Gastrointestinal Syndromes
General Considerations
Classification
The principal symptoms of infections involving the gastrointestinal tract are diarrhea, vomiting, acute abdominal pain, and jaundice. Often, these symptoms occur in combinations, but the clinician can usually make a preliminary diagnosis based on which of these symptoms is predominant.
Gastrointestinal infections are discussed in this chapter under the following syndromes: acute diarrhea, chronic diarrhea, vomiting, acute abdominal pain, and abdominal abscesses. Hepatitis syndromes are discussed in Chapter 13.
Overall Frequency
Acute diarrhea (acute gastroenteritis) was the third most frequent syndrome seen in general practice by Hodgkin.1 Among children younger than 5 years old who are hospitalized, 13% have a discharge diagnosis of diarrhea.1a The Centers for Disease Control and Prevention (CDC) estimates that food-borne illness is responsible for 76 million illnesses, 325,000 hospitalizations, and 5,200 deaths each year in the United States.
Frequency of Various Pathogens
On the basis of reviews of studies done in the United States, including projections and estimates, it is clear that most diarrhea in children is caused by noncultivatible viral pathogens. Usually, diarrhea acquired in the United States is noninvasive (nonbloody), no specific etiologic diagnosis is made, and the child responds to symptomatic treatment. In contrast, bloody diarrhea in children usually has a bacterial cause, and the child may benefit from appropriate antibiotic therapy, depending on the cause.
The frequency also varies with the age of the patient and special exposure situations such as child-care centers, where several pathogen-frequency studies have been done.2,3,4 An increasing percentage of children are enrolled in child-care centers, with an estimated 75% of mothers working outside the home in the year 2000.5 The rate of diarrheal disease in children cared for outside the home is two- to threefold higher than for children who stay at home.6 Outbreaks of diarrheal disease occur at a rate of approximately 1–2 per year for centers that house children who are still in diapers.7
Acute Diarrhea
Diarrhea can be defined as excessively liquid feces. Most epidemiologic studies require at least three loose stools in a 24-hour period for inclusion as a case. Hyperactive bowel sounds and slight abdominal tenderness are usually present. Vomiting may occur briefly at the onset but usually is not persistent in patients who should be classified as having acute diarrhea. Colicky (cramping) abdominal pain may occur.
If the patient does not have vomiting, diarrhea is a better term to use as a preliminary diagnosis than is gastroenteritis, because “gastroenteritis” implies an inflammatory or infectious disease. Although acute diarrhea is usually caused by an infectious agent, it also can be caused by poisoning or have a number of other noninfectious etiologies. Patients with vomiting and diarrhea may reasonably be classified as having gastroenteritis.
Clinical Patterns
Acute diarrhea syndromes can be classified on the basis of the severity of the illness, the appearance of the stool, and the history of contact with others with a similar illness. A secondary classification can be made on the basis of possible etiologic agents (Table 12-1).
Acute Inflammatory Diarrhea
Also called dysentery-like diarrhea, acute inflammatory diarrhea is characterized by mucosal invasion and usually involves the large intestine. The
stools may contain blood, pus, or mucus, and fecal leukocytes may be seen microscopically. Fever, crampy abdominal pain, and urgency of defecation occur frequently. The most common causes are bacteria, which may or may not produce toxins. The most common causes are Salmonella, Shigella, Campylobacter, and Shiga toxin–producing Escherichia coli (especially E. coli O157:H7). In addition to the infectious causes listed in Table 12-1, noninfectious causes, such as inflammatory bowel disease, intussusception, and cow’s milk allergy, should be considered.
stools may contain blood, pus, or mucus, and fecal leukocytes may be seen microscopically. Fever, crampy abdominal pain, and urgency of defecation occur frequently. The most common causes are bacteria, which may or may not produce toxins. The most common causes are Salmonella, Shigella, Campylobacter, and Shiga toxin–producing Escherichia coli (especially E. coli O157:H7). In addition to the infectious causes listed in Table 12-1, noninfectious causes, such as inflammatory bowel disease, intussusception, and cow’s milk allergy, should be considered.
TABLE 12-1. CLASSIFICATION AND COMMON CAUSES OF ACUTE DIARRHEA | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Acute Noninflammatory Diarrhea
This type is characterized by mucosal hypersecretion or decreased absorption without mucosal invasion. It is sometimes referred to as secretory diarrhea, but this implies that the mechanism is known, which is not usually the case. Acute noninflammatory diarrhea usually involves the small intestine. Vomiting and abdominal pain is common, but fever is variable. Stools are watery (without blood or mucus), and fecal leukocytes, if present, are infrequent (< 5 per high-powered field). The most common causes are viruses, such as rotavirus, caliciviruses (which include noroviruses and sapoviruses), enteric adenoviruses (serotypes 40 and 41), and astrovirus. Enteroviruses are so-named because of replication in and recovery from the enteric tract; however, they are not a common cause of diarrhea. Certain toxin-producing bacteria (Clostridium perfringens, Bacillus cereus) also cause noninflammatory
diarrhea. The classic cause of severe secretory diarrhea, Vibrio cholerae, is rare in the United States, even among returning travelers.8
diarrhea. The classic cause of severe secretory diarrhea, Vibrio cholerae, is rare in the United States, even among returning travelers.8
Traveler’s Diarrhea
In areas of poor sanitation, newly arrived individuals often suffer an attack of acute diarrhea, whereas residents of the area are apparently immune because of past infection. Although this may in part be true, it is important to remember that diarrhea is a common cause of morbidity and mortality among residents of the developing world, particularly children younger than 5 years old. Each year, more than 2 million children die of diarrheal diseases, the vast majority in the developing world.9
Traveler’s diarrhea is common, particularly in young children. In a study of 363 children spending at least 14 days in the developing world, the attack rate was 40% for children younger than 2 years old, 9% for children 3–6 years, 22% for children 7–14 years, and 36% in persons 15-20 years old.10
The majority of episodes of traveler’s diarrhea are caused by enterotoxigenic E. coli (ETEC). Other common pathogens are Campylobacter jejuni, Salmonella spp., Shigella spp., rotavirus, caliciviruses, and Giardia lamblia.11
Common-Source Diarrhea
When many individuals develop diarrhea at about the same time, the clinician should suspect a common source of infection, such as food or water. This pattern also can be called diarrheal food poisoning, sewage poisoning, or waterborne diarrhea, but it is useful to use the term common source until the source is identified. Salmonella and C. perfringens are the most common causes of diarrheal food poisoning, whereas Staphylococcus aureus typically produces food poisoning with predominately vomiting and retching. Causes of common-source vomiting are discussed later in this chapter in the section on vomiting syndromes.
Neonatal Diarrhea
Diarrhea occurring in the first month of life is a special case and should be sorted out with the diagnosis of neonatal diarrhea. In the past, enteropathogenic E. coli (EPEC) was a common cause of newborn nursery outbreaks, but this is rarely reported now. Salmonella and Yersinia can cause diarrhea in young infants, but other bacteria rarely do so. Viruses are probably the most common cause. Neonatal diarrhea raises the possibility of necrotizing enterocolitis or of septicemia and its differential diagnosis.
Causes of Acute Noninflammatory Diarrhea
Diarrheagenic Viruses
The majority of acute diarrheal illnesses in the United States studied before 1975 did not have any recognized pathogen recovered when conventional bacterial and viral cultures were done.12 Most of these illnesses were believed to have an infectious etiology on the basis of concurrent findings, such as acute onset, fever, and apparent contagiousness to contacts, rather than on the basis of the ability to demonstrate an infectious agent known to cause diarrhea. This clinical pattern has been called “infectious nonbacterial gastroenteritis” or “viral gastroenteritis,” but “acute diarrhea, probably viral” (without etiologic guesses) is a more accurate problem-oriented diagnosis.
Rotavirus
First recognized by electron microscopy,13 much of the acute diarrhea in infants in the United States is now known to be caused by rotaviruses.14 Rotavirus is the most common cause of diarrheal syndromes severe enough to require hospitalization for intravenous fluid rehydration therapy. Worldwide, dehydration due to rotavirus is estimated to cause more than 800,000 deaths a year.15
Mortality from rotavirus infection is rare in the United States, but it is a common cause of hospitalization.14 Rotavirus is also a common nosocomial pathogen. Rarely, rotavirus infection causes seizures or other central nervous system (CNS) symptoms not related to electrolyte disturbances; rotavirus antigens have been found in cerebrospinal fluid (CSF) using molecular techniques.16
In the late 1990s, a rhesus-reassortant, live-attenuated rotavirus vaccine was briefly placed on the market and recommended as a part of the routine childhood vaccination schedule. The vaccine virus was replication competent and produced protection against severe rotavirus disease (although it did not protect against infection). During the clinical trials, the vaccine was well tolerated, with the major adverse event being fever, which was reported in approximately 15–20% of recipients.17
However, after being placed into routine clinical use, a rare complication of the vaccine was discovered. Some vaccine recipients developed intussusception in temporal association with receipt of the vaccine.18 Case-control studies estimated that, compared with controls, the odds ratio for intussusception among vaccine recipients was between 16 and 22. It was estimated that approximately 1 in every 5,000–10,000 vaccinees would develop intussusception if routine use continued. There is a biologically plausible explanation for the occurrence of intussusception in vaccine recipients; vaccine virus replication in the gut could produce local inflammation and lymph node hypertrophy. This, in turn, could serve as a lead point for the development of intussusception. The vaccine was eventually pulled from the market,19 not because the risk of intussusception was high, but because the morbidity from this rare complication was unacceptable, considering that rotavirus infection in the United States is generally self-limited.
Two new investigational, live-attenuated rotaviruses are currently undergoing large-scale trials. One is a multivalent reassortant vaccine based on a bovine rotavirus genome (Rotateq),19a and the other is a monovalent human rotavirus strain that has been attenuated by serial passage in cell culture (Rotarix).19b
Caliciviruses
These viruses, the prototype of which is norovirus (previously referred to as Norwalk-like virus), most commonly infect older children and adults. Infections occur year-round, but there is an increase in outbreaks during the winter months.20 These viruses are the principal cause of epidemic viral gastroenteritis in all age groups, what is frequently referred to inaccurately as the “stomach flu.” Respiratory symptoms occur in about one-third of children, and the extreme contagiousness suggests possible respiratory transmission, although this has not been proved. Fever and myalgias are common. The incubation period is 12–48 hours. Some patients, especially young children, tend to have more vomiting, whereas adults usually have more diarrhea. Detection in stools by electron microscopy is specific but relatively insensitive. In addition, this test is generally available only in research laboratories.
Astrovirus
Astroviruses are small, double-stranded RNA viruses that are named for their appearance under electron microscopy, which is rather like a star. Infection with astrovirus produces principally a self-limited, watery diarrhea. Illness is generally much milder than that caused by rotavirus. In a prospective study of 214 children from Mexico, the mean duration of illness was 3 days, 20% of the patients reported emesis, and only 7% had fever. Severe illness was not seen.21 A study from Finland characterized the disease in 102 children who had astrovirus detected in diarrheal stools. In this study, 72% had watery diarrhea, 59% had vomiting, and 26% had fever; however, only 5% required oral rehydration, and 3% were hospitalized.22 In both studies, disease peaked in the winter and was most common in toddlers from 13–18 months. Different serotypes co-circulate during the same season.23
Enteric Adenovirus
Adenovirus serotypes 40 and 41 are the fourth most common cause of childhood viral gastroenteritis in most series.24,25 Infections occur year-round, with a slight increase in summer. Children younger than 2 are primarily affected, and transmission occurs from person to person by the fecal–oral route. Respiratory symptoms occur in more than half of patients.26 The incubation period is 3–10 days. The duration of symptoms is typically longer than with other viruses, occasionally lasting as long as 2 weeks.26 In one study, the mean duration of symptoms was 5.4 days.27 An enzyme-linked immunosorbent assay for detection of adenovirus serotypes 40 and 41 in stool is commercially available but not widely used.
Other Possible Causes
Other viruses, such as coronavirus, have been postulated to cause diarrhea, but their etiologic role has not been confirmed. Diarrhea sometimes occurs as a nonspecific finding in children with systemic infections. For example, although influenza virus does not infect the gastrointestinal tract, infants with documented influenza occasionally have diarrhea.28 In the immunocompromised host, cytomegalovirus and some enteroviruses are a possible cause of diarrhea (see Chapter 22).
Other Causes of Acute Noninflammatory Diarrhea
In addition to the diarrheagenic viruses discussed earlier, bacteria can cause noninflammatory diarrhea
as well. This occasionally occurs with the classic causes of bacterial gastroenteritis (Shigella, Salmonella, Campylobacter, and Shiga toxin–producing E. coli). However, it is the usual presentation with a few bacterial causes, such as V. cholerae, S. aureus, C. perfringens, B. cereus, and enterotoxigenic E. coli (ETEC). ETEC is discussed in the section on traveler’s diarrhea. S. aureus is discussed in the section on vomiting syndromes.
as well. This occasionally occurs with the classic causes of bacterial gastroenteritis (Shigella, Salmonella, Campylobacter, and Shiga toxin–producing E. coli). However, it is the usual presentation with a few bacterial causes, such as V. cholerae, S. aureus, C. perfringens, B. cereus, and enterotoxigenic E. coli (ETEC). ETEC is discussed in the section on traveler’s diarrhea. S. aureus is discussed in the section on vomiting syndromes.
Vibrio cholerae
Cholera is the classic cause of secretory diarrhea. Mild illness is similar to that of ETEC infection. However, severe cholera is distinctive, with massive fluid loss (> 1 L per hour) and rapid death in the absence of rehydration. Cholera toxin activates adenylate cyclase in the small intestinal mucosa. The increased intracellular concentration of cAMP stimulates secretion of sodium and chloride into the gut lumen, and water follows passively. The diarrhea is usually watery and flecked with mucus (“rice-water” stools). Vomiting is very common, but fever and abdominal cramps are unusual. In areas with poor sanitation, such as refugee camps, the potential for epidemic spread is devastating. Rapid rehydration with oral rehydration solution (or intravenous Ringer’s lactate for severe cases) can be life saving. Definitive diagnosis requires recovering the organism from stool culture, which is best accomplished by plating the stool on thiosulfate citrate bile-salts sucrose (TCBS) agar. If cholera is suspected, the microbiology laboratory should be notified so that this special culture medium can be used.
Vibrio parahaemolyticus
This is a common cause of diarrheal food poisoning in Japan and has also been recognized in the United States.29 Steamed crabs, boiled shrimp, and clams have been sources of infection with this organism, presumably after inadequate cooking. About 12 hours after ingestion, moderately severe abdominal cramps, diarrhea, some vomiting, headache, chills, and mild fever have been observed. Bloody diarrhea occurs in about 5% of persons.29
Several other species of vibrio can produce a similar illness after ingestion of contaminated and undercooked seafood.
Clostridium perfringens
This organism produces toxins that are elaborated in vivo and can cause diarrheal food poisoning. These heat-labile toxins typically produce an illness about 12 hours after the meal or experimental exposure. Typically, the source is meat or gravy that has been adequately cooked but inadequately refrigerated and served without cooking again.30
The predominant symptoms are watery diarrhea and abdominal cramps. Vomiting and fever are uncommon. Symptoms last 1 to 2 days.
Bacillus cereus
This organism can cause two different syndromes, depending on which of two toxins it elaborates. The strains elaborating preformed enterotoxin produce a syndrome indistinguishable from that of S. aureus food poisoning and are discussed in the section on vomiting syndromes. Other strains produce a heat-labile toxin that is elaborated in vivo and causes a syndrome indistinguishable from that of C. perfringens. As with C. perfringens, meats and gravies are common vehicles.
Enterotoxigenic E. coli (ETEC)
A classification of diarrhea-causing E. coli is shown in Box 12-1. The first four types on the list primarily cause watery diarrhea among persons in the developing world. In contrast, EHEC cause bloody diarrhea and are discussed in the section on acute inflammatory diarrhea.
ETEC are now recognized as a common cause of diarrhea in developing countries and the most common cause of traveler’s diarrhea. A large inoculum (approximately 108 organisms) is required to produce disease. Risk factors for traveler’s diarrhea include eating raw fruits or vegetables (especially salads), ingesting foods or beverages sold by street vendors, and drinking tap water. ETEC are also a cause of acute gastroenteritis in the United States. These pathogens are distinguished by their ability to make toxins. The heat-stable toxin (called ST) causes fluid and electrolyte secretion and is responsible for the watery diarrhea typical of infection with
these bacteria. The heat-labile (LT) toxin is similar to cholera toxin. Adhesion is also necessary for pathogenesis.
these bacteria. The heat-labile (LT) toxin is similar to cholera toxin. Adhesion is also necessary for pathogenesis.
Enteropathogenic E. coli (EPEC)
Although EPEC can infect people of all ages, they are generally thought of as pathogens of the very young. The incidence is highest in infants under the age of 6 months who are not breast-fed. Once a common cause of diarrhea outbreaks in nurseries, this subtype is now rare in the United States. In vitro, they adhere to cells either diffusely or in localized areas; those that adhere locally are the pathogenic strains.
Enteroinvasive E. coli (EIEC)
EIEC display a pattern of enterocyte invasion that is indistinguishable from that of Shigellae. Despite the pathogenetic similarity, disease caused by enteroinvasive E. coli is usually marked by watery, rather than bloody, diarrhea and low-grade fevers. EIEC are primarily a concern in the developing world. A large inoculum of bacteria is required to produce disease. In experimental studies, the disease is made more severe by prior ingestion of sodium bicarbonate, which decreases gastric acidity and thus increases the viability of the ingested bacteria.31
Enteroaggregative E. coli (EAggEC)
EAggEC stick to one another in a “stacked brick” pattern in cell culture. Disease caused by these organisms tends to be of lower severity and longer duration. Diarrhea for 2 weeks or more is not uncommon. However, in careful studies, these organisms are commonly identified in asymptomatic individuals. Like the other types of E. coli listed earlier, they are primarily a concern in developing countries and can be a cause of traveler’s diarrhea.
None of the types of E. coli causing noninflammatory diarrhea can be detected in the clinical microbiology laboratory using routine techniques. Thus, their true incidence is unknown.
Aeromonas Species
Aeromonas species are a possible cause of diarrhea.32 Most studies have demonstrated a higher rate of recovery of the organism in children with diarrhea than without diarrhea.33
The organism is a normal inhabitant of fresh or brackish waters. Young children in the developing world are most commonly affected. It can produce a heat-labile enterotoxin. The diarrhea is usually watery but dysentery occurs occasionally.34 Most infections are self-limited, but persistent or severe infections may respond to trimethoprim-sulfamethoxazole therapy.34
Yersinia enterocolitica
Yersinia enterocolitica is a cause of acute gastroenteritis4,35 that seems to be more common in colder climates of the United States and in Scandinavia. The bacterium grows well at room temperature, and growth is enhanced after storage at refrigerator temperature. Yersinia species have also been incriminated as a possible cause of mesenteric adenitis, as discussed in the section on abdominal pain. About 65% of patients have abdominal pain,36 and in one outbreak from contaminated chocolate milk in Oneida County, New York, 16 (44%) of 36 hospitalized children with yersiniosis had unnecessary appendectomies. The organism can cause bloody stools37 (especially in children)38 or chronic diarrhea but more typically causes watery diarrhea.35 Reactive arthritis can occur as a complication, particularly in adults.35,37,38
The main risk factors are consumption of undercooked pork or unpasteurized milk. Infants may be infected indirectly via the hands of caregivers who prepare chitterlings (pig intestines, “chitlins”).39
Plesiomonas shigelloides
Plesiomonas shigelloides is a member of the vibrio family that appears to be an uncommon cause of diarrhea in the United States, typically related to eating insufficiently cooked shellfish.40 Travel to Mexico and other areas of the developing world is also a risk factor for disease. More common in adults than children, disease caused by this pathogen tends to last longer than disease caused by other enteropathogenic bacteria; in one study, 76% of patients were sick for longer than 2 weeks and 32% for over a month.41 Whether antimicrobial therapy shortens the duration of diarrhea is unclear.42
Most isolates are susceptible to trimethoprim-sulfamethoxazole and to the fluoroquinolones.42
Causes of Acute Inflammatory Diarrhea
Bacteria
Shigella
Until the discovery of the importance of Campylobacter, shigellosis was accepted as the usual cause
of dysentery-like diarrhea in the United States. There are four species of Shigella: S. sonnei, S. boydii, S. flexneri, and S. dysenteriae. S. sonnei causes the mildest symptoms and is by far the most common species found in the United States. S. dysenteriae causes the most severe illness; it occurs in Africa and India but is not endemic in the United States. Among Shigella species, only S. dysenteriae type I is capable of producing Shiga toxin and is thus associated with the hemolytic-uremic syndrome.
of dysentery-like diarrhea in the United States. There are four species of Shigella: S. sonnei, S. boydii, S. flexneri, and S. dysenteriae. S. sonnei causes the mildest symptoms and is by far the most common species found in the United States. S. dysenteriae causes the most severe illness; it occurs in Africa and India but is not endemic in the United States. Among Shigella species, only S. dysenteriae type I is capable of producing Shiga toxin and is thus associated with the hemolytic-uremic syndrome.
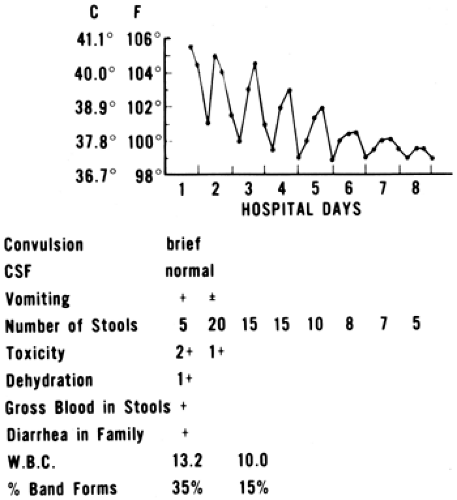
Classically, shigellosis presents with high fever, abdominal pain, and cramping, which may precede the diarrhea by a day or two (Fig. 12-1). This is consistent with observations of experimental shigellosis in human volunteers, in which fever preceded the diarrhea by about 2 days on the average.43 Shigellosis caused by food-borne transmission tends to be with a high inoculum and thus severe symptoms, as listed earlier. However, in toddlers who acquire it via person-to-person transmission, the inoculum is much lower, and the symptoms tend to be milder. This is particularly true with S. sonnei.
In a study of adult prisoners, the infectious dose of Shigella was as few as 200 organisms.43 The mean incubation period to fever was 2 days; to diarrhea, 4 days. The duration of excretion of Shigella without antibiotic therapy was as long as 78 days, with a mean of 27 days. A more recent study confirmed the extremely low infectious dose and high acid-tolerance of this organism; as few as 100 bacteria were sufficient to induce disease.44
In addition to food-borne and waterborne transmission, Shigella infection is spread by the fecal–oral route. Spread within households and day-care settings is common, probably because of the low inoculum required to produce disease45. Antacid use increases the risk of infection, because it enhances the ability of the organism to survive transit through the stomach. Outbreaks in association with innumerable vehicles have been reported. Common sources include salads touched by infected food workers and raw produce such as fresh parsley, lettuce, and green onions.46,47,48 Recreational water fountains and pools have served as a point source for other outbreaks.49
The signs and symptoms of Shigella infection may vary considerably based on the serotype of the infecting organism and the size of the inoculum. In
the United States, most cases are due to S. sonnei, and disease is often acquired by low-inoculum means, resulting in less-severe disease that may be self-limited even without therapy (treatment is still indicated for prevention of spread). Severe cases are typified by sudden onset of high fever and dysentery. There may be other members of the family suffering from diarrheal disease. In rare cases, there is loss of anal sphincter tone. A study of dysentery in Bolivia found that crying during defecation, fever, greater than five loose stools a day, and the finding of more than 50 white blood cells (WBCs) per high power field were associated with the isolation of Shigella from stool cultures.50
the United States, most cases are due to S. sonnei, and disease is often acquired by low-inoculum means, resulting in less-severe disease that may be self-limited even without therapy (treatment is still indicated for prevention of spread). Severe cases are typified by sudden onset of high fever and dysentery. There may be other members of the family suffering from diarrheal disease. In rare cases, there is loss of anal sphincter tone. A study of dysentery in Bolivia found that crying during defecation, fever, greater than five loose stools a day, and the finding of more than 50 white blood cells (WBCs) per high power field were associated with the isolation of Shigella from stool cultures.50
Vomiting may be present early. Appendicitis is occasionally suspected because of the severity of the abdominal pain. Generally speaking, exploratory laparotomy is not indicated in patients with severe abdominal pain in whom the diagnosis of shigellosis has been clearly established. However, surgical complications of Shigella infection occasionally occur, including appendicitis, peritonitis, and intestinal obstruction.51 Lumbar puncture is sometimes done in patients with shigellosis when the diarrhea does not begin until after fever and convulsions. The spinal fluid is usually normal.
The key pathophysiologic event in Shigella dysentery is invasion of the intestinal mucosa. Intense local inflammation is incited by a cytokine cascade. Shigellae that are ingested by macrophages can induce apoptosis in those cells. Once the organism gets into the cytoplasm, it can then spread from cell to cell.52 Although local inflammation and mucosal ulceration can be severe, Shigellae rarely penetrate the lamina propria; therefore, bacteremia is uncommon.53 The components of innate immunity are probably important both in protection from disease and in recovery; high levels of lactoferrin, myeloperoxidase, and leukotriene B4 are found in the stools of patients suffering from Shigella infection,54 and in animal models, lack of natural killer cells predisposes to more severe and progressive disease.55 The pathophysiology behind the CNS effects of shigellosis is not well understood.
Campylobacter
This organism was formerly included in the genus Vibrio. The species associated with diarrhea is C. jejuni.56,57,58,59,60 The species C. intestinalis typically causes bacteremia in humans who are immunocompromised or have a malignancy. C. jejuni was not recognized as a cause of diarrhea before the mid 1970s because special culture techniques are needed to isolate the organism. The frequency of this pathogen as a cause of diarrhea has been underestimated because of the broad spectrum of disease and the difficulty in its isolation. However, it is now known to be more common than shigellosis in the United States. Rates of Campylobacter infection are highest in the state of Hawaii. A case-control study there found two historical events that were significantly associated with the acquisition of disease: (1) consumption of chicken prepared by a commercial food establishment in the 7 days prior to illness, and (2) antibiotic use within 28 days of infection.61 There is a bimodal distribution of Campylobacter illness, with the first peak in infancy and the second in young adulthood.62
The clinical illness typically produced by Campylobacter can mimic shigellosis in its various degrees of severity, including diarrhea with convulsions at the onset of the fever.60 Probably campylobacteriosis was the real cause of most of the past clinical illnesses called “shigellosis” in which no Shigella were cultured. In children, the diarrheal illness is typically associated with mild vomiting, fever with moderate abdominal pain, and frankly bloody stools that begin about 2–4 days after the onset of the other symptoms.58 The diarrhea and abdominal pain last an average of 7 days. Fever has an average duration of about 2 days. The illness may be confused with acute idiopathic ulcerative colitis, especially when it occurs in young adults. Disease is typically milder than that seen with Shigella infection, although severe cases, including two associated with colonic perforation, have been reported.63
Comparatively little is known about the pathogenesis of Campylobacter enteritis. Attachment and invasion are thought to be important. Rarely, asymptomatic individuals can shed Campylobacter organisms in their stool. Molecular comparison of these isolates with those recovered from individuals with illness revealed that there is an identifiable marker found on disease-associated strains.64 This locus has been named the invasion-associated marker. The exact protein encoded and its function have yet to be elucidated.
Waterborne outbreaks of Campylobacter diarrhea have been observed. It is normal flora of domestic livestock, and it can be acquired from ingestion of unpasteurized milk. Multiple studies have documented that the vast majority of commercial chicken is contaminated with Campylobacter.65
Much of the disease caused by Campylobacter in the United States comes from eating improperly cooked or handled chicken. Backyard barbecues are a particular risk, as the organisms can be spread from raw meat to other foods that will not be cooked, such as salads. Unfortunately, one study of 60 “domestic kitchens” showed that many surfaces are contaminated with Campylobacter after preparation of a chicken-containing meal, and that washing with soap and water did not change the rate of recovery of the organisms from surfaces. The situation was improved when hypochlorite was added to the cleaning solution.66 C. jejuni is also found as a cause of diarrhea in puppies and kittens. In one case report, an infant developed Campylobacter bacteremia, and the organism was traced to a puppy in the home. Person-to-person transmission occurs but is uncommon.
Much of the disease caused by Campylobacter in the United States comes from eating improperly cooked or handled chicken. Backyard barbecues are a particular risk, as the organisms can be spread from raw meat to other foods that will not be cooked, such as salads. Unfortunately, one study of 60 “domestic kitchens” showed that many surfaces are contaminated with Campylobacter after preparation of a chicken-containing meal, and that washing with soap and water did not change the rate of recovery of the organisms from surfaces. The situation was improved when hypochlorite was added to the cleaning solution.66 C. jejuni is also found as a cause of diarrhea in puppies and kittens. In one case report, an infant developed Campylobacter bacteremia, and the organism was traced to a puppy in the home. Person-to-person transmission occurs but is uncommon.
