Abstract
Although conservative breast surgery is favored for the treatment of early stage breast cancer, mastectomy still has a place in the armamentarium of the surgeon. In the case of T3, multicentric, and inflammatory breast cancer, it is the treatment of choice. From the patient’s point of view, it is a good choice if they don’t want to or cannot have radiation therapy. It can be accompanied by skin sparing and nipple skin sparing techniques and reconstruction to improve the aesthetic outcome.
Keywords
breast cancer, mastectomy, modified radical mastectomy, simple, total, treatment
With pathologic confirmation of the diagnosis of breast cancer, a complete history, physical examination, and accurate clinical staging evaluation are requisite to therapy of the primary invasive neoplasm. Mammary adenocarcinoma that is 5 cm (T2) or less (T1) in transverse diameter and limited to the central or lateral aspect of the breast with the absence of pectoral fascia, skin fixation, and axillary lymphadenopathy can usually be treated with conservation surgery or total mastectomy with or without node dissection alone. Lesions smaller than 4 cm in diameter may be optionally treated with segmental mastectomy (partial mastectomy, lumpectomy, or tylectomy) and postoperative irradiation, with results comparable to those achieved with radical surgical techniques. Conservation approaches are discussed in Chapter 32 . For cancers larger than 5 cm (T3) in transverse diameter (stage IIIA or IIIB), a combination of radical surgery and radiation therapy, often after neoadjuvant therapy, is essential to achieve locoregional control of the breast, axilla, and chest wall (see Chapter 49 , Chapter 63 , Chapter 65 ).
The significant contributions of investigators to the management of breast cancer in the 20th century established the outcome results for conservation surgical techniques to be equivalent to those of radical approaches with regard to disease-free and overall survival. Thus the procedure to be completed and the anatomic site to receive irradiation for stages 0, I, and II disease depend on the location of the primary neoplasm in the breast, the presence or absence of axillary metastases, phenotype of the index cancer, and the growth characteristics of the index tumor (e.g., extension to musculature of chest wall, skin, axilla).
Moreover, the increasing importance of the primary tumor characteristics and its phenotype relative to its natural history have been established in clinical trials as important criteria for procedure selection. The integration of cellular, biochemical, immunohistochemical, and molecular biologic features of the tumor phenotype will increasingly direct therapies for future decades.
Lesions in the lateral aspect of the breast drain principally through axillary lymphatic channels (see Chapter 2 ). Index tumor presentations in this location can be eradicated from the chest wall by using the modified radical mastectomy with sentinel lymph node biopsy (SLNB; see Chapter 31 , Chapter 42 , Chapter 45 ). This surgical procedure is defined as a total mastectomy with preservation of the pectoralis minor/major muscles and includes dissection of level I and II axillary lymph nodes. These laterally placed neoplasms with histologically positive axillary lymph node metastases may be associated with internal mammary or supraclavicular lymph node metastases in as many as 25% to 30% of patients. Radiation therapy and chemotherapy are used for “grave” presentations of the tumors: skin fixation, nodularity, greater than 20% of nodes dissected histologically involved, more than three histologically involved nodes, and chest wall tumor fixation.
Centrally located lesions that are fixed to the pectoralis major fascia or high-lying (superiorly located) lesions that are fixed to this fascia may be treated with radical mastectomy or with a combination of radical mastectomy and peripheral lymphatic and chest wall irradiation when palpable axillary lymph node metastases smaller than 2 cm are evident. These centrally placed lesions commonly metastasize through lymphatics that parallel the course of the neurovascular bundle medial to the pectoralis minor muscle. This medial neurovascular bundle that contains the lateral pectoral nerve and innervates the pectoralis major muscle is preserved in the modified radical mastectomy to ensure function of the pectoralis major muscle after mastectomy. In the radical mastectomy procedure, this neurovascular bundle, associated lymphatics, and areolar tissue are resected en bloc with the specimen to accomplish adequate surgical extirpation of regional disease.
For medially located neoplasms, the principal lymphatic drainage is through routes that course to lymph nodes near the ipsilateral internal mammary vessels. These medial lesions may be associated with metastasis to the internal mammary lymphatics in 10% to 30% of patients, as previously confirmed by Handley. The presence of pathologically positive axillary metastasis with an associated medial lesion escalates this incidence of internal mammary metastasis to greater than 50%. In the absence of clinically positive axillary metastases, medially located cancers may be adequately treated with segmental (partial) mastectomy or with modified radical mastectomy and peripheral lymphatic irradiation.
Whether the surgeon chooses the conservation or radical approach depends on tumor size and characteristics, general medical status, patient choice, and desire for reconstruction. Regardless of the operative procedure selected, clearance of pathologically “free” margins about the neoplasm in three dimensions is paramount to enhancement of locoregional disease-free survival. Margins of the tumor resection that invade the costochondrium and periosteum of ribs or sternum or the intercostal musculature (as confirmed with magnetic resonance imaging or a chest computed tomography scan) require full-thickness chest wall resection with immediate myocutaneous flap reconstruction. With “clear” margins pathologically, radiation may be administered concomitant with the treatment regimen and depends on the presenting tumor characteristics and location and the presence (number) of metastatic lymph nodes (see Chapter 48 , Chapter 49 , Chapter 63 ). Furthermore, it is common to include chest wall irradiation when axillary metastases are identified pathologically in more than 20% of the removed axillary lymphatics. This principle was originally established because of the high incidence of skin flap recurrence evident with metastatic disease that courses to the axilla through the subdermal lymphatics from medially located primary lesions.
The principal determinant of actuarial survival of the patient after therapy of the primary breast lesion is the pathologic stage of the tumor. As established by the American Joint Committee on Cancer (AJCC), the staging system most commonly used is the tumor, node, metastasis (TNM) system. It is the responsibility of surgeons and radiation oncologists to jointly plan an operative procedure that encompasses, en bloc, the extent of the disease and provides the maximum probability for locoregional chest wall control of the tumor. It is also their responsibility to achieve this end result with minimal morbidity and mortality. These principles are best served by avoiding axillary irradiation after the Patey (complete) surgical dissection of level I to III axillary lymphatics; otherwise the incidence of lymphedema of the extremity of the ipsilaterally irradiated axilla will be increased approximately 7- to 10-fold. After radical resection of lymphatic channels with en bloc dissection of levels I to III, the remaining lymphatics are destroyed with radiation therapy, thus increasing the incidence of lymphedema. In principle, operable breast cancer (stages I and II) treated with total mastectomy and axillary node removal with the radical or modified radical mastectomy should not require postoperative irradiation. Currently, in cases where a SLNB demonstrates metastatic disease, control of the axilla can best be achieved with level I and II dissections; clearance of level III (Patey procedure) is required only if nodes are involved clinically at dissection. In contradistinction, for the treatment of stage III disease with axillary metastases that clinically present with large, matted, or multiple nodes, the radiation oncologist should plan the application of tangential fields to the apex of the axilla, including the peripheral lymphatics and chest wall after the extended simple (total) mastectomy. This therapeutic regimen is essential because level III (apical) lymphatics remain intact after a resection that includes only lymphatics lateral to the border of the pectoralis major and minor muscles. With the extended total mastectomy performed for stage III breast cancer, primary cancer or lymphatics, or both, larger than 1 cm in diameter are unlikely to be sterilized with irradiation alone and require surgical extirpation.
Extension of the primary neoplasm into the axillary space with invasion of the axillary artery, vein, or brachial plexus does not technically allow complete surgical removal and is best treated with regional ionizing irradiation to the axilla, breast chest wall, and supraclavicular sites. Radiotherapy should also be added to low or central axillary nodes that are determined pathologically to have extranodal capsular extension into axillary soft tissues because local and regional control rates are enhanced with this modality despite the significantly increased risk of lymphedema in the ipsilateral arm. In the absence of clinically palpable nodes with primary neoplasms that exceed 5 cm in diameter, neoadjuvant chemotherapy with or without preoperative irradiation often (>80%) induces regression of the primary lesion. Preferably, surgery is performed after neoadjuvant induction with tumor regimen to reduce tumor burden before radiotherapy. The selection of surgical technique depends on the extent (volume) of regression of the primary tumor, the presence or absence of fixation to pectoralis major fascia, location, and the presence or absence of local “grave” signs (ulceration, skin edema and fixation, or satellitosis).
Surgeons should plan the operative procedure with the objective of achieving, at minimum, 1- to 2-cm skin margins with subcutaneous and parenchymal margins of 2 to 3 cm in all directions from the index tumor, which can be accomplished with a radical, modified radical, or extended simple mastectomy. Patients with distant metastases, including supraclavicular lymph node metastases, are best treated with systemic chemotherapy with or without locoregional irradiation. Again, it is the responsibility of surgeons and radiation oncologists to achieve locoregional control except when adequate surgical margins are unobtainable in the absence of tumor regression, thereby reducing the probability of radiotherapeutic responses. The choice of these operative procedures must be individualized for each patient after determination of the site, clinical stage, and histologic type of the primary neoplasm. Similar principles guide the management of inflammatory breast cancers, which may be large, fixed, or ulcerated (see Chapter 63 , Chapter 64 , Chapter 65 ).
As indicated in Chapter 14 , Chapter 22 , Chapter 24 , estrogen receptor (ER) and progesterone receptor (PR) activity, ploidy vc11, jy, HER2/neu status, and cytologic and nuclear grading indicators should be obtained for all pathologically invasive breast cancer specimens to aid the therapeutic planning of endocrine replacement or cytotoxic therapy in the event that adverse prognostic indicators are evident. Prospective data available for analysis suggest that mean survival rates for patients receiving either chemotherapy or hormonal manipulation are greater for patients possessing positive ER/PR activity and favorable biochemical and cellular growth phase indicators than those for patients who do not (negative ER/PR; positive HER2/neu). Regardless of the pathologic stage of the tumor or the receptor activity, the optimal chemotherapeutic regimen for patients with metastatic breast cancer continues to evolve (see Chapter 86 ). Qualitative values for hormone receptor activity of the primary neoplasm and cellular/biochemical and molecular prognosticators are of significant value to the oncologist and should be obtained from the primary lesion and metastatic sites to prospectively guide subsequent therapy.
The proper technique for the processing of tissues that contain ER and PR activity is essential to the design and implementation of future chemotherapy protocols for specific patients; the surgeon’s attention to the preservation and processing of biopsy tissue is crucial. Despite the importance of the ER and PR activity and other cellular/biochemical and oncogene markers to guide future therapies, processing of neoplastic tissue for pathologic examination, in all cases, must take precedence over determination of steroid receptor activity, as well as the procurement of additional tissues to determine cellular, biochemical, and molecular prognostic characteristics that evaluate tumor phenotype (see Chapter 14 , Chapter 22 , Chapter 24 ).
Immunohistologic methodologies have been extensively applied since the mid-2000s with replacement of quantitative steroid receptor analyses with less problematic concern for receptor invalidation. However, surgeons should also be aware of the potential for electrocautery to diminish steroid receptor activity. This was confirmed by Ellis and associates and by Bland and colleagues to be dependent on heat inactivation by the ambient temperature and by devascularization ( Table 29.1 ). The procurement of primary breast cancer tissue for pathologic diagnosis and for determination of immunohistologic (qualitative) steroid receptor activity is best accomplished with the cold scalpel. This technique avoids the possibility of heat induction artifact, tissue necrosis, cellular death, and temperature-dependent inactivation of steroid receptor activity of the procured tissues. Nonetheless, tumor excision with cautery can be used if the operator avoids direct contact of tumor specimen with the cautery blade. The indications and techniques for biopsy of suspicious breast masses are comprehensively reviewed later in this chapter.
| Receptor (n = 11) | ISCHEMIA TIME (MIN) b | ||||
|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 150 | |
| ER | 100 | 79 ± 10 c | 67 ± 11 c | 54 ± 11 c | 56 ± 13 c |
| PR | 100 | 100 ± 21 | 101 ± 26 | 94 ± 14 | 84 ± 27 |
| AR | 100 | 57 ± 12 c | 53 ± 15 c | 28 ± 9 | 42 ± 12 c |
a Ischemia significantly decreased ER levels within the first 30 minutes ( p = .05). ER values had sustained decrease throughout 150 minutes of ischemia. Similarly, AR levels were significantly lower by 30 minutes of ischemia ( p = .002) and remained so throughout 150 minutes of ischemia. The largest decrease in ER and AR levels occurred within the first 30 minutes of ischemia. In contrast, PR levels were unchanged throughout 150 minutes of ischemia.
b Values in last four columns are mean ± SEM, expressed as percent of control at baseline.
Topographic Surgical Anatomy
Chapter 2 provides a detailed review of the anatomy of the breast, including discussions of regional vasculature, neurologic structures, and lymphatic drainage. Hollingshead observed that the fibrous and fatty components of breast tissue occupied the interval between the second or third rib superiorly, with extension to the sixth or seventh ribs inferiorly. The breadth of extension includes the parasternal to the midaxillary lines. The glandular portion of the breast rests largely on the pectoral fascia and the serratus anterior musculature; however, mammary tissue extends typically into the anterior axillary fold (tail of Spence) and may be visible as a well-defined superolateral extension from the upper outer quadrant of breast tissue. Extent of the mammary tissue is ill defined and varies considerably with patient habitus and lean muscle mass.
Parenchymal volume of the gland, with anterior and lateral projections, is variable and depends on lean body mass, habitus, age, and ovarian functional status. Because the ductal and lobular components are almost exclusively sensitive to the trophic effects of secretory estrogen and progestational hormones, the breast remains underdeveloped and rudimentary in the male. In men, short ducts with poorly developed acini are evident. Thus a deficiency of parenchymal fat and nipple-areola development are apparent and contribute to the nonspheroidal or flat appearance of the male breast.
Relative to the male breast, the nonparous breast is hemispheric and somewhat flattened above the nipple. The multiparous breast, on the other hand, is large and is replaced in part with fat, which accounts for its lax, soft appearance; it rarely regains its initial configuration until menopause, when atrophy of glandular tissue is initiated. The breast is circumscribed anteriorly by a superficial layer and posteriorly by a deep layer of the superficial investing fascia of the chest wall. The superficial layer of the superficial fascia of the chest wall derives its anterior boundaries from the fibrous tissue of the tela subcutanea. Haagensen observed the deep layer of the superficial fascia to be contiguous with the pectoral fascia.
After loss of estrogen influence on breast parenchyma and ductal structures, the postmenopausal breast is replaced by fat and is consistently noted to lack supportive parenchymal connective tissue and active (proliferative) glandular components. Spratt and Donegan and Spratt and Tobin note that the nonlactating breast weighs between 150 and 225 g, whereas the lactating organ may weigh as much as 500 g.
Neurologic Innervation of the Pectoral Muscles
For purposes of clarity and consistency, the editors have retained the classic anatomic description and nomenclature for the pectoral (anterior thoracic) nerves and the accompanying neurovascular bundles (see Chapter 2 ). The name of the neurovascular bundle (lateral or medial) is synonymous with its course (position) in the axilla. Classic anatomy teaches that the pectoral nerves are named from the brachial cord (medial or lateral) from which they originate. In the technical description of operative procedures within this chapter and in anatomic descriptions elsewhere in this textbook (see Chapter 36 , Chapter 41 , Chapter 45 , Chapter 49 ), we have retained the classic nomenclature.
As evident in major surgical texts, the anatomy of the medial/lateral pectoral nerves and the innervation of the pectoral muscles have evoked only minimal interest. Major textbooks of surgical anatomy have long considered the names of the medial pectoral and lateral pectoral nerves on the basis of origin from the brachial plexus. Therefore the names in classic anatomic teaching are not correlated with their medial or lateral anatomic positions found during surgery ( Fig. 29.1 ). Moosman, however, completed a detailed study of the pectoral nerves by dissection of 100 adult fixed and fresh cadaver pectoral regions (56 male and 44 female), and he transposed the names of the medial and lateral pectoral nerves according to their anatomic relationship to the pectoral muscles and to the anterior chest wall. These nerves, sometimes called anterior thoracic nerves, originate cephalad and posterior to the axillary vein from an anastomotic nerve loop of variable size between the medial and lateral brachial plexus cords.
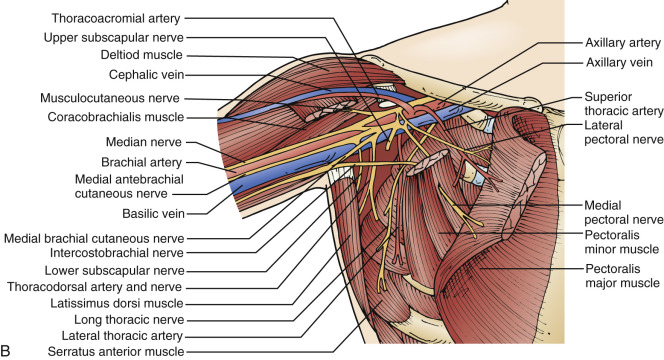
Moosman noted in his anatomic dissections that the lateral pectoral nerve arises anatomically from the lateral cord and in location is medial to the pectoralis minor muscle. In its course, it divides into two to four branches that pass downward and medial to supply the clavicular, manubrial, and sternal components of the pectoralis major muscle. Thereafter the nerve passes through the costocoracoid foramen with the thoracoacromial vessels and enters the interpectoral space to mix with tributaries of vascular origin to the muscle. Moosman observed that this nerve is larger than the medial pectoral nerve because of the greater volume of muscle it innervates.
The medial pectoral nerve is smaller (approximately 1–2 mm in diameter and 10–15 cm in length) than the lateral pectoral nerve, and its origin is medial or posterior to the pectoralis minor. This nerve sends branches to the pectoralis minor and descends on its dorsal surface. Typically this nerve crosses the axillary vein and is accompanied by small tributaries from the axillary or thoracoacromial vessels. It enters the interpectoral space and supplies the lower third of the costoabdominal portion of the pectoralis major muscle. In an extensive review of the anatomy of the medial pectoral nerve, Moosman observed the relationship of this nerve to the pectoralis minor to be one of several variants: (1) as a single descending branch around the lateral border of the lower half of the muscle (38%); (2) division into two branches, with one branch passing through the muscle and the other around its lateral margin (32%); (3) as a single descending branch that passed through the muscle (22%); and (4) as two or three descended branches of varying size, each of which passes through the muscle often at different levels (8%). He observed motor branches to the pectoralis major coursing through the pectoralis minor in 62% of cases.
In rare circumstances the medial pectoral nerve may pass through the medial muscular components of the pectoralis minor or, in other cases, may remain entirely on its medial surface. When numerous branches arise from the major trunks, a more diminutive size can be expected for branches that innervate the pectoralis major. The nerve remains relatively large when it is a single branch, whereas multiple branches passing through the muscle may be of thread size.
Regardless of the anatomic nomenclature used, the surgeon must be cognizant of potential damage to the nerve supply to the pectoralis muscles at all levels of dissection. Manipulation, traction, electrocautery, or resection may destroy the lateral or medial pectoral nerves unless they are carefully separated from nerve branches of variable size.
Vascular Distribution
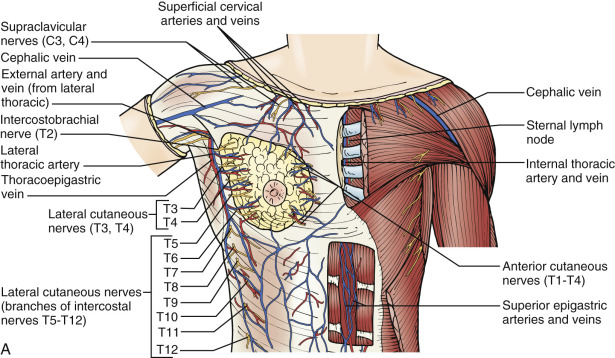
Nutrient arterial supply to the skin and breast is through branches of the lateral thoracic arteries, the acromiothoracic branch of the axillary artery, and the internal mammary artery. The venous drainage system includes the intercostal veins, which traverse the posterior aspect of the breast from the second or third through the sixth or seventh intercostal spaces to terminate and enter posteriorly into the vertebral veins. The intercostal veins may arborize centrally with the azygos system to terminate in the superior vena cava. The deep venous drainage of the breast in large part parallels the pectoral branches of the acromiothoracic artery and the lateral thoracic artery.
The large epithelial and mesenchymal surface area of the superior, central, and lateral aspects of the breast is drained by tributaries that enter the axillary vein. Venous supply from the pectoralis major and minor muscles also drains into tributaries that enter the axillary vein. Perforating veins of the internal mammary venous system drain the medial aspect of the breast and the pectoralis major muscle. This large venous plexus can be observed to traverse the intercostal musculature and terminate in the innominate vein, providing a direct embolic route to the venous capillary network of the lungs. Each plexus of veins in the lateral and medial aspects of the breast is observed to have multiple, racemose anastomotic connections.
Lymphatic Drainage and Routes for Metastases
The rich and elaborate lymphatic drainage generally parallels the arterial and venous supply of the breast. This lymphatic flow is primarily unidirectional, except in subareolar and central aspects of the breast, or in circumstances in which physiologic lymphatic obstruction occurs as a consequence of neoplastic, inflammatory, or developmental processes that initiate a reversal of flow with bidirectional egress of lymph. This bidirectional lymphatic flow (see Figs. 2.13 and 2.15 ) may account for metastatic proliferation in sites remote from the primary neoplasm (e.g., the opposite breast and axilla). The delicate lymph vessels of the corium are valveless and encircle the lobular parenchyma to enter each echelon of the regional lymphatic nodes in a progressive and orderly fashion (e.g., level I → level II → level III). As indicated in Chapter 2 (see Fig. 2.15 , Fig. 2.16 , Fig. 2.17 ), multiple lymphatic capillaries anastomose and fuse to form fewer lymph channels that subsequently terminate in the large left thoracic duct or the smaller right lymphatic duct (see Figs. 2.8 and 2.16 ). As a consequence of the predominant unidirectional flow of lymph, two accessory drainage routes exist for lymph en route to nodes of the apex of the axilla and include the transpectoral and the retropectoral routes, as defined by Anson and McVay. First described by German pathologist Rotter, Rotter nodes are lymphatics of the transpectoral or interpectoral routes that occupy the position between the pectoralis major and minor muscles. Cody and coworkers and Netter report Rotter nodes to be present in up to 75% of individuals, with an average of two to three nodes per patient. Cody and coworkers observed that 0.5% patients with negative nodes and 8.2% of patients who had positive axillary nodes had evidence of Rotter lymph node metastases. This observation was rarely reported by Haagensen. Therefore, although the Patey axillary dissection, included in the Halsted radical and in the modified radical mastectomy, removes the interpectoral Rotter group en bloc, this nodal group plays only a diminutive role in the diagnosis and therapy of breast cancer. The retropectoral lymphatics, however, may play a more important physiologic role in drainage of the breast because they are exposed to the superior and internal portions of the mammary glands. These lymphatics arborize lateral and posterior to the surface of the pectoralis major muscle and terminate at the apex of the axilla. To achieve an adequate en bloc resection of major axillary nodal groups, the surgeon must achieve a thorough conceptualization of breast lymphatic drainage. Familiarization with the anatomy of this area is essential for staging and for curative resection.
Chapter 2 deals with the principal axillary nodal groups as described by Anson and McVay. Fig. 29.2 topographically depicts anatomic levels I to III of the axillary contents with relation to the neurovascular bundle, the pectoralis minor, the latissimus dorsi, and the chest wall. The following axillary nodal groups are included in level I:
- 1.
The external mammary group parallels the course of the lateral thoracic artery from the sixth or seventh rib to the axillary vein. This group occupies the loose areolar tissue inferior and lateral to the pectoralis major muscle in the medial distal axillary space.
- 2.
The subscapular group is contiguous with thoracodorsal branches of the subscapular vessels. This group extends from the ventral surface of the axillary vein to the lateral thoracic chest wall and includes loose areolar tissue on the serratus anterior and subscapularis musculature.
- 3.
The lateral axillary vein group is the most laterally placed nodal group of the axillary space. This group also contains the largest number of nodes in the axilla and is observed to be caudad and ventral to the surface of the axillary vein often lateral to the latissimus dorsi muscle. This nodal group is first encountered in the course of proximate dissection on the anterior-most surface of the latissimus dorsi muscle.
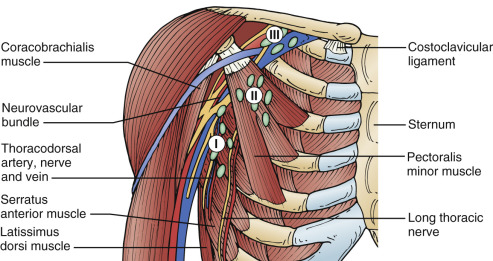
Level II, or the central nodal group, is immediately beneath the pectoralis minor muscle and is the most centrally located of the axillary lymphatic groups. This nodal group is located between the anterior and posterior axillary fold and occupies a superficial position beneath the skin and fascia of the midaxilla. The highest and most medially placed of the lymph node groups is the subclavicular (apical group), designated as level III. This is the cephalomedial lymph nodal group that is located just proximate to the termination of the axillary vein at its confluence with the subclavian vein at the level of Halsted’s (costoclavicular) ligament (condensation of the clavipectoral fascia). Fig. 29.2 depicts the position of these nodes relative to the pectoralis minor muscle and the posterior axillary space. These nodal groups are described relative to topographic anatomic relationships with the pectoralis minor muscle and the medial, lateral, and posterior axillary space. These lymphatics may be different from the nodal groups described by pathologists to indicate the area of metastatic involvement within the axilla.
Evolution of Surgical Techniques for Mastectomy
In 1894 Halsted and Meyer simultaneously reported their radical operations for treatment of cancer of the breast. By demonstrating superior locoregional control rates using en bloc radical resection techniques, these eminent surgeons established the radical mastectomy as the state-of-the-art modality of that era to control cancer of the breast. Subsequently, many modifications of the original incision developed by Halsted have been reported and include those of Meyer, Kocher, Rodman, Stewart, Warren, Greenough, Orr, Gray, and MacFee, to mention only a few variations of the incision. Many of the original incisions were developed to permit multiple approaches for extirpation of the mammae and to allow access to the axillary contents.
The Halsted and Meyer radical mastectomies differed technically in the sequence in which the breast and nodes were removed. Halsted insisted on primary resection of the breast and pectoral muscles before dissection of the axillary contents. In contrast, the Meyer technique (modified Halsted incision) advised the axillary dissection first, followed in sequence by breast and pectoral muscle resections, respectively. As indicated in Fig. 29.3 , the result achieved and the final cosmetic appearance for the Halsted and Meyer mastectomies are similar. Both procedures use a vertical incision to facilitate detachment of the pectoralis major from the clavicle and humerus and removal of the pectoralis minor from the coracoid process of the scapula. Incisions subsequently adopted by various European and American surgeons are indicated in Fig. 29.3 and represent incision modifications for operable breast cancer in each quadrant of the organ. It should be noted that Halsted and Meyer strongly advocated the necessity of en bloc resections for extirpation of the breast and the contents of the axilla but had little appreciation for clinical staging and the ultimate consequences of systemic disease. In their era, no adjuvant modalities (radiation/chemotherapy) existed to provide effective cytoreduction of the advanced primary lesion; thus, advanced stages of disease could be extirpated only with the use of wider skin margins and larger flaps. For this reason, various incision modifications that incorporate breast resection and wound closure were developed.
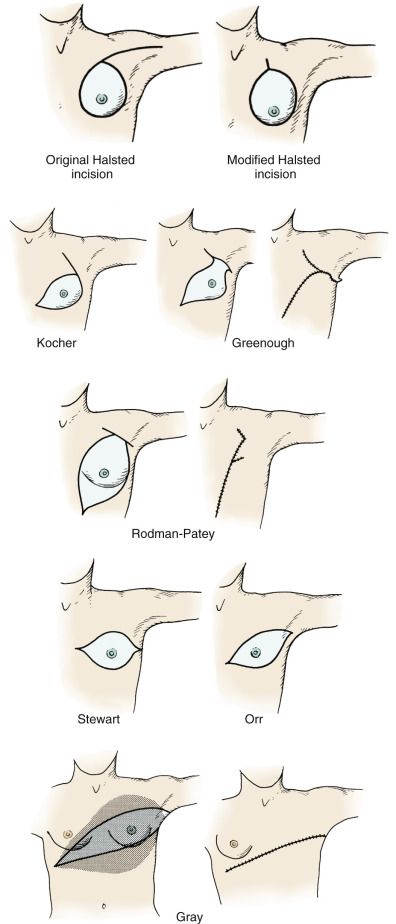
Eminent breast surgeons of the late 19th and early 20th centuries appreciated early in the formulation of therapeutic principles that the total mastectomy incision should incorporate both the nipple and the biopsy site to reduce the possibility of tumor implantation in the wound. In original dissections of the axilla, both Halsted and Meyer advocated complete axillary dissection of all three nodal levels from the latissimus dorsi muscle laterally to the thoracic outlet medially. Both surgeons routinely sacrificed the long thoracic nerve and the thoracodorsal neurovascular bundle en bloc with the axillary contents. Therefore it is not surprising that much of the initial criticism leveled at the radical mastectomy in the treatment of breast carcinoma concerned itself with the limitation of motion in the shoulder and the ipsilateral lymphedema that followed surgery. It also could be argued that survival rates for these patients, especially those with advanced locoregional disease, were not increased in proportion to the resultant disabilities (e.g., the “winged scapula” and shoulder fixation) evident with the procedures. Subsequently, Haagensen advocated preservation of the long thoracic nerve to avoid the winged scapula disability and motor apraxia evident with loss of innervation to the serratus anterior. Furthermore, Haagensen advocated removal of the thoracodorsal neurovascular bundle (with neural innervation to the latissimus dorsi muscle) to allow clearance of the subscapular and external mammary lymphatics that follow the course of this neurovascular structure. However, the majority of breast surgeons currently preserve both the long thoracic and the thoracodorsal nerves in the absence of gross invasion by the neoplasm or nodal fixation to these nerves. These principles are strictly enforced to ensure function of the scapula and to preserve viability and motor innervation of the latissimus dorsi, such that myocutaneous breast reconstruction may be a future option.
It should be noted that contemporary modifications of the Halsted or Meyer radical mastectomy, with preservation of the long thoracic nerve, can be performed with little or no increase in morbidity compared with simple mastectomy. In addition, any argument of the simple versus the radical procedure should be concerned with the long-term survival of the patient, which is the ultimate goal of therapy. To deny a patient the benefit of an adequate operative procedure on the basis of difficulty in placing cosmetic incisions or difficulty with wound closure is tantamount to disregard of an indisputable tenet that portends locoregional recurrence of disease.
The highly regarded and significant contributions of D.H. Patey of the Institute of Clinical Research, Middlesex Hospital, London, should be recognized. His careful clinical development and scientific demonstration of the worth of the “modified radical mastectomy” technique are laudable. In Britain in the 1930s, only a small minority of physicians questioned the absolute necessity of radical mastectomy for carcinoma of smaller size (AJCC stage I–II) with absence of fixation to the pectoral muscles. Three major influences led Patey in the 1930s and 1940s to consider therapeutic alternatives; design of the modified radical mastectomy technique followed. The first and most important consideration was the development and application of modern radiation therapy. The second influence was the growing evidence of dissatisfaction with Sampson Handley’s theory of “lymphatic permeation” as the primary process for the dissemination of carcinoma of the breast—a theory that, in its day, provided a logical pathologic basis for some of the technical details of the radical mastectomy. Third, with newer techniques for the study of lymphatic anatomy, Patey was able to scientifically refute the unproven postulates on which the original radical operations were based. Thereafter Patey and his colleagues developed the technique for in continuity removal of the breast and axillary contents (levels I, II, and III) with preservation of the pectoralis major muscle. This technique removes the pectoralis minor, like the standard radical operation, as the essential operative maneuver to provide access for complete clearance of the axillary contents. Objective demonstration of the efficacy for removal of axillary lymphatics with the technique was later proven with lymphangiography by Kendall and associates in 1963. Although this operation was performed by Patey for the first time in 1932, it was not adopted as a routine alternative to the standard radical mastectomy until late 1936.
Although Patey is credited with the formulation and implementation of the modified radical mastectomy as a standard approach for operable breast cancer, it was Auchincloss and Madden who described and developed technical variants of the modified radical mastectomy. As described earlier, the Patey mastectomy differs from the Halsted mastectomy in that the pectoralis major muscle is preserved. Patey acknowledged the importance of the complete axillary dissection (levels I, II, and III) and appreciated the anatomic necessity for preservation of the medial and lateral pectoral (anterior thoracic) nerves, which may serve as dual innervation to the pectoralis major. In contrast, Auchincloss and Madden advocated modified radical mastectomies with preservation of both the pectoralis major and minor muscles. The similarities of the approaches were that these techniques required total mastectomy with at least partial axillary lymph node dissection. Because these approaches preserved the pectoralis minor, dissection of the apical (subclavicular, level III) nodes was restricted, and in all cases, nodal recovery was less complete than with the Patey modified technique. The advantage of the Auchincloss and Madden procedures may be the greater probability for preservation of the medial pectoral nerve, which courses in the lateral neurovascular bundle of the axilla and may course through the pectoralis minor to supply the lateral border of the pectoralis major muscle. Expectantly, the Madden and Auchincloss techniques dissect only level I and II nodes and preserve level III lymphatics. Even currently, some surgeons advocate preservation of the pectoralis minor and simply detach the tendinous portion of the muscle from the coracoid process of the scapula to allow near-complete dissection of level III nodes to Halsted’s ligament. On completion of the nodal dissection, the tendon of the pectoralis minor was reapproximated to the coracoid with stainless steel wire or nonabsorbable suture.
Randomized clinical trials have established the safety of breast conservation therapy (BCT) compared with mastectomy. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B0-6 trial compared patients with tumors less than 4 cm undergoing partial mastectomy and axillary node dissection or modified radical mastectomy. At 20 years, the was no different in overall survival (OS) or disease-free survival (DFS), but local recurrence was higher in the BCT group compared with the modified radical mastectomy group. Likewise, the MILAN I trial compared radical mastectomy to partial mastectomy followed by radiation in women with invasive breast cancer <2 cm. Local recurrence reflected those seen in NSABP B-06 with a significant difference of 8.8% for BCT versus 2.3% for radical mastectomy, whereas survival was not different. In the European Organization for Research and Treatment of Cancer 10801 trial, patients with breast tumors less than 5 cm were randomized to BCT or modified radical mastectomy. Results were consistent with the previous similar trials demonstrating an increase in local recurrence with BCT, but no significant difference in OS or time to distant metastasis.
Students of breast surgery quickly recognize that incisions for the modified radical mastectomy are less extreme, and wounds are closed primarily. In contrast, radical procedures use wide incision margins, and skin is routinely grafted to wound defects. The application of modern radiobiologic techniques and cytoreductive chemotherapy currently does not require primary incisions that totally ablate the skin of the breast (see Fig. 29.3 ). Before the application of modern adjuvant techniques, incisions for large tumors that were considered locally advanced (because of ulceration, edema, and other “grave” signs) were designed to encompass these lesions with wide (3- to 5-cm) margins.
All skin flaps should be designed so that the incision incorporates skin and parenchyma at minimum of 1 cm from the periphery of the tumor in three dimensions. In principle, less skin is excised when lesions are located deep within the breast and T size is small in transverse diameter (T1 <2 cm). As indicated in Chapter 2 , viable breast tissue is anatomically distributed on the chest wall from the sternum to the axilla and from the clavicle to the aponeurosis of the rectus abdominis tendon. Haagensen demonstrated that small foci of glandular tissue could be histologically identified in close proximity to the dermis just beneath the superficial fascia. Halsted and Haagensen each considered that wide skin excision of at least 5 cm in all directions from the tumor was essential because of the rich superficial lymphatic channels of the central subareolar tissue and subcutaneous dermal lymphatic plexuses of the breast. The rationale of the classic radical and modified radical mastectomies is increasingly being challenged because of the availability of adjuvant modalities that enhance locoregional control with potential lengthening of DFS and OS rates. These tenets form the anatomic and pathologic basis for the skin-sparing mastectomy, which is comprehensively discussed later in this chapter.
Debate continues regarding the thickness of skin flaps that should be elevated in the planning of the total mastectomy as part of the radical or modified radical procedure. Krohn and colleagues report a two-arm study to evaluate the necessity of the “ultrathin” skin flap and the use of autogenous skin graft as methods to enhance local wound control with 5- and 10-year survival. A similar group of women who underwent radical mastectomy with narrow margins of skin excision with primary wound closure without ultrathin flaps had comparable 5- and 10-year survival and local recurrence rates. Wound complications, duration of hospital stay, and subsequent lymphedema, however, were significantly greater in the patients with thinner skin flaps. Most surgeons acknowledge that superior cosmetic results are achievable with well-vascularized flaps and the avoidance of split-thickness skin grafting. We maintain these basic tenets and avoid development of ultrathin flaps. Flaps developed at the plane of insertion of Cooper’s ligament deep to the cutis reticularis with the subcutaneous fat ensure extirpation of the underlying breast parenchyma. In general, flaps of 6- to 8-mm thickness usually ensure generous vascularity and viability of the skin. Subcutaneous fat may be preserved, which is consistent with complete parenchymal resection. Although the deep layer of the superficial investing fascia that intervenes between the subcutaneous fat and the breast tissue is easily identified, the thickness of this well-vascularized flap varies considerably with the patient’s habitus and lean body mass.
Total mastectomy for operable cancer that is not amenable to conservation surgical techniques has been addressed previously. In principle, advanced primary lesions (T2 or T3), with pectoralis major fixation, high-lying lesions, and perhaps some lesions with “grave” signs should be treated with radical or modified radical techniques. As discussed previously, the operations designed by Halsted, his predecessors, and his students reflect the necessity of designing wider flaps with large incisions for advanced primary cancers (T3a, T3b, T4) to technically encompass the primary lesion by at least 5 cm. When considering the advanced primary lesions that were treated in that era without adjuvant techniques, the necessity of larger resections can be rationalized as surgery was the only option for locoregional control.
The design of incisions that allow removal of the entire mammary gland (total or simple mastectomy) must incorporate the nipple-areola complex with the primary tumor in a three-dimensional aspect such that deep and peripheral margins are free of disease. Donegan and coworkers previously determined that incisions that resect skin and breast parenchyma at a distance of more than 4 cm from any margin of the palpable tumor achieve no improved therapeutic success. Currently, many surgeons consider a 1- to 2-cm margin adequate to achieve local control without tumor implantation. Intraoperative frozen section for marginal clearance is appropriate, especially margins 1 cm or less from the index neoplasm. As discussed in Chapter 45 , incisions placed in the breast for suspicious masses must be planned with consideration for the subsequent need for total mastectomy. Incisions should incorporate the primary biopsy scar, which should be well planned at the time of initial biopsy to allow complete extirpation of the neoplasm with the definitive mastectomy. When the primary breast lesion has been totally removed with the original biopsy as an excisional technique, incisions placed for total mastectomy should incorporate the skin and scar of the biopsy site by a minimal 1-cm margin in all three dimensions of the resection. We prefer incisions (Orr/modified Orr) that are slightly oblique from the transverse line and that extend cephalad toward the axilla. However, under no circumstance should the cosmetic design of an incision compromise the successful extirpation of the primary neoplasm. Split-thickness skin grafting has virtually been replaced with myocutaneous flap reconstruction after mastectomy for T3 or T4 primary neoplasms when wider margins and larger flaps are required for wound closure after neoadjuvant therapy.
Figs. 29.4 through 29.10 depict the various locations of breast primaries in which adequate therapy, with or without irradiation and chemotherapy, necessitates total mastectomy, which is completed with conventional technique in which reconstruction is not planned. For all mastectomy procedures, note that wide (radical) skin margin (>5 cm) excisions are not considered essential for locoregional disease control. However, skin margins of at least 1 to 2 cm from the gross index tumor or the surgical biopsy (excision) scar are necessary to ensure final pathology-free margins. Margins in excess of 2 cm are technically feasible for most total mastectomies in which reconstruction (early or delayed) will not be completed. Preoperative consideration should be given to the skin-sparing technique (see discussion later in this chapter) when the patient desires reconstruction.
Design of Incisions for Mastectomy in the Treatment of Breast Cancer
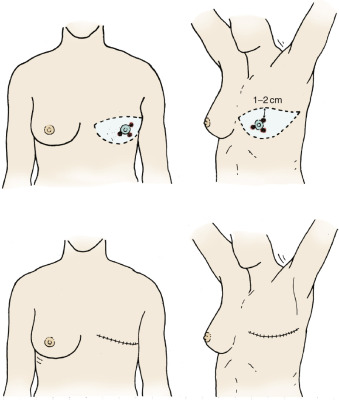
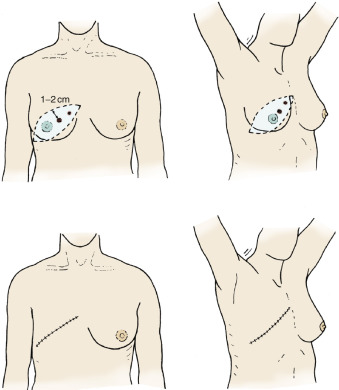
Central and Subareolar Primary Lesions
Fig. 29.4 depicts the design of the classic Stewart elliptical skin incision (see Fig. 29.3 ) that is used for mastectomy of subareolar or central breast primaries. The original biopsy preferably is done through a periareolar incision for lesions in this location. The residual scar should have precise measurements for the cephalad and caudad extents of the incision to include a minimal 1-cm margin. Availability of adequate skin to complete the primary closure is rarely difficult in the pendulous or large breast. For most small breasts, the Stewart (transverse) incision allows primary closure of skin except when more than 4 to 5 cm of skin are encompassed in the primary resection or if evidence of skin devascularization is apparent after completion of the procedure. Loss of skin secondary to loss of vascularity with ultrathin dissection planes or trauma to the flaps may necessitate even wider resections of skin margins.
Fig. 29.5 shows an optional elliptical incision in the contour of the breast for an inner quadrant primary lesion. This incision would perhaps best be described as the modified Stewart incision, which has a predominant extension in a more oblique and cephalad direction toward the ipsilateral axilla. The Stewart incision is commonly preferred by plastic surgeons anticipating delayed reconstruction with myocutaneous flaps, especially when a contralateral simple mastectomy is planned for treatment of high-risk disease or as a prophylactic procedure. Furthermore, this technique is often the choice of oncologic surgeons when radiation to the chest wall is planned before reconstruction.
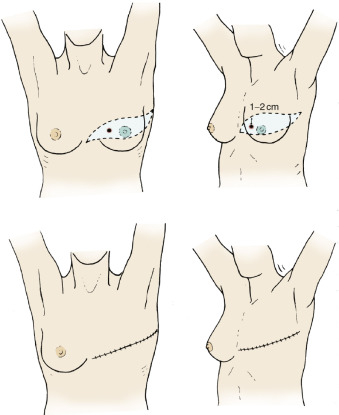
Lesions of the Upper Outer or Lower Inner Quadrants
Figs. 29.6 and 29.7 denote the incision design for operable breast cancer in the upper outer or lower inner quadrants. Minimal skin margins of 1 to 2 cm from the primary neoplasm are incorporated in a modified Orr incision that is slightly oblique from the transverse line with cephalad extension toward the axilla. Similar to the Orr and Stewart incisions, although somewhat more oblique, these incisions lend themselves to cosmetically satisfactory breast reconstruction results using myocutaneous or subpectoral augmentation breast implants.
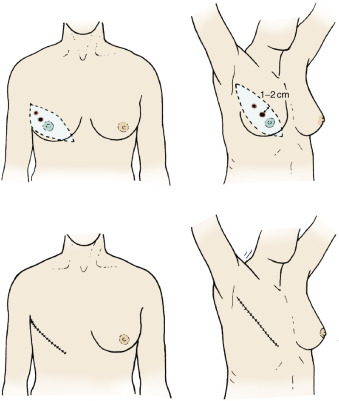
Lesions of the Upper Inner Quadrants
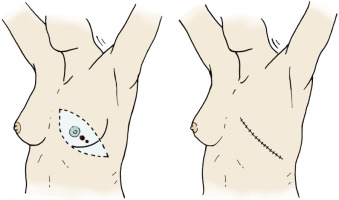
Lesions of the upper inner quadrants of the breast are the most difficult to manage because of their anatomic location. Surgeons should recognize the inherent problems encountered with elevation of skin flaps that allow adequate surgical margins and provide cosmesis for wound closure and potential reconstruction. Surgeons should be able to develop a 1- to 2-cm margin for lesions that are in this quadrant, providing the lesion is not cephalad (infraclavicular). These lesions may be accessed through the modified Stewart incision. Commonly, surgeons encounter the dilemma of designing an elliptical incision that is widely based near the cephalomedial aspect of the breast to incorporate a 1- to 2-cm margin of the tumor with extension laterally and inferiorly such that the incision terminates at the anterior axillary line (see Fig. 29.8 ). Surgeons should plan the cephalic portion of the incision for the superior flap such that adequate access to the pectoralis major and to the axillary contents is ensured.
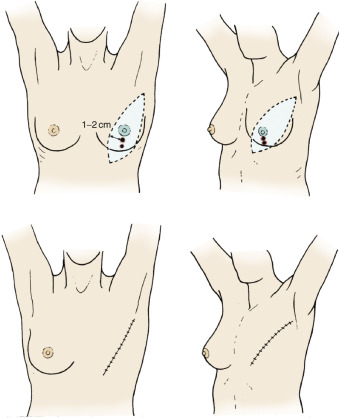
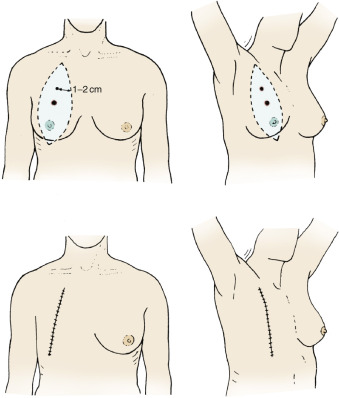
Lesions of the Lower Outer Quadrants
Lesions of the lower outer quadrants of the breast should have an incision design similar to those of the upper inner quadrant, with margins of 1 to 2 cm around the primary lesion (see Fig. 29.9 ) and with maximum extension of the cephalad margin to provide access to flaps for dissection of the pectoralis major and the axillary contents.
High-Lying (Infraclavicular) Lesions
With large lesions (T2, T3, T4) that are high lying, infraclavicular, or fixed to the pectoralis major, incisions designed to provide a minimal 1- to 2-cm margin necessitate skin grafting of the defect or coverage with myocutaneous flaps. The original Halsted and Meyer incisions, with subsequent modifications by Greenough, Rodman, and Gray (see Fig. 29.3 ), were used for treatment of primary lesions of T2, T3, and T4 size. For T1 lesions in this position, design of an elliptical incision placed in a vertical dimension from the clavicle provides adequate access for axillary dissection and clearing of the pectoralis major muscle when indicated but is unequivocally cosmetically deforming. Fig. 29.10 depicts the design of this cosmetically inferior vertical, elliptical incision for these high-lying lesions and the vertical closure. Because these incisions are placed perpendicular to Langer lines, cosmesis is minimized and the planes of cleavage for the medial breast are subsequently ablated.
Skin-Sparing Mastectomy
Total Mastectomy With Limited Skin Excision: Rationale and Technique of the “Skin-Sparing” Total Mastectomy
Wide skin excision is routinely used with every radical and modified mastectomy. A mastectomy with wide skin excision is often inclusive of an excision in excess of 30% to 50% of the breast skin. This is removed as an ellipse, usually measuring 10 cm (width) by 20 cm (length), and is closed primarily. The elliptical excision facilitates removing the dog-ears that are technically created by the wide skin removal and subsequent tension of excessive tissue at the terminal points of skin closure.
Limited skin excision can be defined as excision of the nipple-areola complex, the skin around the biopsy site, and the skin within 1 to 2 cm of the tumor margin. This technique usually sacrifices 5% to 10% of the breast skin, which is either approximated primarily or closed with an autogenous myocutaneous flap that is used to replace the breast volume. Dog-ears do not occur with this technique because the limited skin removal does not initiate skin contracture with closure. Limited skin excision mastectomy has the advantage of providing better reconstructions, particularly with myocutaneous flaps performed immediately after the mastectomy because it saves the entire chest skin and almost the entire skin envelope. Additionally, excision of the open biopsy site is often not applicable because of increasing popularity of a stereotactically controlled core needle aspiration.
Over the course of the 20th century, the clinical guidelines for breast skin excision with mastectomy have developed anecdotally and have been applied by convention. Furthermore, the benefits of wide versus limited skin excision have not been subjected to prospective randomized trials. Because locoregional surgical control of breast cancer has improved over the past 60 years, the extent of the breast skin excision with mastectomy has decreased proportionally. Standards of practice have sequentially evolved as follows: (1) total excision of the breast skin, to (2) wide excision without primary closure, to (3) wide excision with primary closure, and finally to (4) the “skin-sparing total mastectomy.” Indications and techniques for limited skin excision with mastectomy are reviewed here.
Factors Affecting Local Recurrence
With the increasing acceptance and application of breast conservation techniques for the therapy of ductal carcinoma in situ and invasive neoplasms of lobular and ductal origin, it is essential that the surgeon determines whether adequate excision margins are achieved to confer long-term local control. Gilliland and coworkers and Johnson and associates determined the necessity of total mastectomy with and without node dissection; with the exception of advanced disease (T3 and T4 tumors), immediate breast reconstruction can be completed without any effect on the quality or duration of survival. As noted by Johnson and associates, local recurrence is usually a harbinger of systemic disease, and predictably, factors for local recurrence also represent prognostic indicators of survival. The 1992 report by Kurtz reviewed common host and histologic features that influence local failure after breast conservation and irradiation to the intact breast for clinical stage I and II disease. Kurtz determined that the significant features that correlate with increased risk are young age at time of primary therapy and the presence of an extensive intraductal component within the invasive index (primary) neoplasm. In addition, this clinical study determined that adequacy (volume) of the surgical excision, the use of systemic adjuvant therapy, and high-quality radiation therapy techniques all contribute to a reduction in the risk of locoregional recurrence.
Biologic Factors: Effect on Local Recurrence
Contemporary oncologic treatment planning necessitates the incorporation of biologic factors related to the tumor phenotype and cellular characteristics. Aside from nodal status, these parameters exceed all other considerations in assessing various treatment modalities, including total excision of the breast. With the increasing acceptance and application of breast conservation techniques for treatment of both in situ and invasive neoplasms of lobular and ductal origin, it is essential that the surgeon determine whether adequate excisional margins are achieved to confer long-term locoregional control. Many investigators acknowledge the importance of patient selection to achieve optimal DFS relative to locoregional recurrence within the ipsilateral mastectomy site. The large prospective analysis (n = 1036) by the German Breast Cancer Study Group for therapy of T1N0M0 disease concluded that the width of the margin of excision (in centimeters) had no impact on prognosis. However, this analysis of patients who were randomized between breast preservation and mastectomy did establish conclusively that poorer disease-free survival was associated with microscopically involved margins in the conservation technique compared with the mastectomy study group (75% vs. 90% at 3 years). Tumor size and tumor grade were also prognostic factors that accurately predicted recurrence. Age, ER/PR status, menopausal status, histologic tumor type, and type of therapy (mastectomy vs. breast preservation) were not significant predictive factors of recurrence.
The full realization of the impact of contemporary developments in molecular and genetic markers as prognostic indicators of locoregional recurrences has not yet been fully assimilated into oncologic practice. As these tests gain acceptance, recognition of patients with unfavorable disease will be facilitated at the outset after cellular, biochemical, and molecular-genetic analysis of the primary neoplasm. The current method, which uses one variant of the mastectomy for all patients, can then be improved through the design of individual treatment. Moreover, the comprehensive prospective analysis of the NSABP by Fisher and associates suggests that the extent (type) of mastectomy and regional node excision are not associated with significant differences in survival than those from more radical procedures.
Tumor Volume (Size): Effect on Local Recurrence
For patients who are not treated with irradiation after breast conservation surgery, tumor size is an important determinant of risk for local recurrence, as reported in data from the NSABP by Fisher and coinvestigators. Fowble and colleagues and Kurtz and coworkers note that larger neoplasms (e.g., T3, T4) comprise most of these local failures. These findings are further amplified by the large, long-term study by Kurtz of operable T1 and T2 invasive breast cancers. Within the range of tumor diameter appropriate to breast conservation, Kurtz determined that the size of the primary lesion had no apparent influence on the risk for local recurrence, provided that macroscopically complete resection was achieved ( Table 29.2 ). Although the analysis by Kurtz includes radiation of the intact breast with the goal of preserving the organ, other researchers have come to similar conclusions in patients undergoing mastectomy alone. Dao and Nemoto reiterate that this reduction in the local recurrence rate is evident when the adequacy of resection is confirmed.









