INTRODUCTION
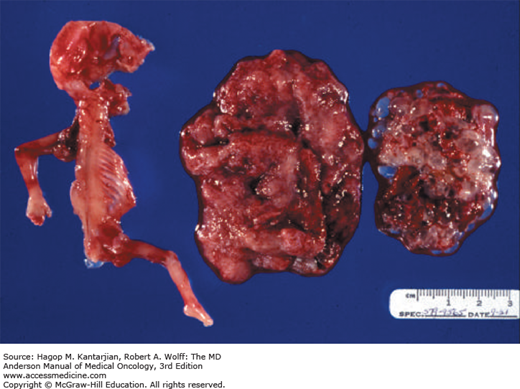
Gestational trophoblastic disease (GTD) comprises a wide spectrum of neoplastic disorders that arise from placental trophoblastic tissue after abnormal fertilization (Fig. 34-1). The spectrum includes benign disease (complete and partial hydatidiform moles) and malignant gestational trophoblastic neoplasia (GTN), which includes choriocarcinoma, placental site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor (ETT) (1). Invasive moles may also occur and are best categorized as malignant, since they can metastasize. Gestational trophoblastic neoplasia is also described as gestational trophoblastic tumor (GTT) and is further designated as nonmetastatic or metastatic (2).
The hydatidiform mole is the most common type of GTD. It is essentially a benign condition with variable potential for malignant transformation. Most molar pregnancies resolve spontaneously after uterine evacuation, with no further events or adverse outcomes. At any time during or after gestation, approximately 20% undergo malignant transformation to invasive nonmetastatic or metastatic GTN and require further treatment (3). Nearly two-thirds of these lesions develop into persistent nonmetastatic GTN; the remaining one-third develop distant metastases (2,4).
These tumors were first described around 400 B.C. by Hippocrates and subsequently termed “dropsy” of the uterus. During the 1950s, the 5-year survival of patients with choriocarcinoma was less than 5%. The treatment of these tumors has been revolutionized with effective chemotherapy, leading to a cure rate approaching 100% and fertility preservation in most patients (1,5,6). Successful outcomes rely on individualized management based on careful staging and treatment planning by a multidisciplinary team.
EPIDEMIOLOGY
The prevalence of GTD depends on geography, maternal age, previous GTD history, socioeconomic factors, dietary factors, and blood grouping. True estimates of the incidence of molar pregnancy are difficult to obtain because of the vast variation in presentation and management of normal and abnormal pregnancies around the world. In North America and in Europe, GTD develops in approximately 1 in 100 to 2,000 pregnancies (1,7,8). A higher rate of 1.5 to 6 per 1,000 pregnancies has been reported in South America. Early observations comparing East and Southeast Asian countries with the United States suggest a 5- to 15-fold higher incidence, as high as 1 in 120 pregnancies in East Asia (1,8,9). Native Alaskans have an incidence three- to fourfold that of white women. Native Americans had a higher incidence than other ethnic groups in New Mexico (10). Not all data confirm the importance of ethnic background. Recent analyses suggest that the incidence in Southeast Asia is similar to that in Europe, which may reflect modifications in diet, improved diagnosis, and improved capture of population statistics (1,2,8).
Extremes of age at conception appear to influence the rate of GTD (1,2,11). Women older than 40 years have a fivefold greater risk of molar pregnancy (11); those younger than 20 years have a 1.5- to 2-fold relative risk (11). Women with a history of hydatidiform mole have a 10-fold greater risk for a second molar pregnancy and a more than 1,000-fold greater risk of choriocarcinoma than women with normal pregnancies (11). The New England Trophoblastic Disease Center demonstrated the increased risk of subsequent molar pregnancy to be 1% (12).
Women of lower socioeconomic status have a 10-fold greater rate of molar pregnancy than more affluent counterparts. This trend has been reported in East Asia, the Middle East, the United States, and Brazil (2,13). This relationship between GTD incidence and geographic region, culture, and socioeconomic status suggests that diet and nutrition may contribute to the etiology. Low β-carotene and high animal fat consumption are associated with GTD. There is strong association between cigarette smoking and GTD.
