ( Table 30.7 ). However, modified radical mastectomy remains the most frequently used surgical therapy for breast cancer by those surveyed. The most significant trend in usage was the decrease in radical mastectomy ( p < .0005); the frequencies in 1972, 1981, and 1990 were 45.3%, 3.4%, and 0.4%, respectively. Since 1972, the radical and extended radical procedures have been virtually replaced by total mastectomy with or without axillary nodal sampling (see Table 30.7 ).
| Type of Operation | PERCENTAGE OF PATIENTS | ||||
|---|---|---|---|---|---|
| 1972 (n = 15,132) | 1976 (n = 24,672) | 1981 (n = 17,692) | 1983 (n = 17,295) | 1990 (n = 24,356) | |
| Partial (segmental) mastectomy | 3.4 | 2.8 | 7.2 | 13.1 | 25.4 a |
| Total mastectomy, no nodes | 11.5 | 7.8 | 5.2 | 4.2 | 4.1 b |
| Total mastectomy, nodes | 32.6 | 55.6 | 78.2 | 75.2 | 65.8 a |
| Radical mastectomy | 45.3 | 27.5 | 3.4 | 1.7 | 0.4 a |
| Extended radical | 1.8 | 1.1 | 0.6 | 0.1 | <0.1 |
| None | 5.4 | 5.2 | 5.4 | 4.6 | 4.2 b |
a The difference for the rates of these operations for 1985 and 1990 was statistically significant ( p < .0005). For extended radical mastectomy, p = .03.
b Denotes p > .05 (not statistically significant). Data from 1972, 1976, and 1981 are included for comparison but were not analyzed statistically.
For the 1990 survey, the criteria on which the surgeon based his or her selection of the operative procedure are depicted in Tables 30.8 and 30.9 . These data illustrate the stage, age distribution (see Table 30.8 ), and geographic variations (see Table 30.9 ) that determine the selection process. Radical and extended radical techniques are rarely used except in advanced local disease (stages IIIA or IIIB). Moreover, most US surgeons have replaced the radical procedure with the modified radical (Patey, Auchincloss-Madden) approach.
| Operation | Median Age (yr) | Stage Unknown (%) | PAJCC STAGE (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | I | IIA | IIB | IIIA | IIIB | IV | No. of Patients | |||
| < Total, no nodes | 69.2 | 34.6 | 22.2 | 4.7 | 2.5 | 1.1 | 0.7 | 4.8 | 19.4 | 3319 |
| < Total, nodes | 59.4 | 6.2 | 7.9 | 19.8 | 11.9 | 8.2 | 3.7 | 3.6 | 5.7 | 5095 |
| Subcutaneous | 56.5 | 0.6 | 2 | 0.2 | 0.2 | 0.3 | 0.2 | 0.4 | 0.6 | 157 |
| Total, no nodes | 71.8 | 9.6 | 11 | 2 | 2.2 | 1.3 | 0.9 | 7.5 | 8.5 | 1519 |
| Total, nodes | 63 | 21.2 | 52.4 | 71 | 81.1 | 87.6 | 90.3 | 75.5 | 39.5 | 28,960 |
| Radical | 60.8 | 0.8 | 0.4 | 0.5 | 0.9 | 1 | 2.2 | 3.1 | 1.8 | 392 |
| Extended | 55 | 0 | 0.1 | 0 | 0 | 0 | 0.2 | 0.3 | 0.2 | 17 |
| Surgery type | 62.4 | 26.1 | 3.8 | 1.3 | 0.9 | 0.5 | 1.5 | 4.4 | 22.5 | 1863 |
| No. of patients | — | 3980 | 2484 | 13,600 | 10,614 | 5871 | 1786 | 1527 | 1773 | — |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








