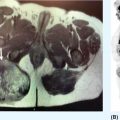
13710 Gastrointestinal Stromal Tumor Gastrointestinal stromal tumors (GISTs) span a wide clinical spectrum that ranges from lesions with essentially no metastatic potential to malignant and life-threatening metastatic tumors. The molecular understanding of the biologic underpinnings of GIST has led and is currently leading to the rational development of therapeutic approaches successfully implemented into the clinic. This chapter reviews clinical presentation, diagnostic approaches, and recommended imaging procedures for GIST, as well as general therapeutic approaches for both primary tumor and metastatic disease. GIST patients should be consulted and/or referred to sarcoma expert centers for disease management and treatment. Patient tolerance of imatinib treatment facilitates its use in the community. However, there are several nuances and steps during the clinical course of the disease that need to be addressed by multidisciplinary teams. Although the majority of GISTs are manageable, prevention and timely dose/schedule adaptation are essential to avoid unnecessary definitive treatment withdrawals from potential effective treatments. Surgery for metastatic disease potentially benefits a subset of GIST patients, and these opportunities should be evaluated in multidisciplinary sarcoma centers. Gastrointestinal stroma tumor, Adjuvant Imatinib, Neoadjuvant Imatinib, tyrosine-kinase inhibitor, tumor heterogeneity clinical trials, gastrointestinal stromal tumor, mutations, real-time biomarker, therapeutic approaches, tyrosine kinase inhibitors Biomarkers, Clinical Trials as Topic, Gastrointestinal Stromal Tumors, Mutation, Protein-Tyrosine Kinases, Therapeutics INTRODUCTION It was not until 1998 that gastrointestinal stromal tumors (GISTs) could be established as a distinctive tumor of mesenchymal origin due to the seminal discovery of the biologic and disease-defining role of KIT receptor tyrosine kinase (RTK) in this disease. Since then, GIST has emerged as a paradigm for clinically effective targeted inhibition of oncogenic driver mutations and a successful model to validate the preclinical concepts for drug response and drug resistance. GIST is currently regarded as the most common malignant gastrointestinal (GI) mesenchymal neoplasm, and it is thought to arise from the interstitial cells of Cajal (ICC). GIST spans a wide clinical spectrum that ranges from lesions with essentially no metastatic potential to malignant and life-threatening metastatic tumors. Activating mutations of KIT and PDGFRA RTKs are early, and possibly initiating, oncogenic events in most GISTs, and they continue to be the crucial drivers of tumor development and maintenance once GISTs have undergone malignant transformation. Throughout, the acquisition of novel genomic events is critical for tumor progression, mainly boosting cell cycle dysregulation and metastases formation. Over the past two decades, we have witnessed a spectacular increase in the overall survival (OS) of patients with this formerly untreatable disease. This is due to the exquisite addiction to oncogenic KIT/PDGFRA, which explains the profound effect of small-molecule KIT inhibitors on GIST cell viability and growth, both preclinically and clinically. However, even in patients with near-complete initial response to imatinib, there are invariably viable residual GIST cells, including imatinib-resistant subclones which harbor KIT secondary mutations, and subsequently manifest as clinical progression, typically in a median time of 20 to 24 months. Secondary mutations in KIT constitute the main mechanism of failure to imatinib in 70% to 90% of GIST patients. These imatinib-resistant secondary mutations cluster in two hotspot regions of the KIT kinase domain: the adenosine triphosphate (ATP)-binding pocket (exons 13 and14) and the activation loop (exons 17 and 18). Treatment strategies after imatinib failure exploit this KIT dependence, and sunitinib and regorafenib, two tyrosine kinase inhibitors (TKIs) with broad KIT inhibitory activity, are approved, respectively, in the second and third line. However, the structural heterogeneity of different KIT-resistant oncoproteins complicates the design of drugs that could effectively bind to and specifically inhibit all mutants. Thus, KIT secondary subclones cannot be completely suppressed by any given currently approved KIT inhibitor, ultimately leading to clinical progression after a median of 4 to 6 months regardless of the TKI used post-imatinib. Therefore, imatinib-resistant disease is not only an unmet clinical need, but also a highly active and evolving area of preclinical and clinical research that will likely continue to shape the field both in the short and long term (Figure 10.1). INCIDENCE AND DEMOGRAPHICS GIST is the most common mesenchymal malignant neoplasm arising in the GI tract, although its estimated incidence is only 1% of all GI tumors. Epidemiologic studies estimate an annual incidence of GIST in the United States of approximately 4,000 to 6,000 new cases, which represents seven to 20 cases per million per year. Recent European population-based, histologically confirmed studies establish the incidence rate of GIST between 1.2 and 1.5 cases per 100,000 per year. Notably, these studies also determined for the first time that GIST is the most frequent histological sarcoma subtype. FIGURE 10.1 Work flow depicting clinical, diagnosis, and treatment management of GIST patients. AFIP, Armed Forces Institute of Pathology; GIST, gastrointestinal stromal tumor; H&E, hematoxylin and eosin; IHQ, immunohistochemistry; NIH, National Institutes of Health. GIST occurs predominantly in middle-aged and older individuals, with a peak of incidence in the seventh decade of life. GISTs are equally distributed across all geographic and racial groups, also without gender predilection. An exception to this is succinate dehydrogenase (SDH)-deficient GIST. SDH-deficient GISTs are an uncommon subgroup (see section “KIT/PDGFRA Wild-Type GIST”), demographically characterized by disease present in patients younger than 40 years—particularly in the second decade—and with female predominance. CLINICAL PRESENTATION As with many mesenchymal neoplasms, there are no specific confirmatory signs or symptoms of GIST diagnosis, and clinical manifestations depend on the tumor size, location of the primary tumor, and presence of metastatic disease. Thus, GISTs are asymptomatic or paucisymptomatic at an early stage of disease and patients may appear completely healthy on physical exam. A constellation of symptoms emerge as the tumor develops, usually associated with the GI portion of origin and involvement of adjacent structures. GISTs may originate throughout the GI tract from the esophagus to the anus, and overt or occult GI bleeding at any point, together with the subsequent anemic syndrome, are the most common manifestations in up to two-thirds of patients. Abdominal mass, abdominal pain, and intestinal obstruction are present in 10% to 40% of the patients. Other symptoms are rather unspecific and may include hyporexia, postprandial fullness or early satiety, bloating, nausea, vomiting, dysphagia, constipation, weight loss, and fatigue. Constitutional symptoms are uncommon at early stages, even in patients with high tumor burden. DIAGNOSTIC APPROACH: BIOPSY AND PATHOLOGIC EVALUATION Tumor Biopsy Imaging results and radiologic findings are commonly highly suggestive of GIST diagnosis, and therefore, the final decision to undertake an image-guided tumor biopsy should be discussed within a multidisciplinary team and based on the confidence of workup results. Tumor biopsy is mandatory to confirm the diagnosis of GIST and the mutational status prior to the initiation of neoadjuvant treatment, when indicated. Likewise, definitive diagnosis is made by tumor biopsy in the case of reasonable questions regarding the radiologic findings. In metastatic GIST patients, image-guided percutaneous core-needle tumor biopsy is appropriate at the time of presentation to establish the diagnosis before initiation of systemic treatment. 139Pathologic Evaluation and Diagnosis GIST shows a broad morphologic spectrum that ranges from spindle cell appearance (77%) to epithelioid (8%) or mixed (15%) histologic types. The epithelioid morphology is more frequently observed in the stomach. Nuclear atypia is relatively uncommon. Mitotic rate is currently obtained as the number of mitosis in an area of 5 mm2, which equals 50 high-power fields (HPF) at a magnification of 40× in microscopes with traditional field size. Approximately two-thirds of GISTs at diagnosis have low or very low mitotic index (≤5 mitosis/5 mm2). Over 95% of GISTs stain for CD117 (KIT), and thus, immunohistochemistry for CD117 is routinely implemented for the diagnosis of GIST. CD117 often shows a diffuse cytoplasmic pattern and, to a lesser extent, membranous or perinuclear expression. Although DOG1 may be included in the initial panel, it is most useful in negative cases for CD117, in which DOG1 is expressed in one-third of the tumors. Other commonly used stains and their percentage of positivity include CD34 (80%), actin (30%), S-100 (10%), and desmin (4%). These markers exclude different entities in the differential diagnosis, such as schwannomas, fibromatosis, leiomyosarcomas, or myofibroblastic inflammatory tumors. Mutational analysis for KIT and PDGFRA mutations can confirm the diagnosis of GIST in challenging cases, particularly in GI mesenchymal neoplasms with a negative expression for CD117 and DOG1. Regardless, for GIST diagnosis, it is strongly recommended to determine the molecular status of KIT and PDGFRA, because the type of