Historically 80% of GTDs are hydatidiform moles, 15% are invasive moles, and 5% are choriocarcinomas. Placental site trophoblastic tumor occurs in 0.2% to 2% of all GTD but is responsible for the highest mortality rate of all GTD histologies (14,15). Choriocarcinoma is associated with an antecedent complete hydatidiform mole in 50% of the cases, a history of abortion in 25%, term delivery in 20%, and ectopic pregnancy in 5%. Occurrence after partial mole is rare (1,2). The precise rate of choriocarcinoma may be underreported because tissue is not recommended for the diagnosis based on the risk of hemorrhage with biopsy (1,2).
In the United States, molar pregnancies are reported in approximately 3,000 patients per year, and malignant transformation occurs in 2% to 19% of these cases (1,2,4). Complete molar pregnancies occur 1 in 15,000 abortions and 1 in 150,000 normal pregnancies. The estimated incidence of twin pregnancy consisting of a molar pregnancy and a normal fetus is 1 per 22,000 to 100,000 pregnancies.
PATHOLOGY
A hydatidiform mole is confined to the uterine cavity. When a hydatidiform mole persists, it is termed malignant and may be designated GTT or GTN and may be metastatic or nonmetastatic. Most malignant GTDs occur after evacuation of a mole and exhibit the histologic features of either hydatidiform moles or choriocarcinomas. Choriocarcinomas are highly malignant and tend to metastasize extensively. Conversely, persistent GTD after a nonmolar pregnancy almost always has the histologic pattern of choriocarcinoma. Invasive moles and placental site tumors are locally invasive but rarely metastatic. Both tumors are rare, but they can be distinguished histologically (2). Specific pathologic criteria exist for each of these histologic subtypes.
Based on morphologic and cytogenetic features, hydatidiform moles are divided into two unique syndromes: complete (classic) and partial (Table 34-1) (See Fig. 34-1). Complete hydatidiform mole is characterized by the lack of a fetus and a characteristic abnormal budding edematous villous structure with nonpatchy trophoblastic hyperplasia, stromal karyorrhectic debris, and collapsed, abnormal villous blood vessels (2). A complete mole is usually detected during the second trimester and is identified by total hydatidiform enlargement of the villi, which are enveloped by hyperplastic and atypical trophoblasts. There is a notable absence of any embryonic or amniotic remnant. In questionable cases, p57 immunohistochemistry can be useful in confirming the diagnosis (16). Approximately 20% of complete moles give rise to persistent trophoblastic disease.
| Complete | Partial | |
|---|---|---|
| Fetal or embryonic tissue | Absent | Present |
| Hydatidiform swelling of chorionic villi | Diffuse | Focal |
| Trophoblastic hyperplasia | Diffuse | Focal |
| Trophoblastic stromal inclusions | Absent | Present |
| Genetic parentage | Paternal | Biparental |
| Karyotype | 46XX; 46XY | 69XXY; 69XYY |
| Persistent β-hCG elevation | 20% | 0.5% |
Partial moles, in contrast to complete moles, are typically accompanied by an identifiable embryo or amniotic membranes (Fig. 34-2). These moles are described as partial because the hydatidiform changes in the villi tend to be focal. They demonstrate patchy villous hydrops with scattered abnormally shaped and scalloped irregular villi with trophoblastic pseudo inclusions and patchy trophoblastic hyperplasia (2). The villous capillaries appear to be functional because they possess the same proportion of nucleated fetal erythrocytes as the embryo. In partial moles, hydatidiform change occurs at a slower rate, and the proportion of relatively normal villi appears to correlate with fetal survival rate.