Salmonella
Salmonellosis is the most frequent cause of diarrheal food poisoning in the United States.67 Salmonellosis was on the increase in the United States for approximately 40 years; however, recent data support a decrease in the incidence of this disease in the years from 1987 to 1997.68 The highest incidence is seen in infancy, with a rate of approximately 159 cases per 100,000 at the age of 2 months.68 Infection with some serotypes is on the rise despite a global downward trend; all such serotypes are associated with exposure to reptiles and amphibians.68a
Because Salmonella spp are commensals in many animals used for food, consumption of contaminated foodstuffs is the usual route of infection. Inadequately cooked poultry and raw or undercooked eggs or egg products are usually impugned. Outbreaks due to alfalfa sprouts, chopped lettuce and tomato, fresh cilantro, and even peanut butter have been reported. These outbreaks are probably secondary to cross contamination from infected workers or irrigation systems. All of these vehicles have been experimentally proven to support the growth of Salmonellae.69,70 Many Salmonella species are remarkably resistant to acid pH, explaining their survival in chopped tomatoes and fresh salsa,71 which naturally have a pH of around 4.3. Interestingly, the serotypes that most often cause human disease are not the same as the serotypes that are found in highest frequency in food animals after slaughter.72
Salmonella species are normal flora in all reptiles and many amphibians. Salmonella is commonly transmitted from pet reptiles (such as turtles, snakes, iguanas, and lizards). Even without direct contact with the reptile, infants can still acquire salmonellosis when a caregiver prepares a bottle after handling a reptile or touching its cage.73 Person-to-person transmission also occurs but is uncommon.
Studies in adult volunteers have demonstrated that, compared with Shigella spp, a much larger number of organisms needs to be ingested to cause disease; infection of children is probably more easily accomplished. The pathogenesis of the disease involves internalization of the bacteria into the epithelial cells of the gut. This has been shown to be a complex sequence of events that terminates with an actin-dependent rearrangement of the cellular cytoskeleton.74 A series of virulence factors that allows the bacterium to survive inside macrophages and resist the bactericidal activity of complement has been described.
In diarrhea caused by Salmonella species, fever is often present, and occasionally blood or mucus is present in the stool (dysentery-like diarrhea). Often, however, the clinical pattern is that of a subacute diarrhea, which does not have an explosive onset but is somewhat persistent and may lead to moderate dehydration after several days. It is most severe in infants, young children, and debilitated elderly adults. Bacteremia may occur without greater severity of illness.75 However, the risk of bacteremia is sufficient that antibiotic therapy has been recommended for infants younger than 3 months old with Salmonella gastroenteritis.75 Young infants may develop meningitis if there is bacteremic seeding of the meninges.
In one outbreak, a healthy child who ingested the largest dose (as quantitated by the amount of ice cream ingested) died, indicating that severity is likely to be proportional to dose.76 In a study of children with leukemia or solid cancers, there were no deaths, and the severity of illness was similar to that in children without cancer.77 However, splenectomized children are at risk for more severe illness (Chapter 22).
Typhoid Fever
Infection with Salmonella typhi causes a systemic illness with bacteremia that is distinct from other salmonelloses. It is uncommon in the United States but is occasionally seen in returning travelers.
Transmission is from food or water that is contaminated by a human carrier. The incubation period is longer than in other Salmonella infections; it is usually about 14 days (range 3–60 days).
Transmission is from food or water that is contaminated by a human carrier. The incubation period is longer than in other Salmonella infections; it is usually about 14 days (range 3–60 days).
Initially, influenza-like symptoms develop with fever, malaise, headache, dry cough, and myalgia. Poorly localized abdominal discomfort and nausea are common. Children may develop diarrhea, but constipation is more common in adults. Young children may present with seizures. Fever rises progressively, until by the second week it is high grade and sustained. On physical examination, abdominal tenderness and hepatosplenomegaly are common. Occasionally, there is a faint maculopapular truncal rash (referred to as rose spots). Complications, such as gastrointestinal bleeding, intestinal perforation, and typhoid encephalopathy, occur in 10–15% of patients.78
Diagnosis is made by culture of blood (60–80% sensitive) or bone marrow (80–95% sensitive). Stool cultures are only positive in about 30% of patients.
For persons traveling to endemic areas, two vaccines are available, each with approximately 80% efficacy.79 The oral, live-attenuated vaccine (Ty21a) is approved for use in immunocompetent persons older than 6 years of age, and the intramuscularly administered subunit vaccine (ViCPS) is approved for persons older than 2 years old. Both have good safety profile.80
Enterohemorrhagic E. coli (EHEC)
The classification of E. coli is shown in Box 12-1. Only EHEC are a cause of inflammatory diarrhea and are discussed here. EHEC are also referred to as Shiga toxin–producing E. coli (STEC).
Enterohemorrhagic E. coli can cause severe, hemorrhagic colitis. These organisms are of special interest because of the propensity of infection with these pathogens to cause a microangiopathic hemolytic anemia. This may lead to a condition known as the hemolytic-uremic syndrome (HUS). Although more than 100 Shiga toxin–producing serotypes of E. coli have been isolated from humans, not all such serotypes have been shown to cause diarrhea or HUS. Non-O157 EHEC appear less likely than E. coli O157:H7 to cause bloody diarrhea. It is likely that some non-O157 EHEC tend to produce bloody diarrhea, others produce nonbloody diarrhea, and others are not human pathogens.81
In the United States, more than 80% of all postdiarrheal HUS is caused by E. coli O157:H7.82 In some European countries, non-O157 serotypes are relatively more common.83 E. coli O157:H7 is estimated to cause more than 70,000 infections, more than 2,000 hospitalizations, and approximately 60 deaths each year in the United States.84 The infectious dose is on the order of several hundred organisms. Children between the ages of 2 and 10 and the elderly are at highest risk.
E. coli O157:H7 is normal intestinal flora in about 1% of healthy cattle,85 and most infections are due to consumption of undercooked ground beef. However, numerous other vehicles have been implicated. Unpasteurized apple cider has been a common vehicle.86,87 Interestingly, in separate outbreaks in the Northwest United States that were traced to steak restaurants, careful epidemiologic investigation revealed that the consumption of meat was not a risk factor for infection; rather, cases were traced to items in the salad bar.88 The largest outbreak occurred in Sakai, Japan, in 1996. In it, approximately 10,000 children were infected by consuming white radish sprouts in their school lunches.89 Houseflies were incriminated as possible vectors in one nursery school outbreak in Japan.90 An experimental study showed that when houseflies were fed E. coli O157:H7, large numbers of bacteria adhered to the mouthparts and proliferated in the “minute spaces of the labellum.” The bacteria were excreted in the flies’ feces for 3 days.90
Well-documented routes of transmission include unchlorinated municipal drinking water and swimming in fecally contaminated lakes or waterparks.91,92,93 Person-to-person transmission is common, especially in the day-care setting, where secondary attack rates as high as 22% have been reported.94
Less than 10% of children symptomatically infected with E. coli O157:H7 develop HUS, which manifests a median of 6 days (range, 2–14 days) after the onset of diarrhea.85 Several retrospective studies have attempted to enumerate risk factors for the development of HUS among patients infected with E. coli O157:H7. One study of 252 children involved in the Japanese outbreak found that an elevated C-reactive protein (CRP) (defined as greater than 1.2 mg/dL), an elevated WBC count (defined as greater than 11,000/mcL), and an elevated body temperature (defined as greater than 38°C) were risk factors, or perhaps more precisely, clinical and laboratory markers of a high risk of HUS.95 Another study, this one involving 221 children,
found that young age was protective, and that a duration of prodromal illness less than 3 days and an elevated WBC count at presentation were predictive of progression to HUS.96
found that young age was protective, and that a duration of prodromal illness less than 3 days and an elevated WBC count at presentation were predictive of progression to HUS.96
Among children with HUS, risk factors for more severe disease include age younger than 2 years, anuria before admission, and elevated WBC count.96a A prospective study of 71 children, 10 of whom developed HUS, found that a high initial WBC count carried a relative risk of 1.3 for progression to HUS. The major finding of this study, however, was that antibiotic treatment of hemorrhagic colitis due to E. coli O157:H7 was the most important risk factor for the development of HUS, with a relative risk of 14. Antibiotic therapy remained an independent risk factor in the multivariate analysis, with a relative risk of 17 (95% confidence interval, 2.2–137).97 This clinical finding is not surprising, given that in vitro studies show that production of Shiga toxin is increased by subinhibitory concentrations of several different classes of antibiotics.85
When ordering a bacterial stool culture, it is important to recognize that not all laboratories screen stool specimens for the presence of E. coli O157:H7.98 The clinician may need to specifically request that stools be plated on sorbitol-MacConkey agar for detection of the organism. An enzyme immunoassay for direct detection of Shiga toxin in stool has been developed. It has a sensitivity of approximately 90% compared with culture for detection of E. coli O157:H7. Its greatest benefit is in detecting non-O157 serotypes. If used, it should be performed in addition to—not in place of—culture on sorbitol-MacConkey agar.99
Clostridium difficile
This organism is the most common cause of antibiotic-associated diarrhea for which an etiology can be determined. Diarrhea as a side effect of either oral or intravenous antibiotic administration is common, occurring in 5–10% of antibiotic courses.100
C. difficile is responsible for 10–20% of antibiotic-associated diarrhea; the remainder are idiopathic. C. difficile is especially likely to be the cause if it is an inflammatory diarrhea with fever, cramps, and bloody stools. The major risk factors are advanced age, hospitalization, and exposure to antibiotics.100 The severity of disease caused by C. difficile is quite variable, from mild diarrhea to extensive pseudomembranous colitis, which has a characteristic endoscopic appearance with gray or yellow plaques.
Diagnosis of C. difficile is usually by enzyme immunoassays that detect either toxin A or both toxins A and B. Particularly in children, tests that detect both toxins are preferred to avoid missing cases.101 A positive test in a young infant with diarrhea does not automatically prove a causal relation.4,102,103,104 The bacteria, with or without the toxins, can be found in from 40–80% of healthy neonates and is recovered from about 3% of older outpatients without diarrhea.105 Therefore, treatment (discussed later) is not automatically indicated. First, any antibiotic should be stopped if possible, as the antibiotic may itself be a cause of diarrhea or may alter bowel flora and so produce diarrhea.
Children with Hirschsprung’s disease can develop an enterocolitis, which may be caused by C. difficile.106 Because C. difficile may be spread from one patient to another via personnel, hospitalized patients should be kept in contact isolation, as should patients with diarrhea of unidentified cause.
Parasites
Entamoeba histolytica
Chronic Diarrhea
Diarrhea can be defined as chronic when it persists for more than 2 weeks. In the United States, non-infectious causes are at least as common as infectious ones, particularly if the diarrhea persists for longer than 4 weeks. The noninfectious causes of chronic diarrhea are numerous and vary depending on the child’s age. In the infant, common causes include disaccharidase deficiency, cow or soy milk protein intolerance, cystic fibrosis, or immunodeficiency. In toddlers, chronic nonspecific diarrhea (toddler’s diarrhea) and celiac disease should be considered. In the older child, inflammatory bowel disease, lactose intolerance, and irritable bowel syndrome are common causes.110 Several excellent reviews on the approach to the patient with chronic diarrhea are available.110,111,112,113
The rest of this discussion focuses on infectious
causes only. The infectious causes of chronic diarrhea can be divided into parasitic and nonparasitic. Parasites are far more common as agents of chronic diarrhea than are viruses or bacteria. However, any of the common causes of acute diarrhea can occasionally cause persistent symptoms, especially Salmonella, Campylobacter, and enteric adenovirus. C. difficile sometimes causes recurrent episodes of diarrhea. Oral vancomycin appears to be superior to metronidazole in treatment of recurrent disease.114 One study suggested that the use of a probiotic agent (Saccharomyces boulardii) provided added benefit.115
causes only. The infectious causes of chronic diarrhea can be divided into parasitic and nonparasitic. Parasites are far more common as agents of chronic diarrhea than are viruses or bacteria. However, any of the common causes of acute diarrhea can occasionally cause persistent symptoms, especially Salmonella, Campylobacter, and enteric adenovirus. C. difficile sometimes causes recurrent episodes of diarrhea. Oral vancomycin appears to be superior to metronidazole in treatment of recurrent disease.114 One study suggested that the use of a probiotic agent (Saccharomyces boulardii) provided added benefit.115
Parasitic Causes of Chronic Diarrhea
Amebiasis
The term amebiasis is usually understood to mean infection with Entamoeba histolytica. This infection is uncommon in the United States but is an occasional cause of chronic diarrhea after infancy.116,117 Often, there is a history of travel to a foreign country, such as Mexico. “Histolytica” refers to the invasive power of this species, which secretes a lytic substance that allows tissue invasion. Other species of amoebae (e.g., Entamoeba coli, E. hartmanni, and E. dispar) may be found in human feces as normal flora.
The motile trophozoite of E. histolytica survives only briefly after defecation. It is destroyed by gastric acid and so is usually not contagious. The cyst is the usual form seen and is the infective form. It also is the usual form recovered from the patient with chronic diarrhea.
Chronic or recurrent diarrhea secondary to amebiasis may be associated with episodes of abdominal pain.116 Mild diarrhea may alternate with constipation. Weakness, weight loss, and anemia may occur. Eosinophilia is unusual and is not striking when present. Fever is usually absent or low-grade (less than 38.4°C [101°F]).
Chronic amebiasis is often considered in the differential diagnosis of ulcerative colitis. There may be blood passed with the stool without much diarrhea.117 Hepatomegaly may be present, as may hepatic abscess.118
E. histolytica is morphologically indistinguishable from the nonpathogenic (and much more common) E. dispar. A commercially available EIA performed on stool is the best means of distinguishing the two.119
Giardiasis
Giardia lamblia is a flagellated protozoan that is the most common gastrointestinal parasitic infection in the United States. It is a relatively frequent cause of diarrhea, especially among those attending day-care centers.120,121 Its usual mode of spread is fecal-oral. Disease in adolescents and adults is related to diaper changing.122 Outbreaks in day-care centers, therefore, are most frequent in classrooms of diapered children.123 It has been transmitted by contaminated water, although the risk of acquiring giardiasis from backcountry water has not been well quantified.124 Dogs can be a source of human infection.121 The incubation period is usually about 1 week but may be as short as 3 days or as long as 3 weeks.
At least two different clinical syndromes are associated with Giardia infection. The acute form features bulky, loose, foul-smelling stools, flatulence, and sometimes abdominal cramping. Fever is uncommon. The chronic form of the disease is insidious; it is marked by waxing and waning symptoms. Long-term bulky diarrhea, belching, flatulence, and poor weight gain are the principal symptoms. Malabsorption and failure to thrive may be the only symptoms. Rarely, vomiting may be associated. Hypogammaglobulinemia is a predisposing factor, with great difficulty eradicating the parasite in these patients.125
The pathophysiology of giardiasis is not completely understood. The trophozoites in the duodenum reach large numbers. They are not invasive, but rather attach themselves to the outside of the endothelial cells. Some experts believe that the cause of the diarrhea and malabsorption is the actual physical obstruction caused by multitudinous trophozoites attached to the absorptive layer of the bowel wall.
The diagnosis can be made by visualizing the cysts in stool. One stool sample provides at least 66% sensitivity for the diagnosis; two samples must be evaluated to elevate the sensitivity to 90% or greater.126,127 Enzyme immunoassays that test for giardial antigens in stool have been developed and are somewhat more sensitive than microscopy.126 Neither test attains 100% sensitivity, because some patients with giardiasis shed the cysts intermittently. If the suspicion of giardiasis is high, a repeat stool sample should be sent. The string test, in which a weighted string is swallowed and allowed to pass through the duodenum, is an
older test that was generally reserved for patients in whom the diagnosis could not be otherwise established. However, the test is quite unpleasant and there is no scientific evidence that it is more sensitive than microscopy or antigen testing. Rarely, diagnosis will be established by esophagogastroduodenoscopy (EGD) with biopsy. Invasive procedures such as EGD, however, are almost never required.
older test that was generally reserved for patients in whom the diagnosis could not be otherwise established. However, the test is quite unpleasant and there is no scientific evidence that it is more sensitive than microscopy or antigen testing. Rarely, diagnosis will be established by esophagogastroduodenoscopy (EGD) with biopsy. Invasive procedures such as EGD, however, are almost never required.
Cryptosporidiosis
This protozoan belongs to the same suborder of parasites as does Toxoplasma.128 Prior to 1976 it was not known to be a cause of gastrointestinal disease. However, it clearly can cause diarrhea outbreaks in day-care centers.129 It is a cause of diarrhea in domestic animals, which can be a source of human infections. A food-borne outbreak in which the source was traced to raw produce in a restaurant has been reported; one of the food handlers at the restaurant was excreting oocysts in the stool.130
Cryptosporidia were also found to be the cause of the largest outbreak of waterborne diarrhea in the history of the United States, when over 400,000 people in Milwaukee became ill from cryptosporidial contamination of the municipal water supply.131 Control of waterborne outbreaks is difficult; the oocysts manage to elude municipal water purification systems and are remarkably resistant to chlorination. In outbreak situations, residents are usually asked to boil water for at least 1 minute prior to consumption. Post-outbreak surveys indicate that the majority of people comply with this request; however, 20% admitted to washing produce with tap water prior to consumption, and 57% used tap water for toothbrushing.132 These exposures are likely to pose significant risk, especially for immunocompromised hosts. The importance of boiling water for all uses needs to be emphasized in outbreak situations. Submicron point-of-use water filters proved to be protective against disease during the Milwaukee outbreak, reducing the infection rate from 50–80% to 18%.133 Filters with pores larger than one micron don’t do anything but provide a false sense of security.
Recreational water exposures such as swimming pools and waterparks are also a common source of infection.93,134,135 Because the infectious dose is as small as 30 oocysts,136 person-to-person transmission is also common, especially in day-care centers.137
After an incubation period of about a week (range, 2–14 days), the illness is marked by watery diarrhea in immunocompetent hosts. Study of the Milwaukee outbreak showed the median duration of illness to be 9 days. Ninety-three percent of patients with proven cryptosporidiosis had watery diarrhea, 84% reported abdominal cramping, 57% experienced low-grade fever, and about half had at least one episode of vomiting.131 In immunocompromised hosts, the disease tends to be more insidious and lasts indefinitely. Patients with advanced HIV disease are particularly prone to problematic cryptosporidiosis. Over time, weight loss progresses to cachexia. Involvement of the biliary tract can cause acalculous cholecystitis. Infection of the respiratory tract can also occur in immunosuppressed hosts. The virulence of cryptosporidia varies by serotype,138 and even within a serotype.
Degree of illness, duration of symptoms, and duration of oocyst shedding vary widely. In a study of traveler’s diarrhea in normal adults, the mean incubation period was 1 week, the mean duration of illness was 12 days, and the majority of patients stopped excreting oocysts about 1 week after the end of symptoms.139 In an outbreak associated with a day-care center, symptoms lasted 1 day to 4 weeks, and oocyst excretion lasted as long as 48 days after the onset of symptoms.129 Interferon-gamma seems to be important in protection against severe cryptosporidiosis.140 The importance of cell-mediated immunity in resolution of infection is attested to by the inability of patients with advanced HIV disease to clear the parasite. Many of these patients mount aggressive antibody responses but are still unable to resolve the disease. Antibodies are not entirely unimportant, however, as witnessed by the fact that patients with antibody-deficiency states tend to suffer more severe disease when infected with cryptosporidia.
Clinicians should be aware that routine ova and parasite examination of stool does not detect the presence of Cryptosporidium—testing for the organism needs to be specifically requested.141 Special stains are required, and most laboratories do not perform them routinely.142 When they do test for Cryptosporidium, three-fourths of laboratories use modified acid-fast staining. The sensitivity of this stain for Cryptosporidium is relatively low, 41–76%, particularly in cases in which the diarrheal illness is mild.141 Auramine-rhodamine staining may be more sensitive but lacks specificity.143 Enzyme-linked immunosorbent and monoclonal antibody
techniques appear to be have the best sensitivity and specificity but tend to be more costly.143,144
techniques appear to be have the best sensitivity and specificity but tend to be more costly.143,144
Cyclospora cayetanensis
Infection with this protozoan causes a watery diarrhea that can last for several weeks. Disease was originally found in returning travelers from areas of endemicity. In one study of traveler’s diarrhea, Cyclospora was found to be the cause in about 2–4% of cases. More than two-thirds of patients had symptoms for greater than 2 weeks, and about 40% had a greater than 3-kg weight loss.145
In the 1990s, several outbreaks were reported in the United States.146 Most outbreaks were due to the consumption of imported produce from Latin America, the largest implicating raspberries from Guatemala.147 Other implicated vehicles include mixed greens and fresh basil.146 In one outbreak, traced to a fruit dessert at a wedding reception, the incubation period averaged 7 days. All those who were infected had watery diarrhea, 93% had weight loss, 91% felt fatigued, and 90% had a loss of appetite. In almost 90% of patients, the symptoms waxed and waned, and in 61% of patients, symptoms lasted longer than 3 weeks.148 In immunocompromised patients, the disease is similar but lasts even longer. One report of cholecystitis from cyclosporiasis in a patient with AIDS has been published.149
Cyclospora oocysts in freshly excreted stool are noninfectious; they require days to weeks in the environment to sporulate into the infectious form. Thus, in contrast to Cryptosporidium, person-to-person transmission has not been documented.
As with Cryptosporidium, routine ova and parasite testing will not detect Cyclospora, and the physician should request that it be looked for when there is a suspicion of cyclosporiasis.146 Safranin is the best stain for demonstrating the oocysts.150 The disease may be underdiagnosed; in one study in Great Britain, only 58% of laboratories were able to correctly identify this pathogen.151 Treatment with trimethoprim-sulfamethoxazole (TMP-SMX) alleviates symptoms and decreases the duration of oocyst shedding.152
Isospora belli
This protozoan is very similar to C. cayetanensis, except that large food-borne outbreaks have not been reported. Infection is more common in tropical and subtropical areas of the world. Disease in immunocompetent patients is marked by watery diarrhea that resolves over several weeks. This pathogen is particularly problematic for patients with immune deficiencies, especially AIDS, in whom it causes massive secretory diarrhea with weight loss and cachexia, which can last for 6–10 months. When HIV infection is aggressively treated, isosporiasis tends to resolve.153 It can be diagnosed by fluorescence microscopy of concentrated feces in a wet mount preparation.154 Alternatively, acid-fast or safranin stains can be used.155 Treatment with TMP-SMX is effective, but relapses are common.156
Microsporidia
Microsporidia is the name given to a group of protozoan parasites. Over 1,200 species have been identified, but only a few have been demonstrated to cause disease in humans. Two species, Enterocytozoon bieneusi and Encephalitozoon intestinalis, primarily cause gastrointestinal infection.157
These pathogens are apparently rare in people with normal immunity, although one report of returning travelers with diarrhea found microsporidiosis in 5 (3%) of 148 of them.158 Aside from this report, however, almost all reported cases are in patients with AIDS. Diagnosis is accomplished by microscopy using a chromotrope-based or fluorescent stain.159,160
Patients with AIDS who are treated with effective antiretroviral regimens respond much more favorably to treatment for microsporidiosis.153 Albendazole is effective against some species (including E. intestinalis),161 but not others (such as E. bieneusi). Cases where cure of E. bieneusi infection was effected with nitazoxanide have been reported but controlled data are lacking.162 Despite reports of thalidomide treatment, this drug should not be used. In vitro, thalidomide does not suppress the growth of any species of microsporidia.163
Dientamebiasis
Dientamoeba fragilis is closely related to the flagellates, such as Trichomonas. It is a rare cause of chronic diarrhea and abdominal pain and sometimes causes acute diarrhea.164 Peripheral eosinophilia is common. The symptoms respond to metronidazole.
Strongyloidiasis
Balantidium coli
Infection with this protozoan pathogen is usually asymptomatic but can result in acute or chronic diarrhea. Infection is most frequent in areas of the developing world in which contact with pigs is common. Diagnosis is by direct visualization of fresh stool, and treatment is with tetracycline or metronidazole.166
Probable Nonpathogenic Protozoa
Several organisms are occasionally reported by the microbiology laboratory when found in stool submitted for routine ova and parasite testing.167 They include Blastocystis hominis, Endolimax nana, and nonpathogenic species of Entamoeba (E. dispar, E. coli, and E. hartmanni). These organisms are detected more frequently in children than adults and may predict the presence of other (pathogenic) protozoa. However, conclusive evidence that they are a cause of gastrointestinal symptoms is lacking.
Postinfectious Lactase Intolerance
The concept that lactose malabsorption is common in the recovery phase of acute bacterial or viral diarrheal illnesses, and that in some children this results in a significant persistence of the diarrhea,168 was a popular notion. Many physicians still prescribe a period of lactose restriction following an acute diarrheal episode. Lactose-free formula has even been developed to fill this clinical niche. Although this concept may still hold true for individual children, in population-based studies the time to recovery from acute diarrheal illness is similar between groups receiving lactose-containing formula or breast milk and those receiving lactose-free milk.169
Thus, routine use of non-lactose-containing formulas is not recommended for children with acute diarrhea. However, the contribution of lactase deficiency in young children with chronic diarrhea in the developing world is thought to be substantial.170 Risk factors for lactose intolerance in this population include age younger than 6 months, low socioeconomic class, and severe dehydration.
Bowel Bacteria Overgrowth
Bacteria such as Proteus or Pseudomonas, which normally are found in the bowel in small numbers, often become the predominant species in a patient with diarrhea. These organisms should not be assumed to be the cause of the diarrhea but probably are a “result” of the diarrhea. Klebsiella, Pseudomonas, and other enteric gram-negative rods occasionally contain plasmids that encode for toxins or invasiveness, much as E. coli do. Therefore, these organisms may occasionally be the cause of diarrhea.171 However, causation is difficult to prove unless isolates are studied for virulence factors in a research laboratory.
Unknown Agents
In surveys of diarrhea using all available techniques to detect the frequency of various agents, there is a moderate percentage of cases with no cause found. Clearly, some agents that cause diarrhea remain to be discovered.
Diarrhea in Compromised Hosts
Hirschsprung’s disease (aganglionic megacolon) is associated with severe enterocolitis in some children, sometimes attributable to C. difficile infection.172
Bone marrow transplant recipients can have diarrhea, especially from adenoviruses, rotaviruses, enteroviruses, and C. difficile (Chapter 22).173 Chronic enterovirus infection and giardiasis are common in children with agammaglobulinemia (Chapter 23).
Diarrhea may be a prominent feature of disseminated histoplasmosis in infants. Despite the presence of massive hepatosplenomegaly, disseminated histoplasmosis is sometimes initially misdiagnosed as infantile inflammatory bowel disease.
Rotaviral infection, giardiasis, cryptosporidiosis, cyclosporiasis, isosporiasis, and microsporidiosis can cause persistent diarrhea in immunocompromised children, such as those with AIDS (Chapter 20). All of these agents can also infect the immunologically normal host as well and were discussed earlier. Disease in immunocompromised hosts tends to be of longer duration. In addition, complications, such as cholecystitis or disseminated infection with protozoan parasites, are more likely to occur.
Laboratory Approach
Several thoughtful reviews are available about the clinical analysis and laboratory approach to children
with diarrhea.174,175,176,177 A variety of tests is available, but many of these are nonspecific. Even the most important test—bacterial stool culture—needs to be used selectively. As with any test that is ordered, careful consideration should be given to whether the results of the test will influence the care of the patient or be of public health significance. For example, rapid testing for rotavirus is widely available, but the management of the young child with rotavirus diarrhea is identical to that of a child with watery diarrhea of any cause. In addition, there are no specific public health implications of detecting a child with rotaviral diarrhea.
with diarrhea.174,175,176,177 A variety of tests is available, but many of these are nonspecific. Even the most important test—bacterial stool culture—needs to be used selectively. As with any test that is ordered, careful consideration should be given to whether the results of the test will influence the care of the patient or be of public health significance. For example, rapid testing for rotavirus is widely available, but the management of the young child with rotavirus diarrhea is identical to that of a child with watery diarrhea of any cause. In addition, there are no specific public health implications of detecting a child with rotaviral diarrhea.
Bacterial Cultures
Indications for this test include
Diarrhea with blood or mucus
Patients sick enough to be hospitalized
Special circumstances: day-care outbreaks, foreign travel, or suggestive exposure history
In acute-inflammatory diarrhea, stool culture is usually the best laboratory procedure and often can influence antibiotic therapy. In acute noninflammatory diarrhea, no laboratory test is of much value in determining the etiology or specific antibiotic therapy, although culture should be done to exclude a treatable bacterial cause when the illness is moderately severe or necessitates hospitalization. In chronic diarrhea, testing for specific parasitic causes (Giardia, Cryptosporidium, and Cyclospora) is reasonable. Patients with severe diarrhea and recent antibiotic exposure should be tested for C. difficile infection. A positive test in a young infant may represent colonization, but in an older child it is usually indicative of a causal relationship.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree