GROWTH HORMONE STRUCTURE
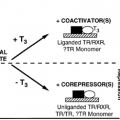
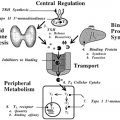
Human GH is heterogeneous and consists of several molecular variants (Fig. 12-2).2 The principal form is a 191-amino-acid, single-chain protein with a molecular weight of ˜22,000. It is the most abundant form (˜90%–95% of pituitary GH), generally referred to as “GH” or “22K GH.” Two disulfide loops are present, and the three-dimensional structure is a twisted bundle of four α-helices (Fig. 12-3).3 Two independent receptor-binding sites are located on opposite surfaces of GH, allowing for ligand-induced receptor dimerization (see later). GH-V (placental GH) has a similar primary structure; it differs from GH-N (22K GH) in 13 of the 191 amino-acid positions (see Fig. 12-2). One important difference is the glycosylation consensus site at asparagine 140; GH-V exists as a glycosylated as well as nonglycosylated protein. The second most abundant GH form after 22K GH is an mRNA splice variant that lacks an internal sequence of 15 amino acids. Its molecular weight is ˜20,000, and hence it is known as “20K GH.” It accounts for 5% to 7% of pituitary GH. Other minor variants include an Nα-acylated and two deamidated variants (see Fig. 12-2). Little is known about the bioactivities or significance of these minor GH forms. In addition to the monomeric forms of GH just described, GH also exists as an oligomeric series of up to at least pentameric GH.4 Both noncovalent and disulfide-linked oligomers occur, and homo- as well as heterooligomers composed of the various monomeric forms exist in the pituitary and plasma. The biologic significance of GH oligomers is unclear, but they likely act as modulators of overall GH activity because of their different affinities for the GH receptor. The existence of so many molecular forms of GH is one reason for the difficulty with GH measurements and the discrepant results obtained by different assays (see later).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree