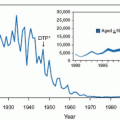
Since the last edition of this text, considerable progress has been made in the prevention of perinatal group B streptococcal (GBS) infections. In the summer of 2002, the Centers for Disease Control and Prevention (CDC) published revised guidelines for the prevention of perinatal GBS disease. A single strategy, based on universal screening by culture at 35 to 37 weeks, was recommended. The American College of Obstetricians and Gynecologists (ACOG) endorsed these recommendations. For the years 2003 to 2005, the overall rate of early-onset disease among newborn infants was 0.33 cases per 1,000 live births—33% lower than during 2000 to 2001
(see Fig. 1.1). However, many questions remain. This heavily revised chapter will detail the 2002 guidelines and provide suggestions for practice beyond the CDC guidelines.
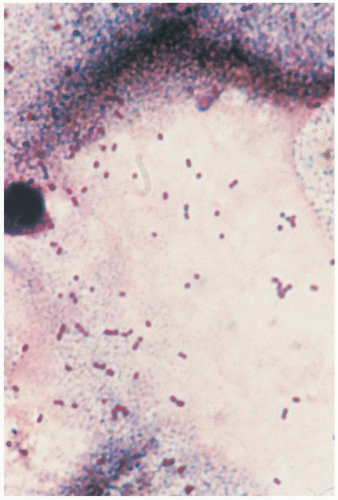
In 1933, Lancefield used serologic techniques to subdivide β-hemolytic streptococci into specific groups, which she named A, B, D, and E. The various groups of streptococci, their taxonomic designations, and hemolytic reactions on blood agar are listed in
Table 1.1. On blood agar, the β reaction shows complete hemolysis around the bacterial colony. The β reaction is a greenish discoloration; there is partial hemolysis around the colony. The β reaction shows an absence of hemolysis around the colony. Most microbiology laboratories report groups C and G “as β-hemolytic streptococci, not groups A, B, or D.”
Although GBS were recognized in veterinary medicine for many years as a cause of bovine mastitis, they were virtually ignored as human pathogens until 1964, when Eickhoff and associates noted the role of GBS in perinatal infections. In the following years, GBS became the leading bacterial pathogens in perinatal infections, replacing Escherichia coli as the most frequent microorganisms associated with bacteremia or meningitis among infants during the first 2 months of life. In 1990, it was estimated that there were 7,600 cases of neonatal GBS disease, accounting for an incidence rate of 1.8 per 1,000 live-born infants. These cases resulted in an estimated 310 deaths from GBS disease among infants younger than 90 days in the United States. Institution of national guidelines, first in 1996 with revisions in 2002, has been accompanied by an 80% reduction in early-onset disease.
Group B streptococcus has been recognized as one of the most important pathogens in obstetric patients as well. This organism causes urinary tract infections, amnionitis, postpartum endometritis, wound infection, intrapartum and/or postpartum bacteremia, and stillbirth. Genital tract colonization is associated with premature rupture of membranes (PROM) and preterm delivery.
EPIDEMIOLOGY
Asymptomatic vaginal colonization with GBS occurs in approximately 20% (range, 5% to 40%) of pregnant women
(Table 1.2). The gastrointestinal tract is the likely reservoir. The reported prevalence of vaginal colonization with GBS in gravid women varies according to geographic locale, age, gravidity, duration of gestation, and location and number of sites cultured. Obtaining specimens for culture from both the anorectum and the distal vagina increases the likelihood of obtaining GBS by a considerable percentage (5% to 25%) over vaginal culture alone. Within the genital tract, the highest isolation rates are reported from the introitus and the lowest from the cervix. Pregnancy does not influence colonization rates.
The choice of culture medium is a crucial determinant of the prevalence of GBS. The highest yield of GBS occurs when a selective broth medium (SBM), such as Todd-Hewitt broth with sheep blood, nalidixic acid, and gentamicin, is used. When selective media are not used for cultures of the genital tract, up to 50% of colonized women will be missed (i.e., the sensitivity will be as low as 50%). The CDC has provided detailed procedures for collecting and processing clinical specimens for GBS culture and performing susceptibility testing to clindamycin and erythromycin
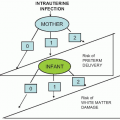
(see Box 1.1).The presence of GBS in the maternal genital tract is the major determinant of both infection in the neonate and colonization of the newborn infant. Vertical transmission from mother to fetus occurs either via an ascending route in utero, most commonly with ruptured membrane (but occasionally with intact membranes), or by acquisition during passage
through the birth canal intrapartum. The risk of transmission has been shown to range from 40% to 70% among neonates born to colonized mothers; only approximately 8% of infants born to noncolonized mothers become colonized. Based on these prospective studies, nearly two thirds of infants born to colonized mothers will be colonized with GBS. In the era before the use of intrapartum prophylaxis, the incidence of early-onset GBS infection (during the first 7 days of life) ranged from 1.3 to 3 per 1,000 live births. For late-onset disease, the range was 1 to 1.7 per 1,000 live births. Only 1% to 2% of infants of colonized women developed early-onset GBS infection.
In addition to maternal-infant transmission, nosocomial acquisition of GBS occurs as well. From 15% to 50% of nursery personnel are carriers of GBS and may be sources for neonatal transmission. Studies have demonstrated nosocomial transmission rates of GBS in neonates born to culture-negative mothers. In addition, cross-contamination of infants may arise via nursery personnel. Nosocomial transmission rates of GBS in neonates born to culture-negative mothers range from 13% to 43%. Whether late-onset GBS infection is largely caused by nosocomial acquisition of GBS is unclear. Both nonmaternal and maternal sources have been implicated.
Risk factors that predispose the neonate to clinical infection have been identified
(Table 1.3). For data from 2003 to 2005, 29% of early-onset cases occurred in infants born preterm (40% in black vs. 24% in white infants).
Before the widespread use of intrapartum prophylaxis, duration of rupture of the membranes (ROM) was defined as an important correlate of neonatal GBS infection. As the duration of ROM increased, attack rates for GBS infection also increased, from 0.8 per 1,000 infants with ROM of 6 hours or less to 10.8 per 1,000 in those with durations greater than 48 hours. In one study, the relative risk of developing GBS early-onset disease for infants with ROM for more than 48 hours was 7.2-fold higher than that for infants with ROM for less than 18 hours. Further, the attack rate of GBS sepsis was 45 per 1,000 infants born to colonized women with perinatal risk factors (birth weight less than 2,500 g, ROM more than 18 hours, or intrapartum fever). The relationship of GBS to premature birth and PROM is discussed in
Chapter 17.
Intrapartum fever, clinical chorioamnionitis, and clinical intra-amniotic infection are perinatal risk factors associated with increased risk for early-onset GBS sepsis. Attack rates for GBS were 6.5 per 1,000 infants among mothers with intrapartum fever versus 1.5 per 1,000 infants of afebrile mothers.
Neonates delivered through birth canals heavily colonized with GBS are significantly more likely to acquire GBS than those born to mothers with light colonization.
Additional risk factors include offspring of mothers who lack type-specific antibodies to GBS. Two mechanisms that result in low levels of neonatal antibody to GBS have been suggested. It either reflects low maternal levels and lack of acquired immunity in the mother or failure of adequate concentrations of these immunoglobulin G (IgG) antibodies to be transported transplacentally to the fetus. The very small preterm infant (younger than 30 weeks) may be significantly compromised by the second mechanism, because nearly two thirds of maternally derived IgG is actively transported from mother to fetus in the last 10 weeks of pregnancy.
In 2003 to 2005, the rate of early-onset disease was 0.37 per 100 live births and the rate of late-onset disease was virtually the same, at 0.36 per 1,000 live-born infants. The survival rate of term infants is 98% but decreases to 90% for infants born at 34 to 36 weeks and to 70% for infants born at less than 33 weeks.
Because bacteremia is present at birth in a large proportion of neonates with early-onset GBS disease, most cases (about 65% to 90%) of GBS early-onset disease have an intrauterine pathogenesis.
The epidemiology and pathogenesis of late-onset GBS infection (occurring more than 7 days after birth) are not as clear as that for early-onset disease. Serotype III strains account for most late-onset GBS infection. The GBS organisms responsible for late-onset disease are acquired either by maternal-neonatal vertical transmission or from nosocomial sources. As noted above, because of the decrease in early-onset disease, early and late disease caused by GBS now occur at virtually equal rates (0.37 and 0.36 cases per 1,000 live-born infants, respectively). The case fatality rate in the 1990s was 4.7% for early-onset and 2.8% for late-onset disease.