mutation in patients with localized or metastatic GIST can be both prognostic and predictive of treatment response to TKIs. SDH-deficient GISTs are characterized by epithelioid morphology, multinodularity, plexiform mural involvement, and low or very low mitotic index. Immunohistochemical staining for SDHB is widely used to confirm the diagnosis. It is strongly recommended to evaluate GIST cases with complex or unusual histopathologic features in referral centers with expertise in sarcoma diagnosis. RECOMMENDED IMAGING PROCEDURES AND CHARACTERISTIC FEATURES Imaging Studies Abdominopelvic CT scan is the initial procedure of choice for GIST workup. CT scans should be performed with both oral and intravenous contrast, which are critical to define the extent and location of the primary tumor and metastases, as well as other characteristics related to the clinical behavior (i.e., invasive components). GIST usually appears on CT scans as well-demarcated spherical masses that arise from the GI wall. They often project exophytically and/or intraluminally, and may have overlying mucosal ulceration. Central and extensive areas of necrosis and hemorrhage are commonly seen in larger GISTs owing to the outgrowth of vascular supplies. Endoscopy and Endoscopy Ultrasound GISTs appear at endoscopy as a submucosal mass protruding into the gastric lumen. Endoscopic ultrasound (EUS) exam further characterizes a gastric mass as suspicious for GIST, as GISTs are characteristically located in the fourth layer of the stomach, corresponding to the muscularis propria. Endoscopic biopsies using standard techniques often do not yield sufficient tissue for a definitive diagnosis; however, they can still be considered in certain cases. MOLECULAR ABERRATIONS DRIVING GIST GROWTH AND SURVIVAL KIT and PDGFRA Primary Mutations Oncogenic activation of KIT or PDGFRA RTKs is central to GIST biology and is present in 85% to 90% of the patients. KIT is a transmembrane receptor that belongs to the type III RTK family, which also includes KIT-homologous receptor PDGFRA, among others. KIT and PDGFRA mutations are mutually exclusive and are likely the initiating event. Constitutive activation of KIT or PDGFRA triggers downstream cell signaling cascades that control critical cell functions, including survival, proliferation, and adhesion. Oncogenic mutations in KIT are found in approximately 80% of GISTs. Two-thirds of GISTs harbor primary mutations in KIT juxtamembrane domain, encoded by exon 11 and distributed in the form of in-frame deletions, insertions, and substitutions, or combinations of these. Similar complexity is found, 140although less frequently, in other KIT regions distributed across the extracellular domain (exon 9), the ATP-binding pocket (exon 13), or the activation loop (exon 17). Primary mutations in PDGFRA (~10%) are found in homologous regions of the gene. Cytogenetic Progression in GIST Clinical and biologic progression of GIST represents a continuum that spans from micro-GISTs (<1 cm) to clinically aggressive and metastasizing GISTs. The crucial transforming and possibly initiating oncogenic event in most GISTs is the mutational activation of KIT or PDGFRA, which continues to be the essential driver of tumor development and maintenance, once the GISTs have undergone malignant transformation. However, KIT mutations alone are insufficient to induce malignant behavior, and additional genetic events are necessary to transform micro-GISTs into tumors with increasingly malignant potential. Thus, there is a well-established multistep cytogenetic progression involving typical chromosomic regions targeting genes not yet fully understood: KIT-activating mutation → 14q deletion (MAX) → 22q deletion → 1p deletion → 9p deletion (CDKN2A) → Xp deletion (DMD) (2–4). Deletion of DMD is almost universally present at a late stage in high-grade, lethal GIST, and conditions the metastatic spread. KIT Secondary Mutations Acquired resistance to first-line imatinib occurs in 70% to 90% of GIST patients through polyclonal expansion of heterogeneous subclones harboring different KIT secondary mutations that confer resistance to imatinib. Such mutations are not random and cluster in two regions of the KIT kinase domain: the ATP-binding pocket (encoded by exons 13 and 14) and the activation loop (encoded by exons 17 and 18). Other mechanisms, most likely involving KIT-independent activation of KIT-downstream signal intermediates (i.e., RAS; PIK3CA, PTEN), might have a potential role in imatinib resistance after several lines of treatment, although they are yet to be fully elucidated. KIT/PDGFRA Wild-Type GIST Wild-type (WT) GIST encompasses 10% to 15% of GISTs with heterogeneous molecular backgrounds sharing a lack of KIT or PDGFRA mutations. SDH-deficient GISTs represent approximately 40% of WT GISTs, and comprise pediatric GIST, Carney tryad, Carney–Stratakis syndrome, and a small proportion of adult WT GISTs. Most SDH-deficient tumors present with the following features: appear at a young age (<40 years), female predominance, gastric origin, epithelioid morphology, multifocal nodular growth pattern, and frequent involvement of local lymph nodes. SDH-deficient WT GISTs normally follow a chronic and indolent course of disease. Loss of SDH function through inactivating mutations or epigenetic silencing triggers a recurrent DNA hypermethylation phenotype. Although the key driver genes and/or pathways remain unclear, hypoxia-inducible factor (HIF) and insulin growth factor receptor (IGFR) pathways have been found to be typically hyperactivated in these patients. WT GISTs that are not SDH deficient may harbor a diversity of genetic events activating the RAS/mitogen-activated protein kinase (MAPK) pathway, uncommon fusions including NTRK, and unknown driver mechanisms. COMMON LOCATION OF PRIMARY DISEASE GISTs are the most common sarcomas of the GI tract and can arise virtually anywhere along the GI tract, although stomach and small intestine are the most common primary tumor locations in 60% and 30% of the patients, respectively. Duodenum and rectum are the less common primary sites (4% each), and more infrequent locations include esophagus, colon, appendix, and extra-GI GIST (i.e., omentum). GENERAL THERAPEUTIC APPROACH FOR PRIMARY TUMOR According to the National Comprehensive Cancer Network (NCCN) and European Society of Medical Oncology (ESMO) guidelines, management of GIST patients should be done in reference centers and/or within reference networks sharing multidisciplinary expertise and treating a high number of patients annually. 141Surgery All GISTs ≥2 cm in size should be resected because of their intrinsic metastatic potential. The standard and potentially curative therapy for localized GIST is surgical resection with the goal of complete removal with negative margins and an intact pseudocapsule. The extent of surgery usually involves a wedge resection of the stomach or bowel, as GIST, unlike epithelial GI tumors, commonly projects exophytically and/or intraluminally, and therefore, wide margins are not needed. Actually, the ideal margin of resection in GIST remains a matter of debate. Hence, while macroscopic incomplete resection is certainly associated with worse outcome, it seems that the presence of microscopically positive margins (R1) does not impact negatively on recurrence-free survival (RFS). Additionally, tumor rupture to the abdominal cavity during perioperative procedures is associated with a risk of recurrence close to 100%, and therefore, it should be avoided. Routine lymphadenectomy is not recommended, given the virtual absence of nodal metastases in GIST, except in rare GIST subtypes such as SDH-deficient GIST. Abdomen and liver inspection should be performed in all cases with an aim to identify and remove any previously undetected peritoneal metastatic deposits. There is no consensus on the management of small GISTs (<2 cm). On the one hand, incidental micro-GISTs (<1 cm) are present in up to one-third of the population and virtually never progress to clinically aggressive GIST. Thus, incidental lesions suggestive of micro-GIST might be initially followed to prove lack of increase in tumor size. By contrast, the clinical behavior of GISTs between 1 and 2 cm is unpredictable in the absence of pathologic evaluation. While surveillance might be considered in gastric GISTs between 1 and 2 cm, small bowel, colon, or rectal GISTs should be resected irrespective of the size, given the higher risk of recurrence and malignant potential. Adjuvant and Neoadjuvant Imatinib The success of imatinib in advanced disease prompted interest in its perioperative use with the final goal of preventing relapse after surgery. This includes both postoperative treatment for patients at high risk of recurrence after complete resection of a primary GIST and preoperative therapy for patients with unresectable disease or borderline resectable tumors. There are several well-defined criteria specifically created to identify the subset of GIST patients with the highest likelihood of relapse and who could obtain more benefit from adjuvant/neoadjuvant imatinib. According to the risk-stratification criteria, GIST patients are classified at low, intermediate, and high risk of recurrence after surgery (see section “Propensity to Metastasize: Risk Factor Stratification in GIST”), and high-risk patients benefit the most from perioperative treatment with imatinib. Adjuvant Imatinib Three years of adjuvant imatinib therapy is associated with reduced recurrence rates and improved OS in patients with high-risk primary GIST, and it is the current standard of care. This recommendation is the result of three randomized Phase 3 clinical trials that evaluated the role of imatinib 400 mg daily in the adjuvant setting: the American College of Surgeons Oncology Group (ACOSOG) trial Z90011, the Scandinavian Sarcoma Group (SSG) XVIII/Arbeitsgemeinschaft Internistische Onkologie (AIO) trial2, and the European Organization for Research and Treatment of Cancer (EORTC) trial 620243. In Z9001, resected GISTs ≥3 cm were randomly assigned to either imatinib or placebo for 1 year after surgery. One-year RFS was significantly improved from 83% in the placebo group to 98% in the imatinib group. No difference in OS was detected between both groups because of the crossover effect and because the trial was not powered to assess this difference. One of the main criticisms to this study was the inclusion of a wide range of GIST patients with different risks for relapse. Regardless, 1 year of treatment with adjuvant imatinib was accepted as the standard treatment after complete resection of GIST. The SSG XVIII/AIO Phase 3 clinical trial subsequently investigated the differences between imatinib 400 mg daily for 1 year and the same regimen for 3 years. For this study, more stringent inclusion criteria were applied, and it only entered high-risk GIST patients based on the National Institutes of Health (NIH) consensus classification or those with tumor rupture. Patients assigned to the 3-year group had a 5-year RFS of 65.6% compared with 47.9% in the 1-year group. Although the trial was not powered to assess differences in OS, the analysis showed that 3-year treatment achieved a 5-year OS of 92% compared with 81.7% in the 1-year group. In view of these results, the NCCN and ESMO guidelines currently recommend 3 years of adjuvant treatment with imatinib in high-risk patients. The NCCN guidelines also recommend considering adjuvant imatinib in intermediate-risk patients on an individual basis. Adjuvant treatment for low-risk patients is not indicated. 142The Phase 3 EORTC 62024 study randomized intermediate- and high-risk GIST patients to 2 years of imatinib or observation after surgical resection. Two years of adjuvant imatinib impacted positively on RFS, with a 3- and 5-year RFS rate in the experimental and placebo groups of 84% versus 66% and 69% versus 63%, respectively. OS did not differ between the treatment arms. Interestingly, this trial changed the primary endpoint to imatinib failure-free survival (IFFS) in order to capture the time when a different TKI was started in the advanced setting. No differences were observed regardless of the treatment group. This trial confirmed earlier studies in terms of RFS, but also observed that most of the benefit was lost after 1 to 3 years from the end of the adjuvant imatinib, thus suggesting that imatinib delayed the relapse. Therefore, this finding raises concerns about the mechanism of action of adjuvant imatinib in GIST, as it is not known whether recurrences are truly being prevented or just delayed. Recently, the PERSIST-5 Phase 2, single-arm clinical trial6 investigated whether longer duration of adjuvant imatinib 400 mg daily (5 years) had an impact on outcomes in completely resected intermediate- and high-risk GIST patients. Half of the patients discontinued the treatment owing to patient choice. Recurrence occurred within 2 years from imatinib discontinuation in those patients completing the 5 years on adjuvant treatment. The definitive answer to this question will come from the SSG XXII trial, which is currently evaluating 3 versus 5 years of adjuvant imatinib in locally resected GIST patients in an international Phase 3 study. Other areas of uncertainty need to be addressed with prospective studies interrogating questions such as whether doses >400 mg should be used in the adjuvant setting and whether adjuvant imatinib might benefit uncommon GIST subgroups (i.e., SDH-deficient GIST). Neoadjuvant Imatinib Current evidence for the use of preoperative imatinib in localized GIST comes from retrospective series and a few prospective, single-arm Phase 2 trials. These studies4,5 showed that most locally advanced GISTs shrink or stabilize in size after 6 to 12 months of preoperative imatinib, allowing subsequent surgery. Therefore, neoadjuvant treatment with imatinib is not a standard of care, and the decision must be made on an individual basis. Several aspects need to be taken into account: first, based on the high rate of responses observed with imatinib in patients with metastatic GIST, preoperative use of imatinib aims to reduce tumor bulk to facilitate complete surgical resection. Thus, it can be considered in patients with rectal or duodenal GIST—to avoid resections that adversely impair the quality of life—and in patients with a large gastric GIST, which likely requires total or subtotal gastrectomy. Second, early assessment of response is required in order to minimize risks, because a delay could impede surgery if imatinib fails to shrink or stabilize the tumor size. Thus, tumor imaging by either PET/CT or CT at baseline and in the first 4 to 8 weeks on imatinib appears to be the most sensitive option, and it is our standard practice. Third, genotyping at baseline and identification of imatinib-nonresponsive mutations (e.g., PDGFRA D842V) are highly recommended. Finally, it should be noted that risk stratification in patients receiving neoadjuvant imatinib will likely be inaccurate, because both pretreatment biopsy and posttreatment specimens do not allow full evaluation of tumor mitotic rate. Thus, careful evaluation is needed for a correct indication for adjuvant imatinib. RECOMMENDED FOLLOW-UP AFTER DEFINITIVE THERAPY Abdominal CT scan is the usual procedure, given the abdominal location and the typical relapse in the peritoneum and liver. MRI is recommended to characterize the liver lesions, complex locations such as rectum, or allergic reactions to iodine contrast agent. PET is indicated when the previous studies are inconclusive. There is a lack of studies assessing the effectiveness of duration, timing, and procedures for the follow-up of GIST patients undergoing treatment for localized disease with or without perioperative treatment with imatinib. In this absence of data, recent guidelines and recommendations advocate adjustments based on risk of recurrence and time after surgery. Localized Disease Annual CT Scan in Low-Risk Patients For intermediate- and high-risk GIST, CT scan can be performed every 3 to 4 months during the first 2 years, every 6 months thereafter, and annually after 5 years of follow-up. It is important to note that once imatinib is withdrawn, recurrence mostly occurs during the following 12 to 24 months, and therefore, follow-up should be maintained during this period. 143Advanced and Metastatic Disease Follow-up should be conducted in GIST patients treated with TKIs approximately every 3 months, but can be prolonged to 4 to 6 months in patients with sustained response to a given TKI, particularly in the long-term responders (>5 years) to imatinib. RECIST (Response Evaluation Criteria in Solid Tumors) is the usual type of measurement in the follow-up, but Choi response criteria should be used to rule out pseudoprogression as a consequence of myxoid degeneration or intratumoral hemorrhage occurring in response to TKIs. PROPENSITY TO METASTASIZE: RISK FACTOR STRATIFICATION IN GIST According to some series, 40% to 50% of GIST patients may develop recurrent disease after surgery despite macroscopically complete resection. All GISTs >2 cm are at risk of recurrence, although their clinical behavior is highly variable. Several prognostic criteria are used among clinicians to estimate the risk of relapse after surgery and/or to predict the potential benefit of adjuvant treatment with imatinib. Primary tumor size, mitotic count, and tumor location are the three main prognostic factors in GIST. Proliferation rate is assessed in GIST with the mitotic count using a total area of 5 mm2 or 25 per HPF in modern microscopes. GISTs located outside the stomach have generally more unfavorable outcomes than GISTs arising in the stomach. Additionally, virtually all patients are considered at risk of relapse in the context of tumor rupture, either spontaneous or due to manipulation during the surgery. Current risk-stratification systems include some or all of the aforementioned prognostic factors. The consensus criteria from the NIH, which is the oldest, stratifies risk based on tumor size and mitotic count. The Armed Forces Institute of Pathology (AFIP) risk criteria added tumor location as a prognostic factor, which improves differentiation between patients with moderate and intermediate risk. Finally, the revised NIH consensus criteria additionally incorporated tumor rupture. Despite these differences, these three methods are comparable and predict reasonably well. These risk-stratification systems are validated only in KIT-mutant GIST—other genotypes are underrepresented. There is a well-established prognostic role of particular mutational status in GIST, although it is not included in any risk evaluation system. GISTs with KIT exon 11 deletions in codon 557 exhibit particularly aggressive behavior. This has been consistently shown in several studies and appears to be particularly helpful in gastric GIST with intermediate risk of relapse by the above-mentioned risk-stratification systems. These reports also agree that GISTs with primary mutations in PDGFRA exon 18 (D842V) are associated with a more favorable prognosis when localized and have a seemingly lower risk of recurrence and distant metastatic spread. COMMON FEATURES OF METASTATIC DISEASE GIST commonly spreads to the liver and/or the abdominal cavity (peritoneum, mesentery, omentum). At the time of diagnosis, approximately 50% of the cases are localized, 20% are locally advanced, and 25% are already present with metastatic disease. THERAPEUTIC APPROACH FOR METASTATIC DISEASE GIST has emerged as a paradigmatic and successful model for rational development of targeted agents against critical driver alterations in cancer. In the past 15 years, we have witnessed how the introduction of KIT inhibitors into the clinic has led to an extraordinary improvement of outcomes in this tumor, formerly deemed chemoresistant. Targeted Therapy Imatinib Imatinib (Gleevec in the United States, Glivec elsewhere; Novartis Oncology, Basel, Switzerland), an orally available, small-molecule inhibitor with activity against KIT, PDGFR, and ABL kinases, was the first TKI that was granted the U.S. Food and Drug Administration (FDA) approval for the treatment of KIT-positive metastatic and/or unresectable GISTs, following the demonstration of sustained response to imatinib 400 mg daily in the landmark B2222 Phase 2 trial.7 Approximately two-thirds of the patients achieved objective radiographic response to imatinib, 15% of patients had prolonged stable disease, and 10% to 15% exhibited primary resistance. Patients with stable disease had similar long-term benefit and favorable survival 144outcomes as those with objective responses. This study showed a median time to treatment progression (TTP) of 24 months and an estimated median OS of 57 months, with no differences between the doses of 400 and 600 mg daily. This benefit is particularly prolonged in a subset of GIST patients, with around one-third achieving progression-free survival (PFS) at 5 years and 5% to 10% achieving it for up to 10 years. Importantly, the KIT genotype predicted GIST outcomes, as KIT exon 11-mutant patients had a substantially greater likelihood of partial response and longer time to treatment failure compared with patients with either KIT exon 9 mutation or KIT/PDGFRA WT. Therapy is generally well tolerated, with diarrhea, edema, and fatigue being the most common treatment-related toxicities. Two subsequent Phase 3 trials8,9 supported these findings and further studied the efficacy of higher doses of imatinib (800 vs. 400 mg daily). Overall, a small but statistically significant PFS advantage was seen in GIST patients treated with imatinib 800 mg daily, with no difference in OS between the two arms, and patients on higher-dose therapy reported more side effects. The presence of KIT exon 9 mutation was the only significant predictive factor for benefit from higher doses. Imatinib at a dose of 400 mg daily is the standard initial dose for the treatment of advanced or metastatic GIST patients. Dose escalation to 800 mg/d is a reasonable option for patients progressing on 400 mg/day—particularly in KIT exon 9 mutant—although there is no data or consensus favoring dose escalation versus initiation of second-line treatment.10–12 The main mechanism of resistance found in 70% to 90% of GIST patients entails the emergence of subpopulations with KIT secondary mutations impeding imatinib binding to the KIT receptor. Resistance involves substantial heterogeneity of secondary mutations between and within metastases from individual patients, which underscores the complexity of resistance as the main treatment challenge in patients with GIST after frontline imatinib treatment. Sunitinib Sunitinib (Sutent; Pfizer Inc., New York, NY, USA) is a multitargeted small-molecule TKI with potent activity against KIT and PDGFRA, among several other kinases, and is currently approved for the treatment of metastatic GIST in patients with imatinib resistance or intolerance. In a pivotal randomized, placebo-controlled Phase 3 trial13 in patients with imatinib-refractory or -intolerant GISTs, 312 patients were enrolled and randomized to receive sunitinib or placebo. Sunitinib dose was 50 mg daily, 4 weeks on and 2 weeks off. Despite a low objective response rate in the sunitinib group (7% response rate), TTP, the primary endpoint, was fourfold longer in the sunitinib arm compared with placebo (27 vs. 6 weeks, respectively). Continuous dosing of sunitinib 37.5 mg daily is also active in the same setting, as shown in a nonrandomized Phase 2 clinical trial14 that yielded a clinical benefit rate of 53% and a PFS of 34 weeks. Regorafenib Regorafenib (Stivarga; Bayer HealthCare Pharmaceuticals Inc., Montville, NJ, USA), an orally active multikinase inhibitor with activity against a variety of kinases including KIT and PDGFRA, obtained worldwide approval for the treatment of GIST after failure of imatinib and sunitinib. A pivotal Phase 3 trial15 randomized GIST patients to regorafenib 160 mg once daily for the first 21 days of each 28-day cycle or placebo. Median PFS was also significantly longer in patients treated with regorafenib (4.8 months) compared to placebo (0.9 month). As observed with sunitinib, the overall response rate was low (4.5%), as most of the benefit was due to disease stabilization. Other Therapies There are currently no other therapies approved for the treatment of TKI-refractory GIST, and therefore, it remains an unmet clinical need. It is highly encouraged to treat these patients in clinical trials. Resumption of imatinib with palliative purposes is a common practice, given the rapid progression and symptom worsening after TKI discontinuation, likely due to the rapid outgrowth of imatinib-sensitive disease. However, a Phase 3 randomized trial16 showed no significant improvement in objective response or in OS compared to placebo in this setting. No improvement in quality of life measurements could be determined either, although other studies have demonstrated the positive impact of TKI maintenance on OS despite progression. Pazopanib has recently proved to be effective in TKI-resistant GIST patients in a randomized Phase 2 trial,17 achieving a significant improvement of median PFS (3.4 months) over placebo (2.4 months), again with <5% response rate, although these results have not been consistent across studies. The following TKIs with KIT inhibitory activity have been investigated in imatinib-resistant GIST, mostly in single-arm Phase 2 trials: sorafenib, ponatinib, nilotinib, dasatinib, and dovitinib. All of them share 145a similar activity profile, with a median PFS between 3 and 5 months and a response rate <10%, regardless of the line of treatment. Interestingly, small-molecule KIT-inhibitor monotherapies have a drug-specific activity profile against a subset of the KIT secondary mutational spectrum, which constitutes the molecular basis for the modest clinical benefit observed with successive lines of treatment in imatinib-resistant GIST. Surgery Resection of metastatic disease in GIST patients is not a standard procedure in all centers; however, in the absence of controlled trials, it can be considered on an individualized basis within multidisciplinary teams in sarcoma expert centers. Resection of metastatic disease aiming to achieve complete response may positively impact on outcomes in GIST patients either responding to TKIs or with focal progression. However, it is not recommended in patients experiencing generalized disease progression while receiving a TKI. Importantly, resection, even if complete, does not eliminate the need for continued treatment with TKI therapy. SUMMARY The molecular understanding of the biologic underpinnings of GIST has led and is currently leading to the rational development of therapeutic approaches successfully implemented into the clinic. All currently approved drugs for GIST are based on KIT inhibition, given the dominance of oncogenically active KIT signaling throughout all steps of GIST oncogenesis from micro-GIST to imatinib-resistant metastatic GIST. However, treatment of GISTs refractory to approved TKIs remains an unmet clinical need, as the heterogeneity of the resistant disease is a challenge. Thus, therapeutic strategies exploiting KIT, KIT-downstream pathways, or adaptive mechanisms to KIT inhibition through combination therapies or novel cross-cutting treatment strategies are being actively sought. Likewise, the high reliance of GIST on KIT or PDGFRA mutations, together with the relevance of KIT secondary genotype for response prediction to current TKIs make GIST an appealing model to validate circulating tumor DNA with the final goal of its implementation as a real-time biomarker for treatment selection. This approach remains under active investigation. In the meantime, GIST patients, as in all rare diseases, should be consulted and/or referred to sarcoma expert centers for disease management and treatment. Patient tolerance of imatinib treatment facilitates its use in the community. However, there are several nuances and steps during the clinical course of the disease that need to be addressed by multidisciplinary teams. For instance, KIT/PDGFRA primary genotype is highly relevant for the indication of imatinib as a neoadjuvant/adjuvant treatment or in the setting of metastatic disease, and should be performed in all cases. Additionally, GIST genotype is increasingly important for the participation in clinical trials. On the other hand, unlike imatinib, the range of side effects of sunitinib and regorafenib is higher owing to the wider spectrum of target inhibition. Although the majority of them are manageable, prevention and timely dose/schedule adaptation are essential to avoid unnecessary definitive treatment withdrawals from potential effective treatments. Likewise, and as mentioned in the section “Surgery,” surgery for metastatic disease potentially benefits a subset of GIST patients, and these opportunities should be evaluated in multidisciplinary sarcoma centers.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree