OUTLINE
Prevalence of Gastrointestinal Symptoms, 191
Implications of Gastrointestinal Symptoms in COVID-19, 191
Mechanism on How SARS-CoV-2 Affects Gut Health, 192
Impact of Gastric Acid Reduction on COVID-19 Outcomes, 193
Impact of COVID-19 in Inflammatory Bowel Disease, 194
Pathogenesis of COVID-19 in Inflammatory Bowel Disease, 195
Impact of Inflammatory Bowel Disease Therapy on COVID-19 Outcomes, 195
Impact of Irritable Bowel Disease Therapies on COVID-19 Antibodies, 195
Impact of COVID-19 on Liver, 196
Impact of COVID-19 on Pancreatic Disorders, 197
Association Between Angiotensin-Converting Enzyme Inhibitor and Angiotensin Receptor Blocker Use and Gastrointestinal Symptoms in COVID-19, 197
Postinfectious Gastrointestinal Symptoms, 198
Conclusion, 198
Prevalence of Gastrointestinal Symptoms
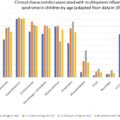
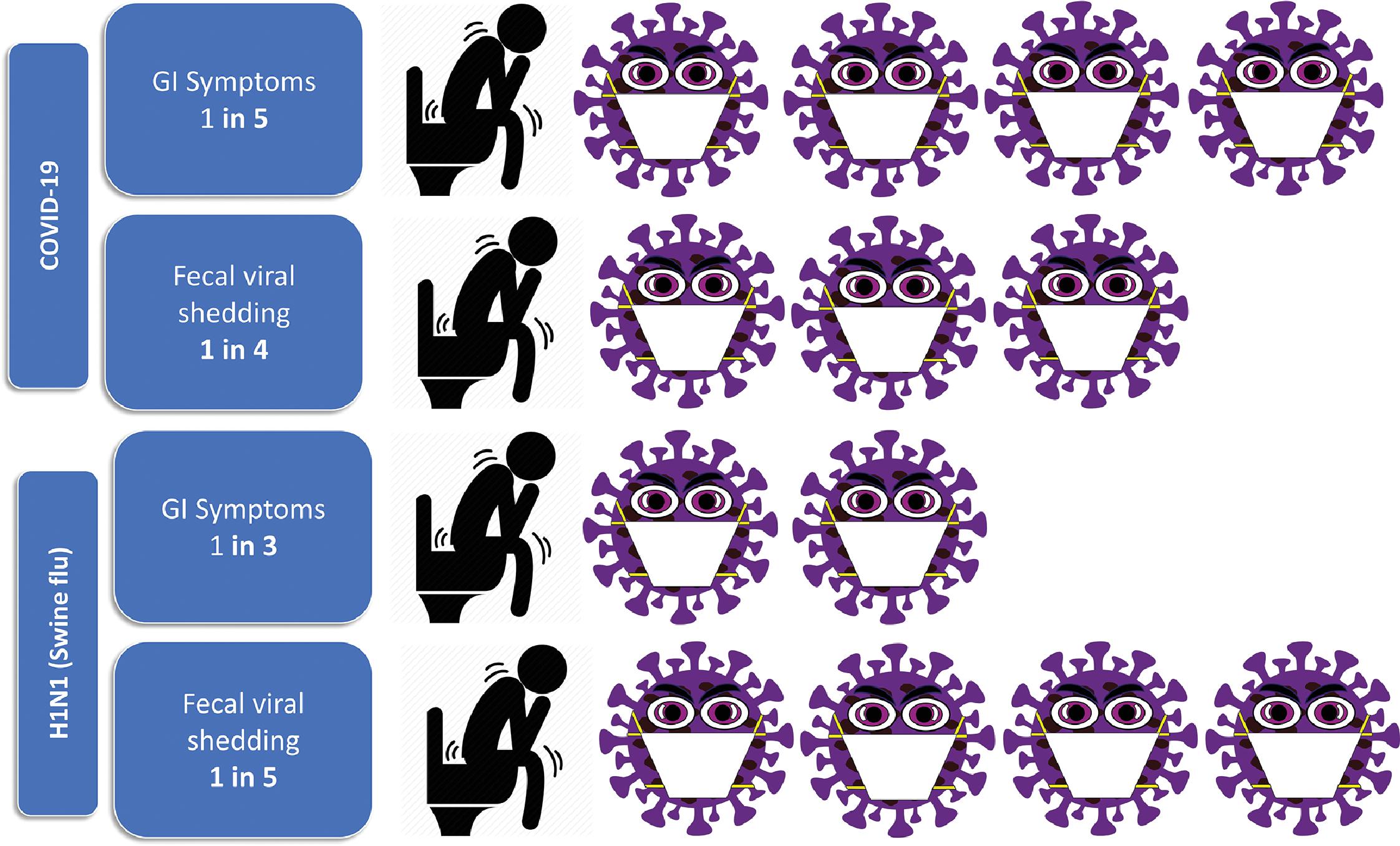
The first reported case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the United States presented on January 19, 2020 with symptoms of cough and nausea and vomiting and diarrhea developed on day 2 of admission. Although the serum remained negative, both the stool and respiratory specimens tested positive by real-time reverse transcriptase polymerase chain reaction (rRT-PCR) for SARS-CoV-2. Multiple studies have since been published reporting gastrointestinal (GI) manifestations of coronavirus disease 2019 (COVID-19). The most common symptoms reported in the literature include diarrhea, nausea, vomiting, abdominal pain, anorexia, and dysgeusia/ageusia. In a study by Han et al., 67 patients had diarrhea as their first symptom of COVID-19 infection and patients with enteric symptoms presented significantly later than those who had respiratory symptoms. More recently, gustatory dysfunction has been described in patients presenting with dysgeusia. In a recent meta-analysis of 25,252 patients with COVID-19, 20.3% presented with GI symptoms and 26.7% had confirmed fecal viral shedding with positive fecal RNA RT‐PCR test ( Fig. 9.1 ). Interestingly, SARS-CoV-2 virus has been shown to persist in stool samples even after respiratory tract specimens were found to be negative, which might suggest prolonged shedding. However, it is important to acknowledge that GI symptoms have always been prevalent in patients with respiratory viral illnesses. In the 2009 H1N1 (swine flu) pandemic, pooled prevalence of GI symptoms was even higher compared with COVID-19 at 31% with confirmed fecal shedding in 20.6% (see Fig. 9.1 ) .
Implications of Gastrointestinal Symptoms in COVID-19
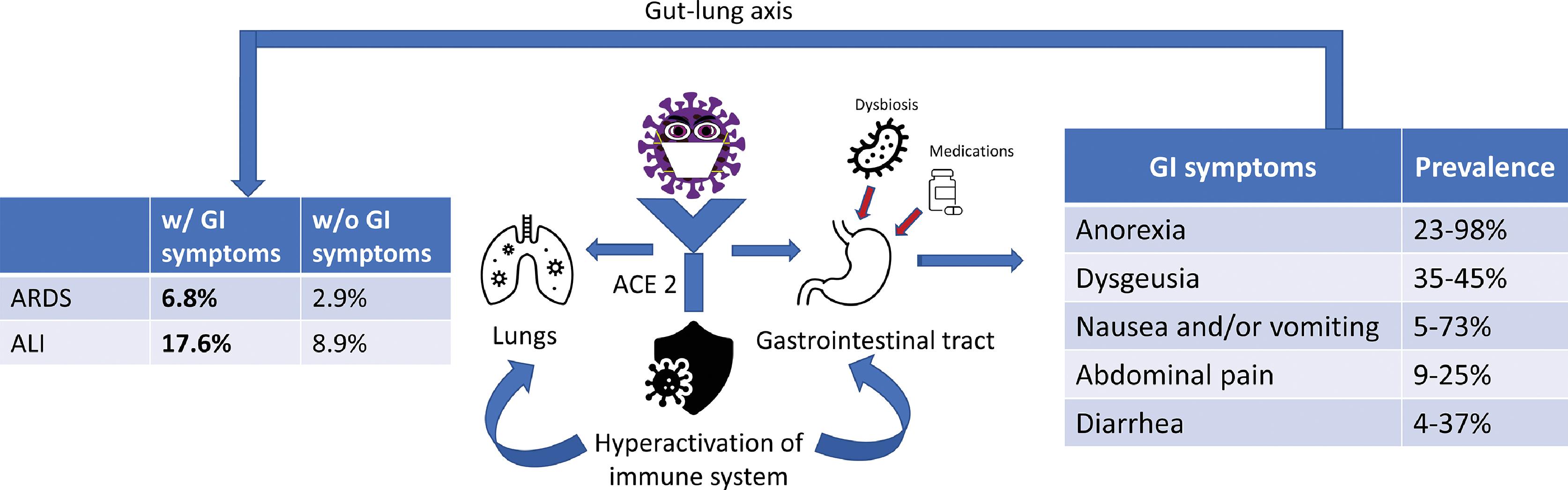
Few studies have suggested that the presence of GI symptoms is associated with worse outcomes in COVID-19 patients. Patients with primarily GI symptoms of COVID-19 have longer duration from illness onset to hospital admission. They are also more likely to have fever (62.4%), have delayed virus clearance, and more likely to have a virus shedding in the stool. A study by Jin et al. compared complications of acute respiratory distress syndrome (ARDS), liver injury, and shock in COVID-19 patients with and without GI symptoms and found significantly higher percentages of ARDS (6.8% vs. 2.9%) and liver injury (17.6% vs. 8.9%) in those with GI symptoms ( Fig. 9.2 ) . They also reported a significantly higher number of patients who required mechanical ventilation and intensive care unit admission in patients with GI symptoms. In another multicenter study, patients without GI symptoms were more likely to be discharged early compared with patients with GI symptoms (60% vs. 34%). Hence, presence of GI symptoms in patients with COVID-19 might be a predictor for worse clinical outcomes.
Mechanism on How SARS-CoV-2 Affects Gut Health
Angiotensin-Converting Enzyme-2 Receptor Mechanism
SARS-CoV-2 has characteristic transmembrane trimeric proteins that enable the binding and fusion of the viral particle to the cellular membrane of the host cell. The virus enters the host cell by attaching to the angiotensin-converting enzyme-2 (ACE2) receptors, which have a high affinity for the spike (S) protein of the virus. These ACE2 receptors are expressed in pulmonary, esophageal, small intestinal, and colonic epithelial cells. GI manifestations of COVID-19 can be explained by the high expression of ACE2 receptors in the gut and extensive infection of the enterocytes. After cell entry, virus-specific RNA and proteins are generated in the host cytoplasm to generate new virions, which are then released into the GI tract. This explains the persistence of viral RNA in stool samples of infected patients.
Another mechanism by which COVID-19 can cause significant damage to the host is the cytokine storm, a hyperactivation of the immune system to evoke a substantial inflammatory response. T-helper cytokines, both Th1- and Th2-type responses, cause changes in contractility of inflamed intestinal smooth muscle. Th1-type cytokines downregulate L-type Ca 2+ channels and upregulate G protein signaling, which contributes to hypocontractility of inflamed intestinal smooth muscle. Conversely, Th2-type cytokines cause hypercontractilty by signal transducer and activator of transcription (STAT) 6 or mitogen-activated protein (MAP) kinase signaling pathways. A cytokine profile characterized by increased interleukin-2 (IL-2), IL-7, granulocyte-colony stimulating factor, interferon-gamma (IFN-γ) inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, and tumor necrosis factor-alpha (TNF-α) has been associated with COVID-19 disease severity. One study from Wuhan, China reported a significant association between elevated inflammatory markers and poor COVID-19 outcomes suggesting hyperinflammation as the main cause of decompensation. For example, mean ferritin serum/plasma levels in nonsurvivors were 1297.6 ng/mL versus 614.0 ng/mL in survivors ( P < .001) and mean IL-6 serum/plasma levels were 11.4 ng/mL in patients who did not survive versus 6.8 ng/mL in those who did ( P < .0001). IL-6 activation and secretion by infected cells causes hyperactivation of the innate immune system and increased vascular permeability as a result of activation of vascular endothelial growth factor (VEGF), which in turn causes shock and ARDS. Tocilizumab, a humanized monoclonal antibody binding the IL-6 receptor (IL-6R), can effectively block IL-6 signal transduction in patients with severe COVID-19. A meta-analysis of 2112 patients enrolled in the tocilizumab group and 6160 patients in the standard of care group found that tocilizumab use was associated with decreased all-cause mortality in patients with signs of hyperinflammation with C-reactive protein (CRP) levels of 100 mg/L or greater, but also with a longer hospital stay when CRP levels were less than 100 mg/L.
Impact of SARS-CoV-2 on Gut Microbiota
Increasing evidence of viral RNA detection in stool samples of infected patients underscores the importance of fecal-oral transmission of SARS-CoV-2. Multiple investigators have reported the presence of ribonucleotides of SARS-CoV-2 in the feces of up to 50% of infected patients. Furthermore, SARS-CoV-2 RNA has been found in specimens from the esophagus, stomach, duodenum, and rectum.
The gut microbiota plays an important role in the GI tract and is responsible for a variety of physiological processes, including protection against infections, regulation of the immune system, and metabolism. A balance in the diversity of gut flora is critical to performing these functions. Interestingly, studies have shown a significantly decreased bacterial diversity in gut flora, increased proportions of opportunistic infections, and a relative low abundance of beneficial symbionts in patients infected with COVID-19 compared with healthy controls. Some have suggested administration of probiotics or consideration of fecal microbiota transplantation (FMT) to improve the microbial dysbiosis in patients with COVID-19, but efficacy is unclear and these treatments have not been rigorously studied. ,
Gut microbiota might also modulate pulmonary immunity through effects on lung microbiota. Gut dysbiosis has been associated with functional disability of alveolar macrophages to perform necessary function, including reduction in reactive oxygen species–mediated killing of bacteria. This association has been termed “gut-lung axis” and is reportedly bidirectional, where not only the microbial components such as endotoxins from the gut can change pulmonary immunity but lung infections also have a clinically significant impact on gut microbiota. To further prove this connection, studies have shown a link between gut dysbiosis and respiratory infections. Gut dysbiosis has been linked to increased risk for respiratory infections, but has also been shown to play a key role in the pathogenesis of sepsis, ARDS, and multiorgan failure in a culture-independent manner. , This association can be explained by the phenomenon of the leaky gut.
An intact intestinal barrier prevents bacterial translocation of gut flora and its metabolic components into the bloodstream and other organs. This protective barrier is dysregulated by unfavorable changes in the gut microbiota. Furthermore, gut dysbiosis also causes decreased production of bacteria-derived short-chain fatty acids such as butyric acid and acetic acid, which are vital in regulating the immune and inflammatory responses. This dysregulation facilitates the translocation of immunogenic bacterial components, including toxins and lipopolysaccharides, leading to uncontrolled immune activation and inflammation. Toll-like receptor 4 (TLR4) activation has been shown to play a pivotal role in this immune system activation. Dysregulated TLR4 activation is involved in acute systemic sepsis, in chronic inflammatory diseases, and in viral infections, such as influenza infection. Fig. 9.2 shows the proposed mechanism of how SARS-CoV-2 affects the lung and GI tract.
Impact of Gastric Acid Reduction on COVID-19 Outcomes
Proton Pump Inhibitors
Proton pump inhibitors (PPIs) are one of the most prescribed group of drugs for the treatment of various gastric acid–associated disorders. Although the impact of acid suppression on COVID-19 is unclear, theoretically, less acidic pH achieved with PPI therapy might not inactivate the virus. Furthermore, SARS-CoV-2 uses ACE2 receptor as the primary target to enter the host cell. These receptors are expressed by the glandular cells of the GI tract, mainly in the stomach, duodenum, and rectum. Activity of ACE2 also varies with pH and temperature. A pH of 7.5 seems optimal for its functioning. Because PPIs decrease acid production, they can theoretically increase binding of the virus to enterocytes and increase GI tract inoculation.
Clinical studies done on the effect of PPIs on COVID-19 have revealed controversial results. One survey-based study enrolled 53,130 participants and revealed that individuals taking PPIs once or twice daily had significantly higher odds of reporting a positive COVID-19 test compared with individuals who did not take PPIs. On the other hand, individuals taking histamine-2 receptor antagonists were not found to have an increased risk. This study was heavily criticized because of numerous methodological inconsistencies with concerns for selection bias and lack of ability to verify the responses in an online survey study. In addition, the number of participants tested for COVID-19 was not mentioned in the study, neither in the overall cohort nor in individual groups. , This raises the possibility of a bias resulting from a disproportionately higher number of people in the PPI cohort having been tested for COVID-19. The demographics of the COVID-19–positive group were also significantly different compared with those of the overall study participants. Patients in the PPI group were younger, and the study did not control of comorbid conditions.
On the other hand, a Korean nationwide cohort study reported that PPI use did not increase susceptibility to COVID-19 infection, but patients currently taking PPIs had 79% greater risk for severe clinical outcomes. More recently, a secondary analysis of a retrospective cohort study aiming to better characterize digestive manifestations in patients hospitalized with COVID-19 across 36 medical centers in North America found that after adjusting for measured baseline confounders, PPI use was not independently associated with the need for mechanical ventilation (odds ratio [OR], 1.02 [0.73–1.43]). Hence, because of conflicting evidence and unclear clear association between PPI and COVID-19 outcomes, these medications, when used for appropriate proven indication, should be continued in the lowest effective dose.
Histamine Receptor Antagonists (H 2 -Blockers)
Histamine and its role in diverse pathophysiological processes have been studied widely in the field of medicine. It is found in a variety of cells and tissues, predominantly in mast cells and basophils. It is stored in cytoplasmic granules and is a part of the cascade of inflammatory markers released in response to immune or nonimmune stimuli, including viral infections. , Histamine performs multiple physiological functions by activating four types of G protein–coupled receptors, designated as H 1 to H 4 . These receptors have been linked to various immune reactions, gastric acid secretion, and allergic reactions. This led to the development of histamine-2 receptor antagonists (H 2 RAs) to treat various allergies and GI disorders. COVID-19 has been associated with mast cell activation, causing histamine release in the early phase of inflammation, and late activation provoked the generation of proinflammatory IL-1 family members, including IL-1, IL-6, and IL-33. Several studies have explored the antiinflammatory properties of antihistamines as a protective mechanism against hyperinflammation related to COVID-19.
Freedberg et al. retrospectively studied 1620 patients admitted with COVID-19. Of those, 84 (5.1%) received famotidine within 24 hours of admission to hospital. The group taking famotidine had a reduced risk for clinical deterioration leading to intubation or death. This was an observational study with limited relevant prior data. Another retrospective, observational study demonstrated improved clinical outcomes, including lower in-hospital mortality, a lower composite of death and/or intubation, and lower levels of serum markers for serious disease in the famotidine group compared with nonusers. In contrast, a territory-wide retrospective cohort study in all patients with COVID-19 from Hong Kong investigated the association between famotidine use and severity of COVID-19. They did not find any statistically significant correlation between famotidine and COVID-19 severity. However, more recently a secondary analysis of a cohort involving more than 1800 patients across 36 medical centers in North America found that H 2 RA use was actually associated with 1.5 higher odds of requiring mechanical ventilation relative to patients not exposed to H 2 RA (confidence interval [CI], 1.11–2.19). This finding persisted after both propensity scores matched analysis and sensitivity analyses and also showed an association between H 2 RA exposure and higher in-hospital mortality (OR, 1.48; 95% CI, 1.04–2.12). It is important to recognize that studies showed a favorable effect of famotidine treatment during active disease, rather than preadmission exposure. The latter showed potential deleterious effects. Given these discrepancies, caution should be used regarding medications aimed at acid secretion in patients with COVID-19.
Impact of COVID-19 in Inflammatory Bowel Disease
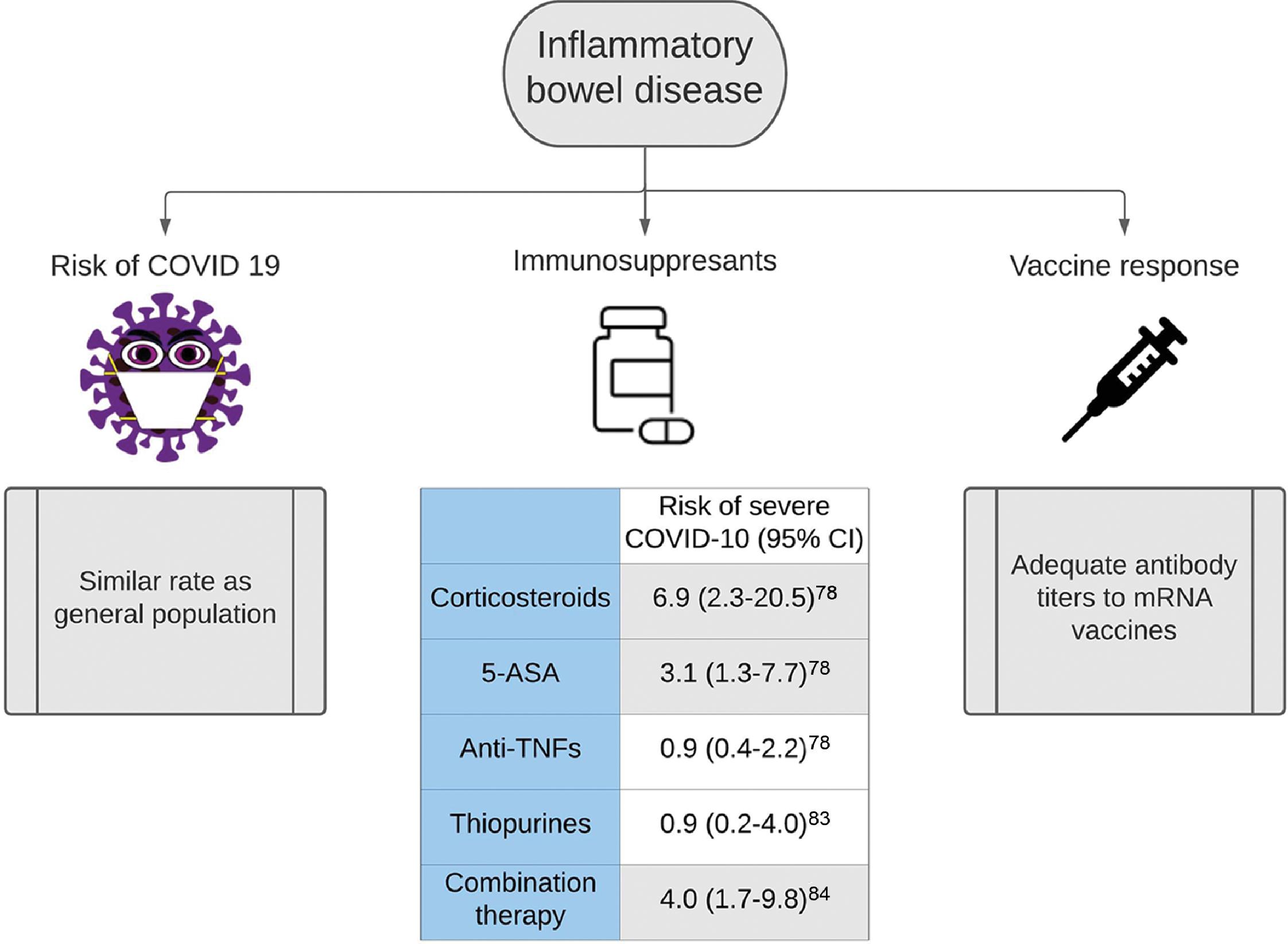
Inflammatory bowel disease (IBD) is a chronic inflammatory disease of the GI tract. There is contradictory evidence regarding the impact on COVID-19 on patients with IBD. According to a study from northern California and the SECURE-IBD registry from France and Italy, IBD patients did not have a higher rates of SARS-CoV-2 positivity than that of the general population, despite 37% of the cohort receiving immunosuppressants and/or biologicals that may affect virus susceptibility. , In fact, data from China through the IBD Elite Union, which covers the seven largest IBD referral centers in China, indicated that patients with IBD had a decreased risk for severe COVID-19 compared with that of the general population. The risk for COVID-19–associated mortality in IBD patients also has been found to be lower compared with that of the general population, with diarrhea being the only prominent symptom of active infection in some patients. The relative risk for acquisition of COVID-19 was not different between ulcerative colitis (UC) and Crohn’s disease (CD), but patients with UC might have higher rates of hospitalization (27.3% vs. 5.3%).
Pathogenesis of COVID-19 in Inflammatory Bowel Disease
The pathogenesis of COVID-19 in IBD patients is complex and multifactorial, including altered immune responses, microbial dysbiosis, environmental stimulants, and malnutrition, leading to dysregulated inflammatory cascades. Patients with active IBD generally present with symptoms of abdominal pain, nausea, vomiting, low appetite, and diarrhea, which overlap with the GI symptoms of COVID-19 infection, making it a diagnostic challenge. Moreover, inflammatory processes in IBD cause damaged mucosal barriers leading to increased permeability, adhesion, and translocation of intestinal microorganisms, dysregulated immune response, and release of proinflammatory cytokines. This allows the virus to enter the lamina propria through increased mucosal permeability, which results in an aggravated immune response. This immune response causes excessive release of cytokines, resulting in cytokine storm.
Cytokines, such as IFN-γ, which are upregulated in IBD, have also been shown to induce ACE2 receptor expression. SARS-CoV-2 relies on these ACE2 receptors for entry into the cytoplasm, and hence blocking the expression of these receptors can theoretically reduce the infectious burden by blocking cellular entry. Moreover, other proinflammatory cytokines such as TNF-α and IL-6 are upregulated in both IBD and COVID-19, and hence antiinflammatory therapies used in IBD can theoretically reduce the severity of COVID-19 by decreasing the risk for a cytokine release “storm.” As a result, COVID-19 may have a milder course in patients with IBD.
Impact of Inflammatory Bowel Disease Therapy on COVID-19 Outcomes
The most widely used IBD therapies include glucocorticoids, biologicals, or novel small molecule inhibitors to control intestinal inflammation. Corticosteroids have been shown to increase the risk for poor outcome for SARS, Middle Eastern respiratory syndrome (MERS), and influenza. These patients were more likely to require mechanical ventilation, vasopressors, and renal replacement therapy. In a study of 525 cases from 33 countries, risk factors for severe COVID-19 among patients with IBD included increasing age (adjusted OR [aOR], 1.04; 95% CI, 1.01–1.02), two or more comorbidities (aOR, 2.9; 95% CI, 1.1–7.8), systemic corticosteroids (aOR 6.9; 95% CI, 2.3–20.5), and sulfasalazine or 5-aminosalicylate use (aOR, 3.1; 95% CI, 1.3–7.7). Due to the possible risk for worse clinical outcomes, the World Health Organization (WHO) recommends against the use of steroids in patients with IBD who are actively infected with COVID-19. Patients with IBD should taper steroids to the lowest possible dose tolerated and discontinue it completely whenever possible. Locally acting steroids, such as budesonide and beclomethasone, have low systemic availability and are associated with significantly fewer side effects when compared with systemic steroids.
Contrary to steroids in IBD, TNF antagonist (anti-TNF) treatment was not associated with severe COVID-19 (aOR, 0.9; 95% CI, 0.4–2.2) and even may be protective. Similarly, a nationwide veterans administration cohort study of 37,857 patients with IBD found that neither immunomodulators (OR, 0.9; 95% CI, 0.23–4.03) nor anti-TNF (OR, 0.58; 95% CI, 0.17–1.9) was associated with an increased risk for severe COVID-19. However, combination therapy of steroids and thiopurines with anti-TNF medications might increase risk for severe COVID-19.
In the absence of robust data, reasonable strategies to minimize the burden of COVID-19 infection in the IBD population include early and careful detection of active infection in patients, restricting the use of corticosteroids, treatment with monotherapy, and continuity of care. Fig. 9.3 shows the impact of COVID-19 in IBD.