Diagnosis of molar pregnancies based on histology alone can be problematic. Negative immunostaining for P57KIP2, an imprinted gene expressed by the maternal allele, is diagnostic of a complete mole, as the placenta of all other gestations demonstrates nuclear staining of cytotrophoblast and villous mesenchyme (2,6). Ploidy analysis can help differentiate partial (triploid) from complete (diploid) mole, but cannot distinguish between this and other etiologies of triploidy. Selective molecular genotyping allows for definitive diagnosis when histologic review is equivocal, but the cost of such testing may prohibit widespread adoption of this technique.
Maturation of mesenchymal elements is only minimally delayed in partial moles, and there is a paucity of fibroblast karyorrhexis. Approximately 2% to 6% of partial moles undergo malignant degeneration (3). Because of this sporadic malignant potential, follow-up and treatment of patients with partial moles are the same as for patients with complete moles.
Locally invasive moles have the same histologic features as complete mole. In addition, they are characterized by myometrial invasion without involvement of intervening endometrial stroma. Invasive moles are typically diagnosed clinically approximately 6 months after molar evacuation when human chorionic gonadotropin (hCG) remains elevated. They tend to invade locally, causing hemorrhage and necrosis. Rarely, uterine perforation results. Hematogenous metastasis may occur, often to the lungs. Occasionally, metastatic deposits display hydropic villi rather than the sheets of anaplastic cells that typify metastatic choriocarcinoma. Invasive moles are scored using the World Health Organization (WHO) scoring system, and treatment is predicated based on the score. If childbearing is complete, hysterectomy is recommended, but if fertility preservation is desired, the appropriate form of chemotherapy based on the WHO score is administered.
Choriocarcinoma is a malignant tumor with a unique histology distinct from that of moles (2). The tumor is grossly red and granular and exhibits extensive necrosis and hemorrhage. On microscopic examination, the neoplasm is composed of a disordered array of syncytiotrophoblastic and cytotrophoblastic elements, absence of chorionic villi, frequent mitoses, and multinucleated giant cells. Direct myometrial and vascular invasion occur early, with resultant metastases to the lungs, vagina, brain, kidneys, liver, pelvis, spleen, and gastrointestinal tract (1,2,6).
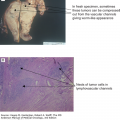
Placental site tumors are rare and most often develop after nonmolar gestations but can occur after evacuation of a complete hydatidiform mole (14). These tumors occur at the placental implantation site and consist of numerous nodules in the endometrium or myometrium. Histologically, these consist of a homogeneous population of mononuclear intermediate trophoblast cells of the placenta infiltrating in sheets or cords between myometrial fibers, sometimes with a few syncytial elements (17). The intermediate trophoblastic cells have oval nuclei with abundant eosinophilic cytoplasm, and no chorionic villi are seen. Syncytiotrophoblastic and cytotrophoblastic populations are absent, and less vascular invasion, necrosis, and hemorrhage are seen than in choriocarcinoma (17). Lymphatic metastasis is common. Placental site trophoblastic tumor secretes placental lactogen and small amounts of β-hCG and is usually diploid (1,2).
Epithelioid trophoblastic tumor is a rare variant of PSTT that mimics carcinoma but histologically is composed of chorionic-type intermediate trophoblast.
PATHOGENESIS
Pathologic characteristics alone generally do not allow adequate discrimination of molar pregnancies. With the advent of cytogenetic techniques, such as chromosomal banding and restriction fragment length polymorphism analysis of DNA, unique chromosomal patterns of molar pregnancies were discovered (18), allowing complete and partial moles to be distinguished from one another (19).
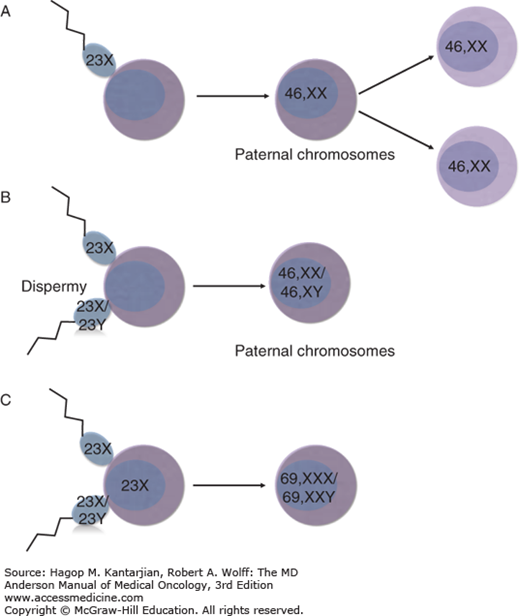
Normal fertilization results from the union of a single sperm and an egg, followed by rapid cellular division and the creation of a diploid embryo (Fig. 34-3). Early embryonic differentiation gives rise to trophoblasts, specialized epithelial cells responsible for developing the placenta and the villi. Gestational trophoblastic tumors arise from abnormal unions of sperm with the ovum, resulting in distinct pathologic characteristics involving activated transcription factors, cytokines, hormone secretion, cell adhesion molecules, and immunologic activity (20).
FIGURE 34-3
Schematic diagram of the pathogenesis of molar pregnancies. (A) Complete mole. Most common pathogenesis, in which a haploid sperm (23X) fertilizes an empty egg which then undergoes duplication (46,XX). (B) Complete mole. Dispermy, in which two spermatozoa (23X or 23Y) fertilize an empty egg yielding a complete mole (46,XX or 46,XY). (C) Partial mole. Two spermatozoa (23X or 23Y) fertilize an ovum (23X) yielding a triploid mole (69,XXY or 69,XXX).
A complete mole contains nuclear chromosomes of paternal origin and mitochondrial DNA of maternal origin (2,21). Chromosomal banding studies reveal that complete moles contain only paternal chromosomes. This finding has been confirmed by showing that when paternal heterozygotes for the human leukocyte antigen (HLA) locus give rise to a mole, the HLA expression of the molar tissue is homozygous (22). Approximately 80% to 92% of complete moles have a 46,XX karyotype derived from fertilization of an empty egg, the chromosomes of which were lost during meiosis, by a haploid sperm (23X) that then undergoes duplication to create a diploid set of identical chromosomes (46,XX) (23). Complete moles can also result from postzygotic diploidization of a triploid conception. Approximately 4% to 20% of complete moles result from dispermy, in which two spermatozoa (each of which may be 23X or 23Y) fertilize an empty ovum, resulting in a 46,XX or 46,XY karyotype containing all paternal nuclear chromosomes (2,24). Most are 46,XY, but approximately 5% of all complete moles are heterozygous 46,XX resulting from such dispermy. A 46,YY mole has not been reported, suggesting that the X chromosome is required for survival. There is no strong evidence that dispermic or Y chromosome–containing moles have greater malignant potential than the monospermic 46,XX karyotype (21).
A partial mole results from the abnormal union of two spermatozoa with one ovum with intact chromosomes, resulting in a triploid karyotype. Occasionally, a normal ovum can be fertilized by an abnormal diploid sperm (2). Therefore, the classic partial mole has a triploid karyotype (69 chromosomes), and both paternal and maternal chromosomes are present. The most common sex chromosome arrangement is XXY, but XXX and XYY do exist (2). Of note, in triploid pregnancies, a partial mole results when the extra haploid chromosome is of paternal origin, and a fetus develops when the extra haploid chromosome is of maternal origin. In the event that a partial mole is reported as diploid, the diagnosis is usually a misdiagnosed complete mole, hydropic abortion, or twin pregnancy (2).
Rarely, familial recurrent hydatidiform mole syndrome may be present, leading to recurrent molar pregnancies. This is an autosomal recessive disorder with mutations in NLRP7 (70% of cases) or KHDC3L (5% of cases) resulting in diploid complete moles of biparental origin, as opposed to exclusively paternal origin. Live birth in these patients is rare, but egg donation from unaffected women may result in successful live birth (25).
Our improved understanding of the activities of proto-oncogenes, tumor suppressor genes, cytokines, and growth factors is contributing to our understanding of GTN and tumor progression (22).
The excess of paternal chromosomes in moles probably contributes to the induction of trophoblastic hyperplasia. The genomic imbalance may cause changes in expression of growth factor genes located on the paternal allele. Both normal placentas and molar pregnancies contain paternal antigens. Upon implantation, an immunologic response is initiated, with infiltration of lymphocytes and macrophages and secretion of cytokines.
The growth of choriocarcinomas may be related to the abundant expression of epidermal growth factor (EGF) receptor. Macrophage-derived cytokines—interleukin-1 (IL-1-α, IL-1-β) and tumor necrosis factor—can suppress cell growth and increase the expression of EGF receptor in choriocarcinoma cell lines, thus acting as paracrine mediators of cell growth (26).
The contribution of several oncogenes to the malignant transformation of GTD has also been examined. Growth regulation in the trophoblast is associated with expression of the transcription factor Mash-2 (27). Complete moles demonstrate increased expression of c-fms RNA compared with that in normal placentas (28). In choriocarcinoma, increased expression of oncogenes has been observed, and progression of some tumors has been associated with inactivation of tumor suppressor genes (29). The significance of these findings is uncertain. Because trophoblasts are by nature rapidly dividing and invasive, increased expression of these oncogenes may be essential for normal cell function. Table 34-2 lists other genes whose overexpression has been implicated in GTD (30,31,32). Human placental growth hormone has recently been detected in all variants of GTD and may serve as a novel biomarker for diagnosis (33).
CLINICAL PRESENTATION
The most common presenting symptom of the complete mole is vaginal bleeding in the first or early second trimester of pregnancy, although the classic signs of a molar pregnancy include vaginal bleeding as well as absence of fetal heart sounds and physical evidence of a uterus that is larger than expected for the gestational age (2).
A constellation of symptoms and signs has historically been associated with molar pregnancy, but such events are becoming less common due to routine ultrasonography in early pregnancy and the resulting early diagnosis of molar pregnancy (34). In the event that molar pregnancy is detected later in the first or early second trimester, patients may present with abdominal pain due to the enlarged uterus, which may be larger than expected for gestational age. Intrauterine blood clots may liquefy and produce the pathognomonic prune juice–like vaginal discharge. Because of recurrent bleeding, patients may also present with iron deficiency beyond that expected for a normal pregnancy. Symptoms of anemia have been noted in approximately 50% of patients at diagnosis (35). Theca lutein cysts, caused by β-hCG–induced hyperstimulation of both ovaries in about 50% of patients, may result in a sensation of pelvic pressure or fullness. Usually, these cysts regress spontaneously after uterine evacuation, although their rupture or tension can cause acute abdominal symptoms occasionally requiring surgery (35).
In the past, 20% to 30% of patients have presented with early pre-eclampsia, thought to be precipitated by the release of large amounts of vasoactive substances from necrotic trophoblastic tissue, but seizures are rare (12). Ten percent of patients have presented with hyperemesis gravidarum, and 7% have presented with hyperthyroidism, presumably due to the structural similarities of β-hCG to thyroid-stimulating hormone (36,37). Thyroid storm has been reported. Other rare presentations include respiratory distress, disseminated intravascular coagulation, and microangiopathic hemolytic anemia (35).
Unlike complete moles, fewer than 10% of patients with partial moles present with an enlarged uterus. An intact fetus can coexist with a partial mole, though this occurs in fewer than 1 in 100,000 pregnancies. Patients with partial moles typically do not have the hormonal symptoms experienced by patients with complete moles, and pre-eclampsia is rare. Among 81 patients with partial moles, none had prominent theca lutein cysts, hyperthyroidism, or respiratory insufficiency and only one had toxemia (37). Patients with partial moles present with signs and symptoms of a missed or incomplete abortion, and the diagnosis of a partial mole is made only after histologic review of curettage specimens.
Although spontaneous remission occurs in approximately 80% of patients with GTD after evacuation, malignant GTD is most commonly diagnosed after evacuation of a molar pregnancy when serum β-hCG titers plateau or rise (2,15). Patients treated surgically for molar pregnancy should be monitored closely for malignant transformation with symptoms, signs, and serum evaluation. Previously referred to as persistent trophoblastic disease, a plateaued or rising β-hCG level (three consecutive measurements) indicates malignant change and the development of GTN.
Fifty percent of all malignant GTNs follow molar pregnancy, 25% follow normal pregnancy, and the remaining 25% follow ectopic pregnancy or abortion. Persistent invasive nonmetastatic GTN usually presents with recurrence of symptoms or signs such as irregular vaginal bleeding, theca lutein cysts, asymmetric uterine enlargement, or persistently elevated serum β-hCG levels. The tumor may even perforate the myometrium causing intraperitoneal bleeding, or the tumor may invade into uterine vessels causing vaginal hemorrhage. Irregular vaginal bleeding may be due to a vascular uterine mass or a vaginal metastasis. Patients can also present with sepsis and abdominal pain, as the uterine tumor presents a nidus for infection (2). Placental site trophoblastic tumor and ETT present in the same manner as invasive moles and nearly always present with abnormal vaginal bleeding, because the disease remains localized to the uterus prior to metastasis (14). Only small amounts of β-hCG are produced relative for their size, and in some cases, the β-hCG is normal (2).
Metastatic GTN occurs in about 20% of patients who have undergone molar evacuation (35). Metastatic GTN most often arises from choriocarcinoma. Molar pregnancy is the most common antecedent of choriocarcinoma, but this tumor may also occur after normal pregnancy, ectopic pregnancy, or abortion. These highly vascular tumors tend to metastasize extensively and may cause spontaneous hemorrhage at the metastatic foci, causing symptoms. The metastases are sometimes histologically identical to molar disease, but the vast majority are choriocarcinomas. Metastatic spread is hematogenous. Because of its extensive vascular network, metastatic GTN often produces local spontaneous bleeding. Common metastatic sites of GTN as reported by the New England Trophoblastic Disease Center are summarized in Table 34-3 (38).
Pulmonary metastases are common, occurring in 80% of patients with metastatic disease (38), and result when trophoblastic tissue enters the circulation via uterine venous sinuses. Most often, this happens spontaneously, but it may also occur after molar evacuation. Because choriocarcinoma is a vascular tumor, hemoptysis is frequent with lung involvement. Other symptoms include chest pain, dyspnea, and cough.
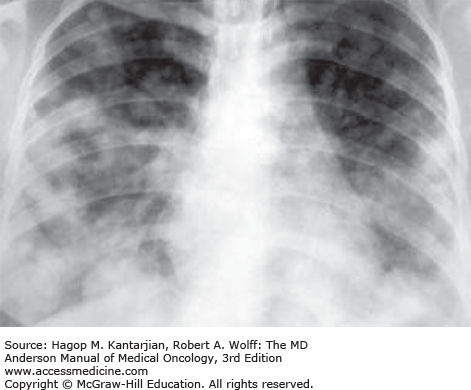
Pulmonary hypertension and pleural effusions may develop. An asymptomatic lesion on a chest x-ray or computed tomography (CT) scan may be the only sign of pulmonary involvement. Radiologic features may be subtle and include alveolar, nodular, and miliary patterns (39). Pulmonary metastases (Fig. 34-4) can be extensive and can cause respiratory failure and death.
Patients may experience right-upper-quadrant pain when hepatic metastases stretch the Glisson capsule. Gastrointestinal lesions can result in severe hemorrhage or perforation with peritonitis, either of which requires emergency intervention. Vaginal examination may reveal bluish metastatic deposits. Biopsy of these and other metastatic sites is contraindicated because severe uncontrolled bleeding may occur (35).
Central nervous system (CNS) involvement from metastatic GTN suggests widespread disease. Central nervous system metastases occur in 7% to 28% of patients with metastatic choriocarcinoma (38,39). The presenting neurologic symptoms include headache, hemiparesis, vomiting, dizziness, coma, grand mal seizure, visual disturbances, aphasia, and slurred speech. Weight loss and anorexia may also occur. In one-third of cases with cerebral metastases, there is no vaginal bleeding (2). Cerebral metastases tend to respond favorably to both radiotherapy and chemotherapy (Fig. 34-5).
DIAGNOSIS
The diagnosis of molar pregnancy is usually made by the histologic examination of curettage specimens. The diagnosis of persistent GTN or malignant GTN is most often indicated by plateauing or rising hCG titers after evacuation during surveillance. Histologic diagnosis of invasive mole, choriocarcinoma, or metastatic deposits should never be attempted because a biopsy of these neoplasms can cause massive, life-threatening hemorrhage. The hCG level in this setting is sensitive enough to make the diagnosis of malignant GTN without histologic confirmation. Beause PSTT and ETT do not typically arise after molar pregnancy, their diagnosis is usually made upon examination of the curettage, biopsy, or hysterectomy specimens (2). Presenting symptoms of PSTT are most commonly irregular vaginal bleeding, amenorrhea, and a pelvic mass (17).
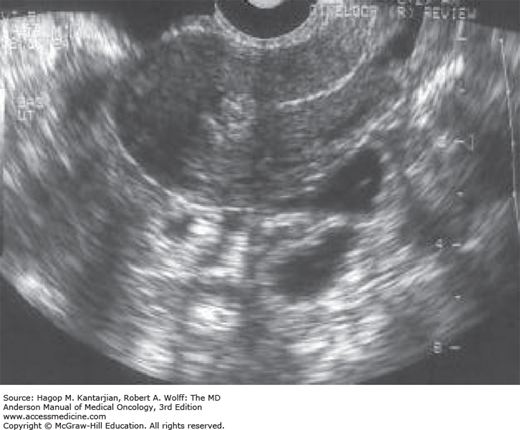
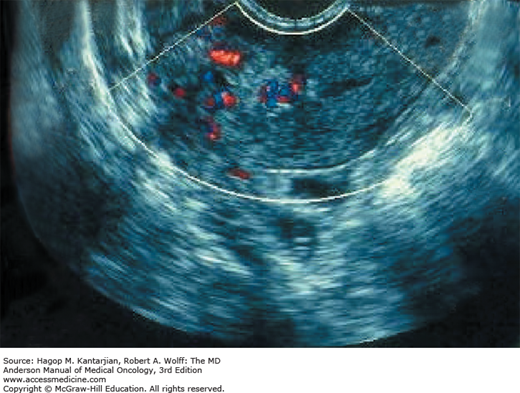
Ultrasonography is useful for the confirmation of molar pregnancy but is not diagnostic, because diagnosis depends on histologic examination of evacuated material. Ultrasonography is the first modality of radiographic imaging used when GTD is considered and may reveal the classic “snowstorm” appearance, which is due to the numerous chorionic villi exhibiting diffuse hydatidiform swelling (40) (see Fig. 34-5; Figs. 34-6 and 34-7). Such a classic appearance is less common now, because the diagnosis is often made in the first trimester prior to the development of significant hydropic change. More common is a mixed echogenic vascular mass. In the setting of partial mole, fetal parts may also be detected (1).
A chest x-ray should be performed in all patients because 70% to 80% of patients with metastatic GTN have lung involvement (35) (see Fig. 34-4). Brain imaging is not routinely warranted in the absence of symptoms or pulmonary abnormalities, because 97% to 100% of patients with CNS disease from choriocarcinoma have concomitant pulmonary metastases (41).
An abnormal chest x-ray associated with a β-hCG level that plateaus or rises during treatment is an indication for a more extensive evaluation for metastatic disease. Computed tomography scans of the brain, abdomen, and pelvis should be performed to evaluate other likely sites of metastatic spread.
Magnetic resonance imaging (MRI) is the preferred modality for localized disease to delineate invasiveness and tumor vascularity (Fig. 34-8) (42).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree