Compared with epithelial ovarian cancers, nonepithelial ovarian tumors are uncommon. They include malignancies of germ cell origin, sex-cord–stromal cell origin, metastatic carcinomas to the ovary, and a variety of extremely rare ovarian cancers, for example, sarcomas and lipoid cell tumors.
Nonepithelial malignancies of the ovary account for approximately 10% of all ovarian cancers (1,2). Although there are many similarities in the presentation, evaluation, and management of these patients, these tumors also have unique features that require a special approach (1–5).
Germ Cell Malignancies
Germ cell tumors are derived from the primordial germ cells of the ovary and occur with only about one-tenth the incidence of malignant germ cell tumors of the testis. Although they can arise in extragonadal sites such as the mediastinum and the retroperitoneum, the majority of germ cell tumors arise in the gonad from undifferentiated germ cells. The variation in the site of these cancers is explained by the embryonic migration of the germ cells from the caudal part of the yolk sac to the dorsal mesentery before their incorporation into the sex cords of the developing gonads (1,2).
Germ cell tumors are a model of a curable cancer. The management of patients with ovarian germ cell tumors has largely been extrapolated from the much greater experience of treating males with the more common testicular germ cell tumors. There have been many randomized trials for testicular germ cell tumors, which have provided a strong evidence base for treatment decision making (6,7). The outcome of patients with testicular germ cell tumors is better in experienced centers, and it is reasonable to suggest the same will be true for the less common ovarian counterparts. The cure rate is high, and attention is now being directed at reducing toxicity without compromising survival. There are still a small number of patients who die from the disease, and studies are in progress to try to improve the outcome for this high-risk, poor-prognostic subset (6,7).
In one of the largest reported series, which included 113 patients with advanced ovarian germ cell tumors treated with cisplatin-based chemotherapy, Murugaesu et al. (8) reported that stage and elevated tumor markers were independent poor prognostic indicators. These findings are important because they identify similar prognostic factors for ovarian and testicular germ cell tumors, and are in accordance with the clinical observation that testicular and ovarian germ cell tumors behave similarly. This is relevant for the management of patients with ovarian germ cell tumors because it may help to identify a poor prognostic subset of patients who require more intensive treatment (8).
Table 12.1 Histologic Typing of Ovarian Germ Cell Tumors
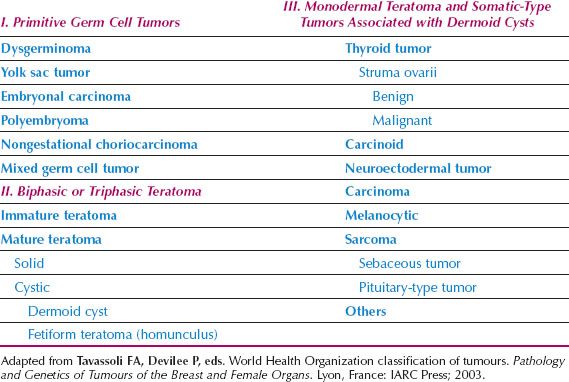
Classification
A histologic classification of ovarian germ cell tumors is presented in Table 12.1 (1,9). Both α-fetoprotein (AFP) and human chorionic gonadotropin (hCG) are secreted by some germ cell malignancies. An elevated AFP and β-hCG can be clinically useful in the differential diagnosis of patients with a pelvic mass, and in monitoring patients after surgery. Placental alkaline phosphatase (PLAP) and lactate dehydrogenase (LDH) are elevated in up to 95% of patients with dysgerminomas, and serial monitoring of serum LDH levels may be useful for monitoring the disease. PLAP is more useful as an immunohistochemical marker than as a serum marker. The classification of germ cell tumors is based both on histologic features and immunohistochemical expression of tumor markers (Fig. 12.1) (10,15).
In this scheme, embryonal carcinoma, which is composed of undifferentiated cells that synthesize both hCG and AFP, is the progenitor of several other germ cell tumors (4,10). More differentiated germ cell tumors—such as the endodermal sinus tumor (EST), which secretes AFP, and choriocarcinoma, which secretes hCG—are derived from the extraembryonic tissues; immature teratomas are derived from the embryonic cells and do not secrete hCG, but may be associated with an elevated AFP. Elevated hCG levels are seen in 3% of dysgerminomas and the level is typically less than 100 International Unit. AFP is never elevated in pure dysgerminomas (1).
Epidemiology
Although 20–25% of all benign and malignant ovarian neoplasms are of germ cell origin, they account for only about 5% of all malignant ovarian neoplasms (1). In Asian and black societies where epithelial ovarian cancers are much less common, they may account for as many as 15% of ovarian cancers. In the first two decades of life, almost 70% of ovarian tumors are of germ cell origin, and one-third of these are malignant (1,2). Germ cell tumors account for two-thirds of the ovarian malignancies in this age group. Germ cell cancers also are seen in the third decade, but thereafter they become quite rare.
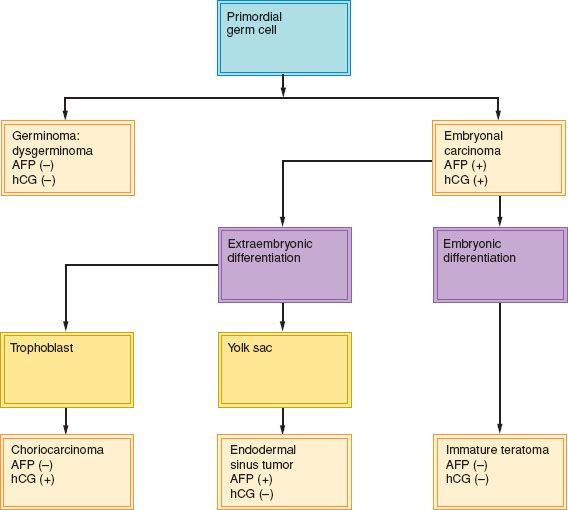
Figure 12.1 Relationship between examples of pure malignant germ cell tumors and their secreted marker substances.
Clinical Features
Symptoms
In contrast to the relatively slow-growing epithelial ovarian tumors, germ cell malignancies grow rapidly, and often are characterized by subacute pelvic pain related to capsular distention, hemorrhage, or necrosis. The rapidly enlarging pelvic mass may produce pressure symptoms on the bladder or rectum, and menstrual irregularities also may occur in menarchal patients. Some young patients may misinterpret the symptoms as those of pregnancy, and this can lead to a delay in diagnosis. Acute symptoms associated with torsion or rupture can develop. These symptoms may be confused with acute appendicitis. In more advanced cases, ascites may develop, and the patient may present with abdominal distention (3).
Signs
In patients with a palpable adnexal mass, the evaluation can proceed as outlined in Chapter 11. Some patients with germ cell tumors will be premenarchal. If the lesions are principally solid, or a combination of solid and cystic on an ultrasonographic evaluation, a neoplasm is probable and a malignancy is possible (Fig. 12.2). The remainder of the physical examination should search for signs of ascites, pleural effusion, and organomegaly.
Diagnosis
Adnexal masses measuring 2 cm or more in premenarchal girls or complex masses 8 cm or more in premenopausal patients will usually require surgical exploration (Fig. 12.3). In young patients, preoperative blood tests should include serum hCG, AFP, LDH and CA125 levels, a complete blood count, and liver function tests. A radiograph of the chest is important because germ cell tumors can metastasize to the lungs or mediastinum. A karyotype should ideally be obtained preoperatively on all premenarchal girls because of the propensity of these tumors to arise in dysgenetic gonads, but this may not be practical (3,11). A preoperative computed tomographic (CT) scan or magnetic resonance imaging (MRI) may document the presence and extent of retroperitoneal lymphadenopathy or liver metastases, but unless there is very extensive metastatic disease, is unlikely to influence the decision to operate on the patient initially. If postmenarchal patients have predominantly cystic lesions up to 8 cm in diameter, they may undergo observation or a trial of hormonal suppression for two cycles (12).
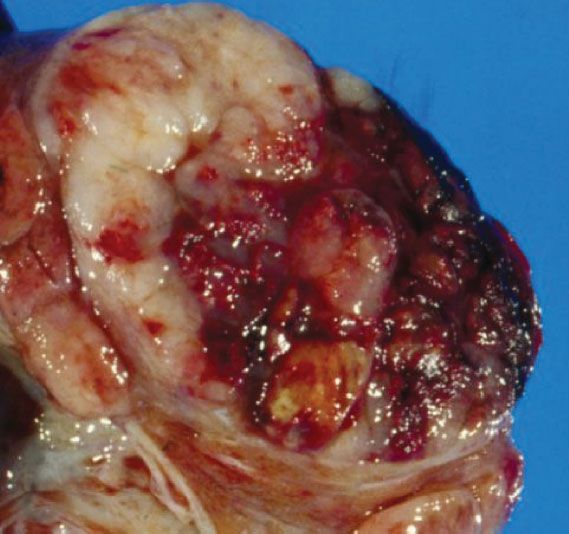
Figure 12.2 Dysgerminoma of the ovary. Note that the lesion is principally solid with some cystic areas and necrosis. (From Berek JS, Longacre TA, Friedlander M. Ovarian, fallopian tube and peritoneal cancer. In: Berek JS, ed. Berek & Novak’s Gynecology. 15th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2012.)
Dysgerminoma
Dysgerminomas are the most common malignant germ cell tumor, accounting for approximately 30–40% of all ovarian cancers of germ cell origin (2,10). They represent only 1–3% of all ovarian cancers, but represent as many as 5–10% of ovarian cancers in patients younger than 20 years of age. Seventy-five percent of dysgerminomas occur between the ages of 10 and 30 years, 5% occur before the age of 10 years and they rarely occur after age 50 (1,4). They typically occur in young women and 20–30% of ovarian malignancies associated with pregnancy are dysgerminomas.
Approximately 5% of dysgerminomas occur in phenotypic females with abnormal gonads (1,11). Dysgerminomas can be associated with patients who have pure gonadal dysgenesis (46XY, bilateral streak gonads), mixed gonadal dysgenesis (45X/46XY, unilateral streak gonad, contralateral testis), and the androgen insensitivity syndrome (46XY, testicular feminization). Therefore, in premenarchal patients with a pelvic mass, the karyotype should be determined, particularly if a dysgerminoma is considered as the likely diagnosis (Fig. 12.4).
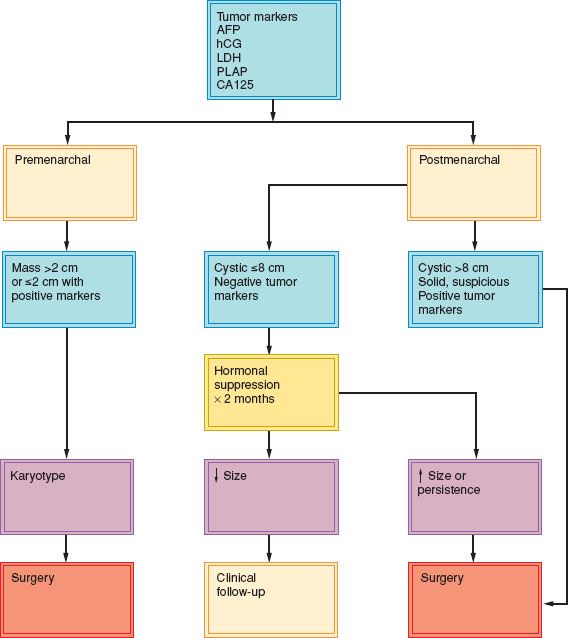
Figure 12.3 Evaluation of a pelvic mass in young female patients.
In most patients with gonadal dysgenesis, dysgerminomas arise in a gonadoblastoma, which is a benign ovarian tumor composed of germ cells and sex-cord stroma. If gonadoblastomas are left in situ in patients with gonadal dysgenesis, more than 50% will subsequently develop ovarian malignancies (13).
Approximately 65% of dysgerminomas are stage I at diagnosis (1,3,5,14,16,18–20). Eighty-five to ninety percent of stage I tumors are confined to one ovary, while 10–15% are bilateral. All other germ cell tumors are rarely bilateral.
In patients whose contralateral ovary has been preserved, a dysgerminoma can develop in 5–10% of them over the next 2 years (1). This figure includes patients who have not received systemic chemotherapy, as well as patients with gonadal dysgenesis.
In the 25% of patients who present with metastatic disease, the tumor most commonly spreads via the lymphatics, particularly to the higher para-aortic nodes (18). They can also spread hematogenously, or by direct extension through the capsule of the ovary with exfoliation and dissemination of cells throughout the peritoneal surfaces. Metastases to the contralateral ovary may be present when there is no other evidence of spread. An uncommon site of metastatic disease is bone, and when metastasis to this site occurs, the metastases are seen typically in the lower vertebrae. Metastases to the lungs, liver, and brain are rare and seen most often in patients with long-standing or recurrent disease. Metastasis to the mediastinum and supraclavicular lymph nodes is also usually a late manifestation of disease (14,16).
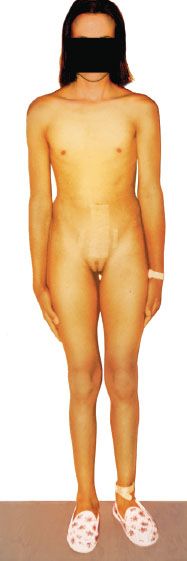
Figure 12.4 A 16-year-old girl with 46XY gonadal dysgenesis, showing lack of secondary sexual features, who developed dysgerminoma.
Treatment
The treatment of patients with early dysgerminoma is primarily surgical, including resection of the primary lesion and limited surgical staging–washings, omental biopsy, careful palpation of all peritoneal surfaces and retroperitoneal nodes, and biopsy of anything suspicious. Chemotherapy is administered to patients with metastatic disease. Because the disease principally affects young women, special consideration must be given to the preservation of fertility (17,18). A comparison of outcomes based on treatment at the Norwegian Radium Hospital clearly demonstrates the superiority of chemotherapy over radiation. Survival was better and morbidity was lower in the group treated with chemotherapy (17). An algorithm for the management of ovarian dysgerminoma is presented in Figure 12.5.
Surgery
The minimum operation for ovarian dysgerminoma is unilateral oophorectomy (19,21). If there is a desire to preserve fertility, as is usually the case, the contralateral ovary, fallopian tube, and uterus should be left in situ even in the presence of metastatic disease, because of the sensitivity of the tumor to chemotherapy. If fertility preservation is not required, it may be appropriate to perform a total abdominal hysterectomy and bilateral salpingo-oophorectomy in patients with advanced disease (5), although this will be appropriate in only a very small minority of patients. In patients whose karyotype contains a Y chromosome, both ovaries should be removed, although the uterus may be left in situ for possible future embryo transfer. Cytoreductive surgery is of unproven value, but bulky disease that can be readily resected (e.g., an omental cake) should be removed at the initial operation. It is important not to undertake surgery that is potentially morbid and may delay the initiation of chemotherapy.
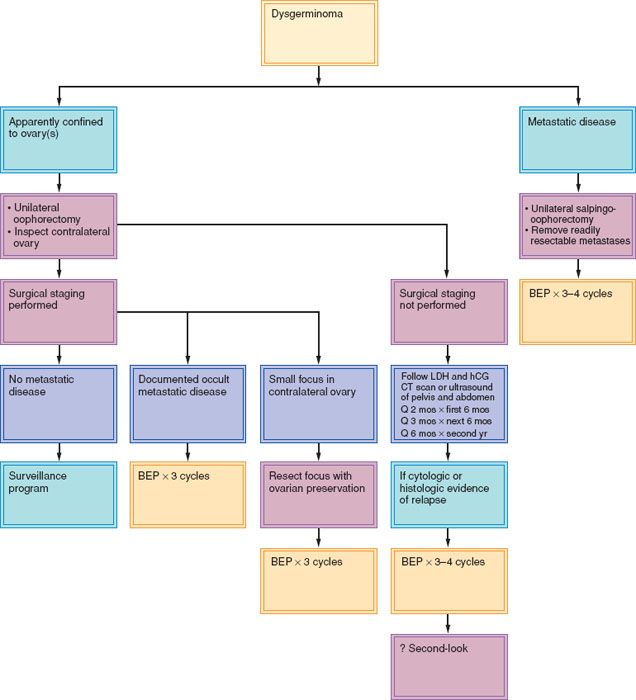
Figure 12.5 Management of dysgerminoma of the ovary. BEP, bleomycin, etoposide, and cisplatin; CT, computed tomogram.
In patients in whom the dysgerminoma appears on inspection to be confined to the ovary, a careful staging operation should be undertaken to determine the presence of any occult metastatic disease. These tumors often metastasize to the para-aortic nodes around the renal vessels. Peritoneal washings should be taken for cytology, and a thorough exploration made of all peritoneal surfaces and retroperitoneal lymph nodes, with biopsy or resection of any noted abnormalities. The contralateral ovary should be carefully inspected because dysgerminoma is the only germ cell tumor that tends to be bilateral, and not all of the bilateral lesions have obvious ovarian enlargement. Therefore careful inspection and palpation of the contralateral ovary and excisional biopsy of any suspicious lesion are desirable (5,19–21). If a small contralateral tumor is found, it may be possible to resect it and preserve some normal ovary.
Many patients with a dysgerminoma will have a tumor that is apparently confined to one ovary and will be referred after unilateral salpingo-oophorectomy without surgical staging. The options for such patients are (i) repeat laparotomy for surgical staging, (ii) regular pelvic and abdominal CT scans, or (iii) adjuvant chemotherapy (18). Because most dysgerminomas are confined to the ovary at presentation and are rapidly growing tumors, the author’s preference is to offer regular and close surveillance to such patients (23,24) (Fig. 12.1).
Radiation
Loss of fertility and second malignancies are important late effects of radiation therapy, so it is no longer used for primary treatment (17). Radiation can be used selectively to treat recurrent disease (5,17,20). Dysgerminomas are very sensitive to radiation therapy, and doses of 2,500 to 3,500 cGy may be curative; however, it is uncommonly used because these tumors are very sensitive to platinum-based chemotherapy, and have a high likelihood of cure.
Chemotherapy
Chemotherapy is regarded as the treatment of choice (21,22,25–32,34). The obvious advantage is the preservation of fertility in most patients, and the reduced risk of second malignancies compared with radiation (21,35–39).
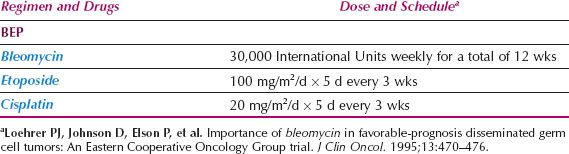
The most frequently used chemotherapeutic regimen is BEP (bleomycin, etoposide, and cisplatin). In the past, VBP (vinblastine, bleomycin, and cisplatin), and VAC (vincristine, actinomycin, and cyclophosphamide) were commonly used but are now rarely used (21,22,25–28) (Table 12.2).
The Gynecologic Oncology Group (GOG) studied three cycles of EC: Etoposide (120 mg/m2 intravenously on days 1, 2, and 3 every 4 weeks) and carboplatin (400 mg/m2 intravenously on day 1 every 4 weeks) in 39 patients with completely resected ovarian dysgerminoma, stages IB, IC, II, or III (31). The results were excellent, and GOG reported a sustained disease-free remission rate of 100%.
For patients with advanced, incompletely resected germ cell tumors, the GOG studied cisplatin-based chemotherapy on two consecutive protocols (25). In the first study, patients received four cycles of vinblastine (12 mg/m2 every 3 weeks), bleomycin (20 unit/m2 intravenously every week for 12 weeks), and cisplatin (20 mg/m2/d intravenously for 5 days every 3 weeks). Patients with persistent or progressive disease at second-look laparotomy were treated with six cycles of VAC. In the second trial, patients received three cycles of BEP initially, followed by consolidation with VAC, which was later discontinued in patients with dysgerminomas (25). VAC does not appear to improve the outcome following the BEP regimen and is no longer used.
Table 12.2 Combination Chemotherapy for Germ Cell Tumors of the Ovary
A total of 20 evaluable patients with stages III and IV dysgerminoma were treated in these two protocols, and 19 were alive and free of disease after 6 to 68 months (median = 26 months). Fourteen of these patients had a second-look laparotomy, and all findings were negative. A study at MD Anderson Hospital (28) used BEP in 14 patients with residual disease, and all patients were free of disease with long-term follow-up. In another series of 26 patients with pure ovarian dysgerminomas who received BEP chemotherapy, 54% of whom had stage IIIC or IV disease, 25 (96%) remained continuously disease free following three to six cycles of therapy (34).
These results indicate that patients with an advanced-stage, incompletely resected dysgerminoma have an excellent prognosis when treated with cisplatin-based combination chemotherapy (32–38). The optimal regimen is three to four cycles of BEP based on the data from testis cancers (33,39,40) with the number of cycles depending on the extent of disease and the presence or absence of visceral metastases. If bleomycin is contraindicated or omitted because of lung toxicity, consideration should be given to four cycles of cisplatin and etoposide rather than three cycles of BEP.
There is no need to perform a second-look laparotomy in patients with dysgerminomas (41–43). The role of surgery to resect residual masses following chemotherapy for dysgerminomas is not clear, as the vast majority of these patients will only have necrotic tissue and nonviable tumor. In general, these patients should be closely monitored with scans and tumor markers. A positron emission tomography (PET–CT) scan should be considered in patients who have bulky residual masses larger than 3 cm more than 4 weeks after chemotherapy. A positive PET–CT scan appears to be a sensitive predictor of residual seminoma in males in these circumstances, (44) with residual disease being evident in 30–50% of patients. If the PET–CT is positive or if there is a suggestion of progressive disease on scans, ideally there should be histologic confirmation of residual disease before embarking on salvage therapy (45).
Recurrent Disease
Although recurrences are uncommon, 75% will occur within the first year after initial treatment (1–4), with the most common sites being the peritoneal cavity and the retroperitoneal lymph nodes. These patients should be treated with either chemotherapy or radiation, depending on the location of disease and the primary treatment. Patients with recurrent disease who have had no therapy other than surgery should be treated with chemotherapy. If previous chemotherapy with BEP has been given, an alternative regimen such as TIP (paclitaxel, ifosfamide, and cisplatin), a commonly used salvage regimen in testicular germ cell tumors (46), may be tried.
These treatment decisions should be made in a multidisciplinary setting with the input of physicians experienced in the management of patients with germ cell tumors. Consideration may be given to the use of high-dose chemotherapy with peripheral stem cell support in selected patients. A number of high-dose regimens have been used in phase II studies, and the choice depends on the previous chemotherapy, the time to recurrence, and the residual toxicity from the previous therapy (47,48). It is unclear whether high-dose chemotherapy is superior to conventional dose chemotherapy as first-line salvage therapy for patients with relapsed disease. The only randomized trial, conducted by the European Group for Blood and Marrow Transplantation (EBMT)-IT-94, did not demonstrate superiority for three cycles of VIP or vinblastine-ifosfamide-cisplatin (VeIP) followed by high-dose chemotherapy compared with four cycles of conventional dose chemotherapy. An international randomized trial (TIGER) plans to randomize 390 patients with recurrent germ cell tumors to four cycles of conventional dose cisplatin-based chemotherapy with TIP, compared with two cycles of paclitaxel-ifosfamide followed by three cycles of high-dose carboplatin and etoposide with autologous stem-cell support (TI-CE) (48).
Radiation therapy may be considered in selected patients with dysgerminomas with a localized recurrence, but this has the major disadvantage of causing loss of fertility if pelvic and abdominal radiation is required, and may also compromise the ability to deliver further chemotherapy if unsuccessful (17).
Pregnancy
Because dysgerminomas tend to occur in young patients, they may coexist with pregnancy. When a stage IA cancer is found, the tumor can be removed intact and the pregnancy continued. In patients with more advanced disease, continuation of the pregnancy will depend on gestational age. Chemotherapy can be given in the second and third trimesters in the same dosages as given for the nonpregnant patient without apparent detriment to the fetus (35,49). Relatively few patients have been treated with BEP during pregnancy and some fetal malformations and complications have been reported, underscoring the importance of ensuring that only patients who definitely require chemotherapy during pregnancy should be treated (50).
Prognosis
In patients with stage IA dysgerminoma, unilateral oophorectomy alone results in a 5-year disease-free survival rate of greater than 95% (5,20). The features that have been associated with a higher tendency to recurrence include tumors larger than 10 to 15 cm in diameter, age younger than 20 years, and microscopic features that include numerous mitoses, anaplasia, and a medullary pattern (1,10).
Kumar et al. abstracted data on malignant ovarian germ cell tumors from the Surveillance, Epidemiology, and End Results (SEER) program from 1988 through 2004 (51). There were a total of 1,296 patients with dysgerminomas, immature teratomas, or mixed germ cell tumors, 613 (47.3%) of whom had lymphadenectomies. Lymph node metastases were present in 28% of dysgerminomas, 8% of immature teratomas, and 16% of mixed germ cell tumors (p < 0.05). The 5-year survival for patients with negative nodes was 95.7% compared to 82.8% for patients with positive nodes (p < 0.001). The same group updated the results recently and reported on 1,083 patients with ovarian germ cell tumors who had surgery and who were believed to have disease clinically confined to the ovary (52). This included 590 (54.5%) who had no lymphadenectomy and 493 (45.5%) who had a lymphadenectomy. Of the latter, 52 (10.5%) were upstaged to FIGO stage IIIC due to nodal metastases. The 5-year survival was 96.9% for patients who did not have a lymphadenectomy, 97.7% for those who did, and 93.4% for patients who were found to have stage IIIC disease after lymphadenectomy. These survivals were not statistically different, and underscore the excellent prognosis for patients with dysgerminomas (22,25–32,34–38).
Immature Teratomas
Immature teratomas typically contain immature neuroepithelium and may be pure immature teratomas or occur in combination with other germ cell tumors as mixed germ cell tumors. The pure immature teratoma accounts for fewer than 1% of all ovarian cancers, but it is the second most common germ cell malignancy and represents 10–20% of all ovarian malignancies seen in women younger than 20 years of age (1). Approximately 50% of pure immature teratomas of the ovary occur between the ages of 10 and 20 years, and they rarely occur in postmenopausal women.
Semi-quantification of the amount of neuroepithelium correlates with survival in ovarian immature teratomas and is the basis for the grading of these tumors (53–55). Those with less than one lower-power field (4) of immature neuroepithelium on the slide with the greatest amount of immature neuroepithelium (grade 1) have a survival of at least 95%, whereas greater amounts of immature neuroepithelium (grades 2 and 3) appear to have a lower overall survival (approximately 85%) (55). This may not apply to immature teratomas of the ovary in children, because they appear to have a very good outcome with surgery alone, regardless of the degree of immaturity. These findings are from an era when not all patients would have received platinum-based chemotherapy (56,57).
Some pathologists have recommended a two-tiered grading system, suggesting that immature teratomas be categorized as either low grade or high grade because of the significant inter- and intraobserver difficulty with a three-grade system (55). This is the authors’ current practice.
Immature ovarian teratomas may be associated with gliomatosis peritonei, which has a favorable prognosis if composed of completely mature tissues. Recent reports have suggested that these glial “implants” are not tumor derived, but represent teratoma-induced metaplasia of pluripotential müllerian stem cells in the peritoneum (56,58,59). The researchers have exploited a unique characteristic of ovarian teratomas. The latter typically contain a duplicated set of maternal chromosomes, and are therefore homozygous at polymorphic microsatellite loci, while DNA from matched normal tissue contains genetic material of both maternal and paternal origin, so exhibits heterozygosity at many of these same polymorphic microsatellite loci.
Malignant transformation of a mature teratoma is a rare event. Squamous cell carcinoma is the most frequent subtype of malignancy, but adenocarcinomas, primary melanomas, and carcinoids may also rarely occur (see below) (30). The risk is reported to be between 0.5% and 2% of teratomas, and usually occurs in postmenopausal patients.
Diagnosis
The preoperative evaluation and differential diagnosis are the same as for patients with other germ cell tumors. Some of these tumors will contain calcifications similar to mature teratomas, and this can be detected by a radiograph of the abdomen or by ultrasonography. Rarely, they are associated with the production of steroid hormones and can be accompanied by sexual pseudoprecocity (4). AFP may be elevated in some patients with a pure immature teratoma but hCG is not elevated.
Treatment
Surgery
In a premenopausal patient where the tumor appears confined to a single ovary, unilateral oophorectomy and limited surgical staging should be performed. In the rare postmenopausal patient with an immature teratoma, a total abdominal hysterectomy and bilateral salpingo-oophorectomy may be performed. Contralateral involvement is rare, and routine resection or wedge biopsy of the contralateral ovary is unnecessary (2). Any suspicious lesions on the peritoneal surfaces should be sampled and submitted for histologic evaluation. The most frequent site of dissemination is the peritoneum and, much less commonly, the retroperitoneal lymph nodes. Blood-borne metastases to organ parenchyma such as the lungs, liver, or brain are uncommon. When present, they are usually seen in patients with late or recurrent disease and most often in tumors that are high grade (4).
It is unclear whether debulking of metastases improves the response to combination chemotherapy (60,61). Cure ultimately depends on the ability to deliver chemotherapy promptly. Any surgical resection that may be potentially morbid and therefore delay chemotherapy should be resisted, although surgical resection of any residual disease should be considered at the completion of chemotherapy.
Chemotherapy
Patients with stage IA, grade 1 tumors have an excellent prognosis, and no adjuvant therapy is required. In patients with high-grade, stage IA immature teratomas, adjuvant chemotherapy has commonly been given, although this has been questioned, as excellent results have been also reported with close surveillance and treating only patients who have a recurrence (17,18,26–28,42,57,62–76).
The most frequently used combination chemotherapeutic regimen in the past was VAC (70–72), but a GOG study reported a relapse-free survival rate in patients with incompletely resected disease of only 75% (72). The approach over the last 20 years has been to incorporate cisplatin into the primary treatment of these tumors, and most of the experience has been with the VBP in the past and BEP more recently (65).
The GOG prospectively evaluated three courses of BEP therapy in patients with completely resected stage I, II, and III ovarian germ cell tumors (30). Overall, the toxicity was acceptable, and 91 of 93 patients (97.8%) with nondysgerminomatous tumors were clinically free of disease. In nonrandomized studies, the BEP regimen is superior to the VAC regimen in the treatment of completely resected nondysgerminomatous germ cell tumors of the ovary. Some patients can progress rapidly postoperatively, and, in general, treatment should be initiated as soon as possible after surgery, preferably within 7 to 10 days, in those patients who require chemotherapy.
The switch from VBP to BEP has been prompted by the experience in patients with testicular cancer, where the replacement of vinblastine with etoposide has been associated with a better therapeutic index (i.e., equivalent efficacy and lower morbidity), with less neurologic and gastrointestinal toxicity, and improved outcomes (65,66). Furthermore, the use of bleomycin appears to be important in this group of patients. In a randomized study of three cycles of etoposide plus cisplatin with or without bleomycin (EP vs. BEP) in 166 patients with germ cell tumors of the testes, the BEP regimen had a relapse-free survival rate of 84% compared with 69% for the EP regimen (p = 0.03) (40).
Cisplatin is superior to carboplatin in metastatic germ cell tumors of the testis. One hundred and ninety-two patients with good prognosis germ cell tumors of the testes were entered into a study of four cycles of etoposide plus cisplatin (EP) versus four cycles of etoposide plus carboplatin (EC). There were three relapses with the EP regimen versus seven with the EC regimen (41). A German group randomized patients to (i) a BEP regimen of three cycles at standard doses given days 1 to 5 versus (ii) a CEB regimen of carboplatin (target AUC of 5 [mg/dL × min] on day 1), etoposide 120 mg/m2 on days 1 to 3, and bleomycin 30 mg on days 1, 8, and 15 (77). Four cycles of CEB were given, with the omission of bleomycin in the fourth cycle so that the cumulative doses of etoposide and bleomycin in the two treatment arms were comparable. Fifty-four patients were entered on the trial; 29 were treated with BEP and 25 with CEB chemotherapy. More patients treated with CEB relapsed after therapy (32% vs. 13%). Four patients (16%) treated with CEB died of disease progression in contrast to one patient (3%) after BEP therapy. The trial was terminated early after an interim analysis. The inferiority of carboplatin was confirmed in a larger randomized trial reported by Horwich et al. (78). In view of these results, BEP is the preferred treatment regimen (65,79–81). The 3-day schedule has been found to be equivalent to a 5-day schedule for BEP chemotherapy. A cycle of BEP consisted of etoposide 500 mg/m2, administered at either 100 mg/m2 days 1 through 5 or 165 mg/m2 days 1 through 3, cisplatin 100 mg/m2, administered at either 20 mg/m2 days 1 through 5 or 50 mg/m2 days 1 and 2. Bleomycin 30,000 International Unit is administered on days 1, 8, and 15 during cycles 1 through 3.
Recurrent Disease
The principles and approach are identical to the management of recurrent dysgerminoma, as discussed above.
Second-look Laparotomy
Second-look operation for ovarian germ cell tumors (43,44) is not indicated in patients who have received adjuvant chemotherapy (i.e., stage IA, grades 2 and 3). However, surgery should be considered in patients with metastatic immature teratomas who have residual disease at the completion of chemotherapy because they may have residual mature teratoma and are at risk of growing teratoma syndrome, a rare complication of immature teratomas (90–92). Furthermore, cancers can arise at a later date in residual mature teratoma, and it is important to resect any residual mass and exclude persistent disease, as further chemotherapy may be indicated.
The principles of surgery are based on the much larger experience of surgery in males with residual masses following chemotherapy for germ cell tumors with a component of immature teratoma (93). Mathew et al. (94) reported their experience of laparotomy in assessing the nature of postchemotherapy residual masses in ovarian germ cell tumors. Sixty-eight patients completed combination chemotherapy with cisplatin regimens, of whom 35 had radiologic evidence of residual masses. Twenty-nine of these 35 patients underwent laparotomy, and 10 patients (34.5%) had viable tumor, including seven cases (24.2%) of immature teratoma. Nineteen patients (65.5) had no evidence of malignancy, including three (10.3%) cases showing mature teratoma, and 16 (55.2%) showing necrosis or fibrosis only. None of the patients with a dysgerminoma or embryonal carcinoma and a radiologic residual mass of less than 5 cm had viable tumor present, whereas all patients with primary tumors containing a component of teratoma had residual tumor, strengthening the case for surgery in patients with metastatic immature teratoma and any residual mass (94,95).
Prognosis
The most important prognostic feature of the immature teratoma is the grade of the lesion (1,53). In addition, the stage of disease and the extent of tumor at the initiation of treatment also have an impact on prognosis (4). Overall, the 5-year survival rate for patients with all stages of pure immature teratomas is 70–80%, and it is 90–95% for patients with surgical stage I tumors (42,53,62).
The degree or grade of immaturity generally predicts the metastatic potential and prognosis. The 5-year survival rates have been reported to be 82%, 62%, and 30% for patients with grades 1, 2, and 3, respectively (53) but many of these patients were treated in an era before optimal chemotherapy was available, and these figures do not match current experience and more recently published data (67). For example, Lai et al. (96) reported on the long-term outcome of 84 patients with ovarian germ cell tumors, including 29 immature teratomas, and the 5-year survival was 97.4%.
Occasionally, these tumors are associated with mature or low-grade glial elements that have implanted throughout the peritoneum. Such patients have a favorable long-term survival (4). Mature glial elements can grow and mimic malignant disease and may need to be resected to relieve pressure on surrounding structures.
Endodermal Sinus Tumor
Endodermal sinus tumors (ESTs) have also been referred to as yolk sac carcinomas because they are derived from the primitive yolk sac (1). They are the third most frequent malignant germ cell tumor of the ovary.
ESTs have a median age of 18 years at diagnosis (1–3,97,98). Approximately one-third of the patients are premenarchal at presentation. Abdominal or pelvic pain occurs in approximately 75% of patients, whereas an asymptomatic pelvic mass is documented in 10% of patients (3).
Most ESTs secrete AFP and rarely may also elaborate detectable alpha-1-antitrypsin (AAT). There is a good correlation between the extent of disease and the level of AFP, although discordance also has been observed. The serum level of AFP is useful in monitoring the patient’s response to treatment, as well as in follow-up (97–104).
Treatment
Surgery
The treatment of an EST consists of surgical exploration, unilateral salpingo-oophorectomy, a frozen section for diagnosis, and limited surgical staging. A hysterectomy and contralateral salpingo-oophorectomy should not be done (4,100,101). Conservative surgery and adjuvant chemotherapy allow fertility preservation as with other germ cell tumors (21). In patients with metastatic disease, all gross disease should be resected if possible. At surgery, the tumors tend to be solid and large, ranging in size from 7 to 28 cm (median = 15 cm) in the GOG series. Bilaterality is not seen in EST, and the other ovary is involved with metastatic disease only when there are other metastases in the peritoneal cavity. Most patients have early-stage disease: 71% stage I, 6% stage II, and 23% stage III (101,105).
Chemotherapy
All patients with ESTs should be treated with chemotherapy shortly after recovering from surgery. Prior to the routine use of combination chemotherapy, the 2-year survival rate was approximately 25%. After the introduction of the VAC regimen, the survival rate improved to 60–70%, which highlights the chemosensitivity of the majority of these tumors (71,72). All patients should be treated with a cisplatin-based regimen such as BEP, which is considered the standard of care. The chance of cure now approaches 100% for patients with early-stage disease, and is at least 75% for patients with more advanced-stage disease (101).
The optimal number of treatment cycles has not been established in ovarian germ cell tumors, but it is reasonable to extrapolate from the much larger experience in testicular germ cell tumors where three cycles of BEP is considered optimal for good prognosis, low-risk patients and four cycles for patients with intermediate to high-risk tumors (7). In patients for whom bleomycin is omitted or discontinued because of toxicity, four cycles of cisplatin and etoposide are recommended. An alternative approach is to use VIP (etoposide, ifosfamide, and cisplatin) in patients with more advanced disease in whom bleomycin is contraindicated. Four cycles of VIP are equivalent to four cycles of BEP, but it is more myelotoxic and requires growth-factor support (6,7,108). These patients should only be treated by clinicians experienced in the management of germ cell tumors as the outcomes of patients in inexperienced hands are compromised.
Neoadjuvant chemotherapy followed by fertility-sparing surgery may also be a reasonable option for patients with advanced ovarian germ cell tumors not suitable for optimal cytoreduction, as shown in a recent study of 21 patients from India (109).
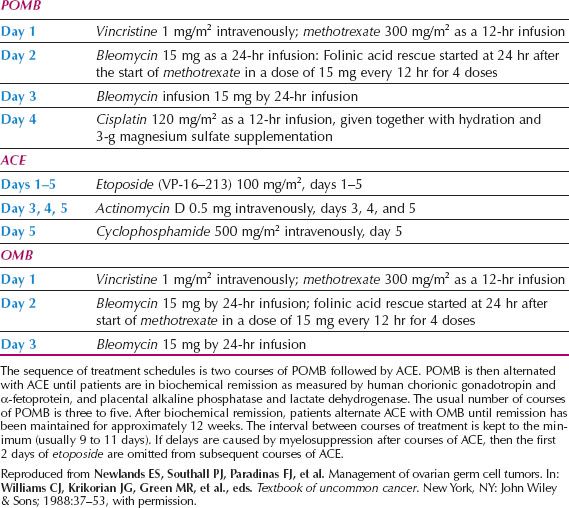
A number of years ago, the group from the Charing Cross Hospital in London developed the POMB-ACE (cisplatin, vincristine, methotrexate, bleomycin, actinomycin D, cyclophosphamide, etoposide) regimen for high-risk germ cell tumors of any histologic type (104) (Table 12.3). Their results appear to be superior to BEP in patients with poor prognostic features, but this has not been confirmed in randomized controlled trials. This protocol introduces seven drugs into the initial management, which is intended to reduce the chances of developing drug resistance, which may be particularly relevant for patients with large volume metastatic disease. The authors have used the POMB-ACE regimen as primary therapy for selected patients with liver or brain metastases, and this regimen is still used at Charing Cross.
The POMB schedule is only moderately myelosuppressive, so the intervals between each course can be kept to a maximum of 14 days (usually 9 to 11 days), thereby minimizing the time for tumor regrowth. When bleomycin is given by a 48-hour infusion, pulmonary toxicity is reduced (108). With a maximum of 9 years of follow-up, the Charing Cross group has seen no long-term side effects in patients treated with POMB-ACE. Children have developed normally, menstruation has been physiologic, and several women have completed normal pregnancies. There are a number of groups investigating different regimens to treat patients with testicular germ cell tumors who fall into the intermediate or high-risk prognostic subsets, and these regimens include accelerated BEP every 2 weeks.
Table 12.3 POMB-ACE Chemotherapy for Germ Cell Tumors of the Ovary
Second-look Laparotomy
A second-look operation is not required in patients with EST whose AFP values return to normal and remain normal (102,103). There have been reported cases in which the AFP measurement has returned to normal in spite of persistent measurable disease; some of these cases have been mixed germ cell tumors and in patients with residual masses, surgery may be required (103).
Rare Germ Cell Tumors of the Ovary
Embryonal Carcinoma
Embryonal carcinoma of the ovary is an extremely rare tumor that is distinguished from a choriocarcinoma of the ovary by the absence of syncytiotrophoblastic and cytotrophoblastic cells. The patients are very young, their ages ranging between 4 and 28 years (median = 14 years) in two series (105). Older patients have been reported (106). Embryonal carcinomas may secrete estrogens, with the patient exhibiting symptoms and signs of precocious pseudopuberty or irregular bleeding (1). The presentation is otherwise similar to that of the EST. The primary lesions tend to be large, and approximately two-thirds are confined to one ovary at the time of presentation. These lesions frequently secrete AFP and hCG, which are useful for following the response to subsequent therapy (102).
The treatment of embryonal carcinomas is the same as that for ESTs (27,76,107).
Choriocarcinoma of the Ovary
Pure nongestational choriocarcinoma of the ovary is an extremely rare tumor. Histologically, it has the same appearance as gestational choriocarcinoma metastatic to the ovaries (110). The majority of patients with this cancer are younger than 20 years. The presence of hCG can be useful in monitoring the patient’s response to treatment. In the presence of high hCG levels, isosexual precocity has been seen, occurring in approximately 50% of patients whose tumors appear before menarche (111,112).
There are only a few limited reports on the use of chemotherapy for these nongestational choriocarcinomas, but complete responses have been reported to the MAC regimen (methotrexate, actinomycin D, and cyclophosphamide) as described for gestational trophoblastic disease (110). These tumors are so rare that no good data are available, but the options also include the BEP or POMB-ACE regimens. The prognosis for ovarian choriocarcinomas has been poor. The majority of patients have metastases to organ parenchyma at the time of initial diagnosis, and they should be managed as high-risk germ cell tumors.
Polyembryoma
Polyembryoma of the ovary is another extremely rare tumor, which is composed of “embryoid bodies.” This tumor replicates the structures of early embryonic differentiation (i.e., the three somatic layers: Endoderm, mesoderm, and ectoderm) (1,10). They occur in very young, premenarchal girls with signs of pseudopuberty, and AFP and hCG levels are elevated. Women with polyembryomas confined to one ovary may be followed with serial tumor markers and diagnostic-imaging techniques to avoid cytotoxic chemotherapy. In patients who require chemotherapy, the BEP regimen is appropriate (71).
Mixed Germ Cell Tumors
Mixed germ cell malignancies of the ovary contain two or more elements of the tumors described above. In one series (107), the most common component of a mixed germ cell tumor was dysgerminoma, which occurred in 80%, followed by EST in 70%, immature teratoma in 53%, choriocarcinoma in 20%, and embryonal carcinoma in 16%. The most frequent combination was a dysgerminoma and an EST. The mixed germ cell tumors may secrete either AFP or hCG—or both or neither—depending on the components.
These tumors should be managed with combination chemotherapy, preferably BEP. The serum marker, if positive initially, may become negative during chemotherapy, but this may reflect regression of only a particular component of the mixed lesion. Therefore, a second-look laparotomy may be indicated if there is residual disease following chemotherapy, particularly if there was an immature teratomatous component in the original tumor.
The most important prognostic features are the size of the primary tumor and the relative percentage of its most malignant component (107). In stage IA lesions smaller than 10 cm, survival is 100%. Tumors composed of less than one-third EST, choriocarcinoma, or grade 3 immature teratoma also have an excellent prognosis, but it is possibly less favorable when these components comprise the majority of the tumor.
Surveillance for Stage I Ovarian Germ Cell Tumors
Surveillance is a common approach to the management of young men with apparent stage I testicular germ cell tumors. There is a large body of evidence to support this approach, as well as guidelines on what constitutes appropriate surveillance (6,7). Although as many as 20–30% of patients will relapse, almost all will be cured with salvage chemotherapy with BEP, and the potential adverse effects of chemotherapy can be avoided in most patients.
Although this is a very common approach in young men, it has not been widely adopted in females with ovarian germ cell tumors. However, some data are now available to support surveillance in selected patients whose disease is confined to the ovary. Cushing et al. reported a study of 44 pediatric patients with completely resected ovarian immature teratomas who were followed carefully for recurrence of disease with appropriate diagnostic imaging and serum tumor markers (113). Thirty-one patients (70.5%) had pure ovarian immature teratomas with a tumor grade of 1 (n = 17), 2 (n = 12), or 3 (n = 2). Thirteen patients (29.5%) had an ovarian immature teratoma plus microscopic foci of yolk sac tumor. The 4-year event-free and overall survival for the ovarian immature teratoma group and for the ovarian immature teratoma plus yolk sac tumor group was 97.7% (95% confidence interval, 84.9–99.7%) and 100%, respectively. The only yolk sac tumor relapse occurred in a child with ovarian immature teratoma and yolk sac tumor who was then treated and salvaged with chemotherapy (113).
The Charing Cross Group initially reported a prospective study of 24 patients with stage IA ovarian germ cell tumors who were also enrolled in a surveillance program. The group consisted of nine patients (37.5%) with dysgerminoma, nine (37.5%) with pure immature teratoma, and six (25%) with ESTs (with or without immature teratoma). Treatment consisted of surgical resection without adjuvant chemotherapy, followed by a surveillance program of clinical, serologic, and radiologic review. A second-look operation was performed, and all but one patient were alive and in remission after a median follow-up of 6.8 years. The 5-year overall survival was 95%, and the 5-year disease-free survival was 68%. Eight patients required chemotherapy for recurrent disease or a second primary germ cell tumor. This included three patients with a grade II immature teratoma, three patients with a dysgerminoma, and two patients with dysgerminoma who developed a contralateral dysgerminoma 4.5 and 5.2 years after their first tumor. All but one, who died of a pulmonary embolus, was successfully salvaged with chemotherapy (114).
The same group updated its experience and reported on the safety of the ongoing surveillance program of all stage IA female germ cell tumors (24). Thirty-seven patients (median age 26, range 14 to 48 years) with stage I disease were referred to Mount Vernon and Charing Cross Hospitals between 1981 and 2003. Patients underwent surgery and staging followed by intense surveillance, which included regular tumor markers and imaging. The median period of follow-up was 6 years. Relapse rates for stage IA nondysgerminomatous tumors and dysgerminomas were 8 of 22 (36%) and 2 of 9 (22%), respectively. In addition, one patient with mature teratoma and glial implants also relapsed. Ten of these 11 patients (91%) were successfully cured with platinum-based chemotherapy. Only one patient died from chemoresistant disease. All relapses occurred within 13 months of initial surgery. The overall disease-specific survival of malignant ovarian germ cell tumors was 94%.
More than 50% of patients who underwent fertility-sparing surgery went on to have successful pregnancies. They concluded that surveillance of all stage IA ovarian germ cell tumors was safe and feasible, and that the outcome was comparable with testicular tumors. They questioned the need for potentially toxic adjuvant chemotherapy in all patients with nondysgerminomas who have greater than 90% chance of being salvaged with chemotherapy if they relapse.
This strategy is appealing and is supported by a larger pediatric literature, but there is much less experience in adults. It deserves further study, but this will require international collaboration. If a surveillance program is to be instigated, it is essential that the protocols used by the Charing Cross group are closely adhered to and that patients understand that the data for adults are limited.
The surveillance policy is very strict and includes a CT scan of chest, abdomen, and pelvis after surgery, if not done preoperatively. At 12 weeks following surgery, a repeat MRI/CT of the abdomen and pelvis or a second-look laparoscopy should be performed if there has been inadequate initial staging. If all of these are negative, MRI/CT imaging should be repeated at 12 months. Patients are reviewed monthly in year one, every 2 months in year two, every 3 months in year three, and so on until year five, after which they are seen every 6 months for another 5 years. A pelvic ultrasound and chest x-ray should be done on alternate visits. Tumor markers including AFP, β-hCG, CA125, and LDH measurements should be done every 2 weeks for 6 months, and then monthly for 6 months, every other month in year two, every third month in year three, and so on until year five when they are repeated every 6 months for 5 years (24,114).
Late Effects of Treatment of Malignant Germ Cell Tumors of the Ovary
Although there are substantial data regarding late effects of cisplatin-based therapy in men with testicular cancer, much less information is available for women with ovarian germ cell tumors. The toxicity of BEP chemotherapy has been well documented in men and includes significant pulmonary toxicity in 5% of patients, with fatal lung toxicity in 1%; acute myeloid leukemia or myelodysplastic syndrome in 0.2–1% of patients; neuropathy in 20–30%; Raynaud phenomenon in 20%; tinnitus in 24%; and high-tone hearing loss in as many as 70% of patients. In addition, late effects occur on gonadal function, there is an increased risk of hypertension and cardiovascular disease, and some degree of renal impairment occurs in 30% of patients (115,116). These side effects underscore the importance of limiting BEP to three cycles for low-risk and to four cycles for high-risk patients, and emphasize the need for these patients to be referred to major referral centers (95,117).
Gonadal Function
An important cause of infertility in patients with ovarian germ cell tumors is unnecessary bilateral salpingo-oophorectomy and hysterectomy. Although temporary ovarian dysfunction or failure is common with platinum-based chemotherapy, most women will resume normal ovarian function, and childbearing is usually preserved (11,20,21,35–39). In one representative series of 47 patients treated with combination chemotherapy for germ cell malignancies, 91.5% of patients resumed normal menstrual function, and there were 14 healthy live births and no birth defects (21). Factors such as older age at initiation of chemotherapy, greater cumulative drug dose, and longer duration of therapy all have adverse effects on future gonadal function (36,95,117,119,120).
A large study of reproductive and sexual function after platinum-based chemotherapy in ovarian germ cell tumor survivors was recently reported by the GOG, and 132 survivors were included in the study. Surprisingly, only 71 (53.8%) had fertility-sparing surgery; of these, 87.3% were still having regular menstrual periods. Twenty-four survivors had 37 offspring after cancer treatment (80,118).
Secondary Malignancies
An important cause of late morbidity and mortality in patients receiving chemotherapy for germ cell tumors is the development of secondary tumors (95). Etoposide in particular has been implicated in the development of treatment-related leukemias.
The chance of developing treatment-related leukemia following etoposide is dose related. The incidence of leukemia is approximately 0.4–0.5% (representing a 30-fold increased likelihood) in patients receiving a cumulative etoposide dose of less than 2,000 mg/m2 (121) compared with as much as 5% (representing a 336-fold increased likelihood) in those receiving more than 2,000 mg/m2 (122). In a typical three- or four-cycle course of BEP, patients receive a cumulative etoposide dose of 1,500 or 2,000 mg/m2, respectively.
Despite the risk of secondary leukemia, risk–benefit analyses have concluded that etoposide-containing chemotherapy regimens are beneficial in advanced germ cell tumors; one case of treatment-induced leukemia would be expected for every 20 additionally cured patients who receive BEP as compared with PVB (cisplatin, vinblastine, and bleomycin). The risk–benefit balance for patients with low-risk disease, or for high-dose etoposide in the salvage setting, is less clear (122).
Sex-Cord–Stromal Tumors
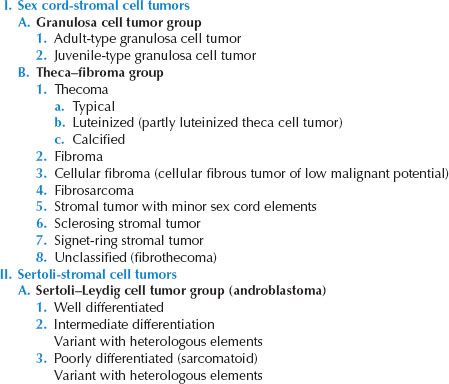
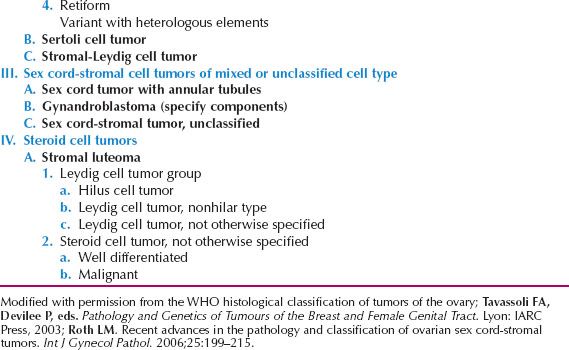
Sex-cord–stromal tumors of the ovary account for approximately 5–8% of all ovarian malignancies (1–4,123–128). They are derived from the sex cords and the ovarian stroma or mesenchyme, and are usually composed of various combinations of elements, including the “female” cells (i.e., granulosa and theca cells) and “male” cells (i.e., Sertoli and Leydig cells), as well as morphologically indifferent cells. A classification of this group of tumors is presented in Table 12.4 (9,129).
Granulosa–Stromal-Cell Tumors
Granulosa–stromal-cell tumors include granulosa cell tumors, thecomas, and fibromas. The granulosa cell tumor is a low-grade malignancy. Thecomas and fibromas are benign but rarely may have morphologic features of malignancy and then may be referred to as fibrosarcomas (131).
Granulosa cell tumors, which may secrete estrogen, are seen in women of all ages, and are classified as either Adult Granulosa Cell tumors or Juvenile. Five percent of cases are found in prepubertal girls; the others are distributed throughout the reproductive and postmenopausal years (127,128,130,132). They are bilateral in only 2% of patients.
Of the rare prepubertal lesions, 75% are associated with sexual pseudoprecocity because of the estrogen secretion (128). In the reproductive age group, most patients have menstrual irregularities or secondary amenorrhea. In postmenopausal women, abnormal uterine bleeding is frequently the presenting symptom. Endometrial cancer occurs in association with granulosa cell tumors in at least 5% of cases, and 25–50% are associated with endometrial hyperplasia (1,125,127,128,130). Rarely, granulosa cell tumors may produce androgens and cause virilization.
The other symptoms and signs of granulosa cell tumors are nonspecific and the same as most ovarian malignancies. Ascites is present in approximately 10% of cases, and rarely a pleural effusion (127,128). Granulosa tumors tend to be hemorrhagic; occasionally they rupture and produce a hemoperitoneum.
Table 12.4 Classification of Sex Cord-Stromal and Steroid Cell Tumors of the Ovary
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree