Gastrointestinal and hepatic complications in cancer patients
Robert S. Bresalier, MD  H. Franklin Herlong, MD
H. Franklin Herlong, MD  Boris Blechacz, MD, PhD
Boris Blechacz, MD, PhD
Overview
Gastrointestinal and hepatic complications represent some of the most common and potentially life-threatening disorders associated with treatment of the cancer patient. The expansion of therapeutic options for these patients has been accompanied by a growing number of direct and indirect consequences that effect the rapidly dividing cells of the GI tract. Cytotoxic, immunologic, and infectious insults often combine to increase toxicity. Recognition of these complications, together with proper evaluation and management, is key to the well being of these patients.
A growing spectrum of treatments is available to combat cancer. Chemotherapy, radiotherapy, and molecular targeted therapies including immunotherapies lead to adverse effects in several organs systems, including those of the gastrointestinal (GI) tract. GI complications are very common in patients undergoing cancer treatment. Some of these complications can be life threatening and require prompt and appropriate diagnosis and treatment. This chapter addresses common GI complications that result from cancer treatment and focuses on the evaluation and management of these problems.
Esophageal disorders
Esophagitis
Esophagitis in patients with cancer may be due to the direct cytotoxic effects of chemotherapy or radiation, or by infections due to immunosuppressive effects of cancer therapy (Table 1). Cell death leads to mucosal atrophy, ulceration, and initiation of the inflammatory response. Reactive oxygen species, proinflammatory cytokines, and metabolic byproducts of colonizing organisms may also play a role in amplifying tissue injury.1, 2 Synergy between chemotherapy and radiotherapy may increase the severity and extent of esophagitis observed with combined modality therapy. Esophagitis may also be due to pill-induced injury, acid reflux disease, and graft-versus-host disease (GVHD) in hematopoietic stem-cell transplant recipients. When esophagitis is suspected, particularly in an immunocompromised patient, prompt evaluation with endoscopy with biopsies and/or brushings is indicated to allow for early diagnosis and therapy.
Table 1 Common causes of esophagitis in patients receiving cancer therapy
| Infectious agent/injury | Endoscopic appearance | Treatment |
| Candida albicans | White plaque-like lesions with surrounding erythema on the esophageal mucosa | Systemic antifungal treatment with fluconazole, itraconazole, voriconazole, or echinocandins |
| Herpes simples virus | Small vesicles, coalescing to form ulcers | Acyclovir and foscarnet sodium |
| Cytomegalovirus | Linear or serpiginous ulcers | Ganciclovir, foscarnet sodium and valganciclovir |
| Varicella–Zoster virus | Small vesicles, similar to HSV ulcers | Intravenous acyclovir |
| Polymicrobial oral flora | Bacteria mixed with necrotic epithelial cells in biopsy samples | Broad-spectrum antibiotics |
| Injury due to chemotherapy and radiation | Friable mucosa with erythema and edema | Lidocaine hydrochloride, narcotic analgesics, proton pump inhibitors, and endoscopic dilation/stents (for strictures), PEGa |
a Percutaneous gastrostomy.
Radiation-induced esophagitis
Radiation-induced esophagitis can occur during external beam radiation treatment of lung, head and neck, and esophageal cancers. Acute radiation esophagitis is primarily due to injury to the rapidly dividing cells of basal epithelial layer, which subsequently leads to thinning and denudation of esophageal mucosa. The severity of esophagitis depends on radiation dose and is exacerbated by the concurrent use of chemotherapeutic agents such as cisplatin.3–5 Patients generally complain of odynophagia, dysphagia, and chest pain. Endoscopy findings include erythema, edema friable mucosa, ulcerations, or stricture formation. Treatment of acute esophagitis includes the use of local anesthetics such as oral viscous lidocaine hydrochloride, systemic narcotic analgesics, and acid suppression with proton pump inhibitors. Symptoms in some patients are so severe as to require temporary percutaneous gastrostomy (PEG) placement. Some patients undergoing extensive head and neck surgery and chemoradiation may benefit from PEG placement before treatment in anticipation of severe symptoms. A recent consensus statement of the Multinational Association of Supportive Care in Cancer (MASCC) and the International Society of Oral Oncology (ISOO) suggested the use of intravenous amifostine to prevent esophagitis induced by concomitant chemotherapy and radiation in patients with nonsmall cell lung cancer.1 Esophageal strictures are treated by endoscopic dilation. In patients with tracheo-esophageal fistula due to esophageal cancer, covered stents (self-expanding metal or plastic stents) are the treatment of choice, and can achieve fistula closure in 70–100% of patients.6
Fungal infections
Esophageal candidiasis is very common in immunocompromised patients, with C. albicans being the most common causative organism for esophageal and oropharyngeal candidiasis (OPC). Patients complain of odynophagia and/or dysphagia. On endoscopy, esophageal candidiasis is identified by white plaque-like lesions with surrounding erythema on the esophageal wall. Esophageal biopsies or brushings may confirm the presence of invasive yeast or hyphal forms of C. albicans. An empiric course of antifungal therapy is recommended in immunocompromised patient with odynophagia or dysphagia. Endoscopy should be performed if symptoms do not improve within 72 h. The general duration of antifungal treatment is 14–21 days. Candida esophagitis in immunocompromised patients requires systemic antifungal therapy; it cannot be treated with topical agents.7–10 Patients unable to tolerate oral agents require intravenous therapy. The treatment of esophageal candidiasis includes azoles, echinocandins, or amphotericin B.7–15 Azoles inhibit cell membrane formation by inhibiting the synthesis of ergosterol, a principal component of fungal cell membrane. Fluconazole, an azole, is the recommended first-line agent due to its efficacy, ease of administration, and low cost. For patients with fluconazole-refractory esophageal candidiasis who can tolerate oral therapy, newer azoles (voriconazole and posaconazole) are available. Itraconazole has been found to be as effective as fluconazole for the treatment of esophageal candidiasis, however its use is limited by significant nausea and by its potential for drug interactions owing to inhibition of the cytochrome p 450 enzymes.
Patients requiring intravenous therapy should be treated with fluconazole or one of the echinocandins (caspofungin, micafungin, or anidulafungin), rather than amphotericin B, because of their better toxicity profiles. Echinocandins inhibit synthesis of β(1,3)-D-glucan, an essential component of the fungal cell wall. Mammalian cells do not require β(1,3)-D-glucan, thereby limiting potential toxicity. Relapse rates are higher with echinocandins compared to azoles, and these are used as second-line therapy if treatment with azoles has failed. Amphotericin B is reserved for esophageal candidiasis during pregnancy and in individuals with drug-resistant candidiasis. OPC is a local infection. Risk factors include radiation, chemotherapy, antibiotics, and steroids. Treatment is with local agents such as nystatin or clotrimazole troches. Patients at risk of developing OPC may be given antifungal prophylaxis. Topical antifungals, such as clotrimazole or miconazole, are effective for prophylaxis.16
Viral infections
Viral infections of the esophagus are caused by herpes simplex virus (HSV), cytomegalovirus (CMV), and, rarely, varicella-zoster virus (VZV).8, 17 Patients usually present with odynophagia and dysphagia. Less-frequent symptoms include nausea, vomiting, heartburn, epigastric pain, and fever. In the case of HSV esophagitis, some patients may have coexistent herpes labialis or oropharyngeal ulcers. Diagnosis is made by endoscopy and biopsy (Figure 1). In the early stage, HSV lesions may appear as small vesicles, although they are rarely seen. The vesicles eventually coalesce to form large ulcers, which are usually less than 2 cm in size. The ulcers are well circumscribed with normal-appearing intervening mucosa. CMV will cause ulcers that are linear or serpiginous and deeper than HSV-related ulcers. Exudates may also be present. Biopsies taken from the edge of an HSV-related ulcer will show intranuclear inclusions and multinucleated giant cells. Inclusions can also be detected by immunohistochemistry using monoclonal antibodies to HSV. Viral cultures are helpful in identifying resistant strains in patients who do not respond to acyclovir. VZV can produce esophagitis in adults with herpes zoster, usually in the setting of disseminated infection. Endoscopically, VZV ulcers are similar to those seen with HSV. On biopsy specimens, distinction from HSV will require immunohistochemistry or culture.
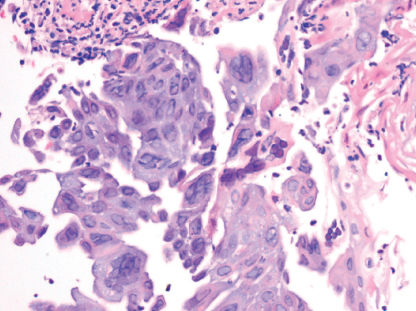
Figure 1 Herpes simplex virus (HSV) esophagitis. High-power view of esophageal mucosa shows squamous cells with ground glass nuclear viral inclusions and multinucleated giant cells in a background of neutrophilic exudates.
CMV infects endothelial cells and fibroblasts, but not epithelial cells as with HSV and VZV. Routine biopsies in a CMV-infected patient show intranuclear inclusions in fibroblasts and endothelial cells. Immunohistochemistry with anti-CMV antibodies is also helpful for diagnosis.
For patients with HSV esophagitis, acyclovir (400 mg orally five times daily for 14–21 days or 5 mg/kg intravenously every 8 h for 7–14 days) is the therapy of choice.8, 9, 18, 19 Acyclovir-resistant HSV result from mutations in the thymidine kinase (TK) gene of HSV. Viruses with TK mutations are generally cross-resistant to valacyclovir, but remain susceptible to agents that act directly on DNA polymerase such as foscarnet (80–120 mg/kg/day IV in 2–3 divided doses until clinical response). Cases of severe persistent infection with acyclovir-resistant HSV occur almost exclusively in immunocompromised hosts. Famciclovir or valacyclovir can be considered in patients able to tolerate oral therapy, although there is limited clinical experience with these drugs for the treatment of HSV-associated esophagitis. VZV esophagitis is initially treated with intravenous acyclovir as these patients usually have disseminated infection. After clinical improvement, treatment may be changed to oral agents used for HSV esophagitis. CMV esophagitis is treated with intravenous ganciclovir (5 mg/kg twice daily) or foscarnet sodium (68 mg/kg IV every 8 h or 90 mg/kg every 12 h) for 3–6 weeks.8, 20–22 The patient may be switched to valganciclovir 900 mg every 12 h once the patient can absorb and tolerate oral therapy. Valganciclovir is an oral precursor of ganciclovir. Valganciclovir is an oral precursor of ganciclovir. At a dose of 900 mg daily, valganciclovir produces systemic drug exposure equivalent to 5 mg/kg of intravenous ganciclovir. The role maintenance treatment after the clearance of infection is not well defined.
Bacterial infections
Bacterial esophagitis can occur in the immunocompromised patient and is usually polymicrobial and derived from oral flora. The diagnosis is made by endoscopic biopsies and treatment is broad-spectrum antibiotics.
Pill-induced esophagitis
Pill-induced esophagitis can occur in patients taking medication at bedtime with insufficient liquid or in the recumbent position. The most common medications associated with this disorder include potassium chloride, tetracyclines, aspirin, nonsteroidal anti-inflammatory drugs, quinidine, iron, and alendronate. Injury is caused by prolonged contact of the caustic contents of the medication with the esophageal mucosa. Patients will often present with sudden onset of odynophagia, which may be severe enough to make even the swallowing of saliva difficult and painful. Endoscopy is helpful in making a diagnosis; but more importantly, it serves to rule out other diagnoses such as infectious esophagitis and malignancy. On endoscopy, there is usually a discrete, single ulcer located in the proximal esophagus. On occasion, the injury appears as a nodular, polypoid lesion, suggestive of a neoplasm, or as a stricture (Figure 2). Esophageal biopsies are nonspecific and may show acute inflammatory changes only. There is no specific therapy for this disorder, as pill-induced ulcerations can heal spontaneously within a few days without any intervention. Strictures will require endoscopic dilation
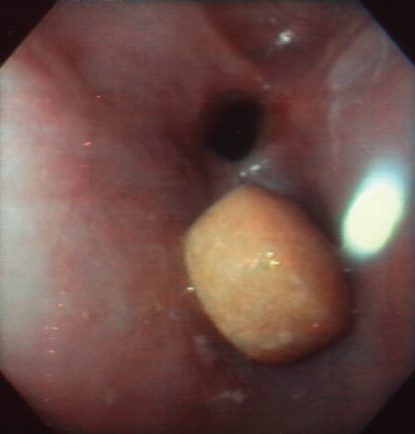
Figure 2 Pill-induced esophageal damage. A pill is seen at endoscopy lodged above an esophageal stricture.
Malignant dysphagia
Patients with esophageal cancer often present at an advanced, incurable stage. For those who are not candidates for chemoradiation or surgery and for those who develop recurrent dysphagia after treatment, a variety of endoscopic techniques have been developed to improve esophageal luminal patency.
Esophageal dilation can be performed with through-the-scope balloons, mercury-filled rubber bougies, or wire-guided polyvinyl bougies (Savary-Gilliard dilators), but dilation, to be successful, must be repeated every few weeks and the procedure carries a risk of perforation. Self-expanding metal stents (SEMS) are used increasingly as an effective nonsurgical option for the palliation of obstructive, advanced esophageal tumors.23–27 SEMS are made of a variety of metal alloys in different shapes and sizes to adjust to the length and position of the malignant stricture. Furthermore, approved devices are available in the uncovered, partially covered, and fully covered design. Additionally, they vary as to construction (nitinol vs surgical steel vs plastic) and function (fully patent vs “antireflux”). SEMS are placed under endoscopic guidance with or without fluoroscopy (Figure 3). Newly designed double-layered nitinol (Niti-S) stents are associated with longer survival time and fewer complications than previous designs. Once a metal stent is deployed, it cannot be removed. There have been several randomized trials comparing different stents in patients with malignant esophageal strictures.24 Advantages of SEMS include relative ease of insertion, larger stent diameters, and low risk of perforation with elimination of the need for excessive dilation. Disadvantages include high cost, tumor ingrowth and overgrowth, stent migration, maldeployment, inadequate expansion, airway obstruction, and hemorrhage. The rate of tumor ingrowth has now been reduced with the introduction of stents covered by a polyurethane coating. Covered stents are the device of choice in the management of patients with tracheo-esophageal fistulas.6 A recent additional option for palliation of esophageal cancer has resulted from the development of self-expanding plastic stents (SEPS). SEPS have been reported in some cases to have a higher failure rate of stent placement and higher migration rate, and to conform less easily to strictures. Stents are also effective at providing symptom relief in patients with esophageal malignancies after chemoradiation. The risk of complications, such as esophagorespiratory fistula, increases with radiation dose. Biodegradable stents, which gradually dissolve over time, are being developed. Stents have also been proposed as a bridge to surgery in those receiving neoadjuvant therapy.
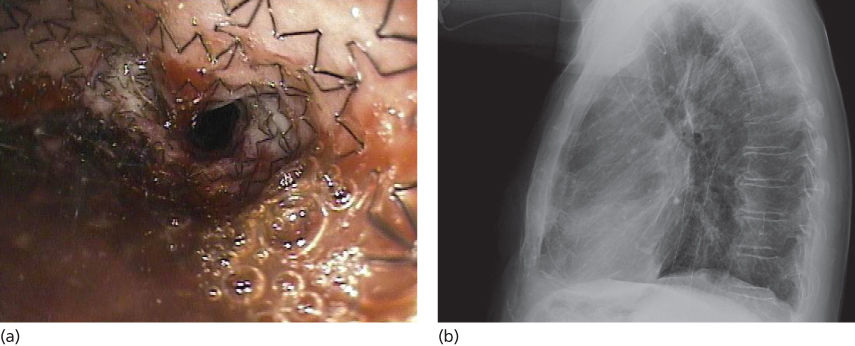
Figure 3 Self-expanding esophageal stent. (a) Endoscopic view of a self-expanding stent deployed in the esophagus for treatment of an esophageal stricture. (b) Chest radiograph showing stent deployed in the esophagus.
Ablative techniques such as lasers, photodynamic therapy (PDT), and high-dose brachytherapy have been successfully applied for palliation of malignant esophageal obstruction, but for the most part have been supplanted by SEMS. Laser energy produced by the neodymium:yttrium-aluminum garnet crystal, or Nd:YAG, delivered endoscopically through a quartz fiber has been extensively used for the palliation of esophageal cancer in the past. This approach may require prior esophageal dilation to allow passage of the endoscope. Major complications include esophageal perforation, development of tracheo-esophageal fistula, hemorrhage, and bacteremia. Disadvantages of Nd:YAG laser therapy include difficulties in treating long, tortuous lesions or lesions located in the proximal esophagus, number of treatment sessions required, and high cost. PDT uses a photosensitizing agent and low-power laser to achieve tumor necrosis and luminal patency. Porfimer sodium (Photofrin®) is the only photosensitizer approved by the Food and Drug Administration for the treatment of esophageal cancer. Photofrin® is administered as an intravenous bolus and is selectively retained at a high concentration by neoplastic tissue. Approximately 48 h after injection, patients are exposed to monochrome laser light at 630 nm via cylindrical diffuser attached to the tip of a quartz optical fiber placed through the accessory channel of an endoscope, initiating a photochemical reaction in the tissue leading to the formation of oxygen radicals, ischemia, and tumor necrosis.
A recent Cochrane Collaboration analysis extensively reviewed 53 studies on interventions for dysphagia in esophageal cancer.28 This analysis concluded that SEMS are safe, effective, and quicker in palliating dysphagia compared to other modalities, but that high-dose intraluminal brachytherapy is a suitable alternative and may provide additional survival benefit with a better quality of life. Combinations of brachytherapy and SEMS may reduce the need for reinterventions. Rigid plastic stent insertion, dilation alone or in combination with other modalities, and chemotherapy alone were not recommended for palliation of dysphagia owing to a high incidence of complications and recurrent dysphagia.
Diarrhea
Chemotherapy and radiation
Diarrhea is a common complication of cytotoxic therapy, and has been described with fluoropyrimidines (5-fluorouracil and capecitabine), irinotecan, methotrexate (MTX), and cisplatin (Table 2).29–31 Diarrhea also commonly occurs in patients receiving small-molecule epidermal growth factor receptor-tyrosine kinase inhibitors (erlotinib and sorafenib). Diarrhea can be very debilitating and in severe cases it can lead to treatment delays, reduced quality of life, and diminished compliance. It is the dose-limiting factor and the major toxicity of regimens containing a fluoropyrimidine and/or irinotecan. The severity of chemotherapy-induced diarrhea is often described, particularly for study purposes, using the National Cancer Institute Common Toxicity Criteria (NCI CTC). Grading is based on number of stools per day, presence of nocturnal stools, and the need for parenteral support or intensive care.
Table 2 Differential diagnosis of diarrhea in the cancer and hematopoietic cell transplant patient
| Chemotherapy-related (fluoropyrimidines, irinotecan, methotrexate, cisplatin, small-molecule epidermal growth factor receptor-tyrosine kinase inhibitors: erlotinib and sorafenib), and others |
| Colitis secondary to immune-modulatory agents (ipilimumab, nivolumab, lambrizumab), and others |
| Radiation therapy |
| Conditioning regimen |
| Graft-versus-host disease |
| Infection |
| Bacterial (including C. difficile) |
| Viral (including CMV) |
The severity of diarrhea with 5-FU is increased by the addition of leucovorin. Moreover, diarrhea can be worse when 5-FU is administered by bolus injection as opposed to intravenous infusion. Irinotecan can cause an early-onset diarrhea accompanied by abdominal cramping, lacrimation, salivation, and other symptoms that appear cholinergic-mediated. The late diarrhea associated with irinotecan is unpredictable and can occur at all dose levels. It is seen less often when given in the every 3-week schedule compared to every week. Significant diarrhea has been reported with a combination of irinotecan, 5-FU, and leucovorin compared to 5-FU and leucovorin alone. Grade 1 to 2 diarrhea has been reported in up to 56% of patients receiving erlotinib, and 34% of patients taking sorafenib.32, 33
Radiation therapy can produce injury to the GI mucosa. Symptoms typically occur during the third week of fractionated radiotherapy. Pelvic or abdominal radiation can lead to acute enteritis, characterized by abdominal cramping and diarrhea in approximately 50% of patients. These symptoms are made worse by concomitant chemotherapy.1
Opioid agonists are the cornerstone of therapy for chemotherapy-induced diarrhea.34 Loperamide and diphenoxylate are both widely used and are approved by the US Food and Drug Administration (FDA) for this indication. Loperamide is more effective. For mild-to-moderate diarrhea, an initial dose of 4 mg loperamide hydrochloride may be given, followed by a further 2 mg every 4 h or after every stool. Severe diarrhea often requires a more aggressive regimen, with an initial dose of 4 mg loperamide hydrochloride followed by a further 2 mg every 2 h or 4 mg every 4 h until the patient is diarrhea-free for 12 h. This high-dose loperamide has been used effectively for the control of irinotecan-induced diarrhea. Octreotide, a synthetic long-acting somatostatin analog, has been used as second-line therapy in opioid-resistant patients.1 It decreases the secretion of vasoactive intestinal peptide, prolongs intestinal transit time, and reduces secretion of intestinal fluid and electrolytes. The recommended initial dose of octreotide is 100–150 g given subcutaneously three times per day, or 25–50 g every hour if given as an intravenous infusion. Octreotide can be titrated to higher doses (500–2500 g three times daily) for the treatment of those individuals who do not respond to lower doses.
Other drugs used as adjunctive therapy in chemotherapy or radiation-induced diarrhea include absorbents such as kaolin and charcoal, deodorized tincture of opium, paregoric, and codeine phosphate.
Because of the well-recognized risk of diarrhea associated with irinotecan, several recent studies have investigated prophylactic regimens to prevent chemotherapy-induced diarrhea. Long-acting, slow-release formulation of octreotide (octreotide LAR) can be administered by intramuscular injection once a month. Once steady-state levels have been achieved, a 20-mg intramuscular dose of octreotide LAR every 4 weeks produces the same pharmacologic effects as 150 µg octreotide tid by SC injection and dramatically reduces fluctuations in peak and trough octreotide concentrations. Additionally, octreotide LAR (at a starting dose of 20 mg) effectively controls diarrhea associated with carcinoid syndrome,31 and monthly doses of 20–30 mg are currently being investigated for the treatment and prevention of chemotherapy-induced diarrhea.
For patients undergoing hematopoietic stem-cell transplantation (HCT), diarrhea may be due to the conditioning regimen (total body irradiation and/or high-dose chemotherapy). Pretransplant conditioning regimens can injure the GI mucosa, causing secretory diarrhea that resolves after mucosal restitution. After day 20, acute GVHD is the most common cause of diarrhea in these patients. GVHD will be discussed separately.
Clostridium difficile-associated diarrhea
If diarrhea is not directly the result of chemotherapy or radiation and particularly if it occurs in a hospital setting, Clostridium difficile infection should be considered as this is the most common cause of infectious diarrhea in hospitalized patients. Although commonly associated with use of antibiotic therapy, risk factors for C. difficile diarrhea or colitis also include bowel surgery, immunocompromised state, and any process that suppresses the normal flora including antifungal and chemotherapeutic agents. Cancer patients receiving chemotherapy appear predisposed to C. difficile-induced diarrhea even in the absence of antibiotics. In a study of such patients, MTX, doxorubicin, and cyclophosphamide were the drugs most frequently associated with C. difficile infection. Clinical presentation may vary from mild diarrhea without colitis, colitis with systemic manifestations, pseudomembranous colitis with or without protein-losing enteropathy, and fulminant colitis with development of toxic megacolon. Diagnostic testing for C. difficile has rapidly evolved.35 Previously rapid EIA tests for toxin A or B were the most widely used diagnostic tests. These tests have a sensitivity of 75–95% and specificity of 83–98%. Two major advances in the laboratory diagnosis of C. difficile are the use of glutamate dehydrogenase (GDH; an enzyme produced by C. difficile), detection in stool (75% to >90% sensitivity with a negative predictive value of close to 100%), and nucleic acid amplification tests (PCR) for toxin genes. Endoscopically, pseudomembranes can be seen as adherent yellow plaques that vary in diameter from 2 to 10 mm (Figure 4). The rectum and sigmoid colon are typically involved, but in approximately 10% of cases, colitis is only present in the more proximal colon and can be missed during sigmoidoscopy.
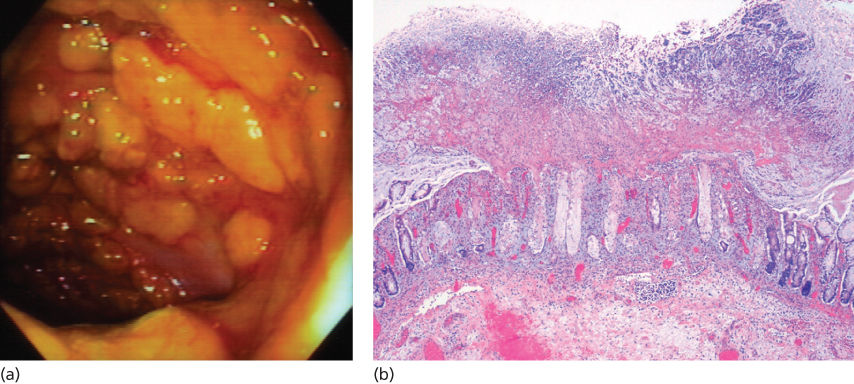
Figure 4 Pseudomembranous colitis. (a) Pseudomembranes adherent to the colonic mucosa seen at colonoscopy. (b) Low-power view of colonic mucosa shows a typical volcano (mushroom)-like appearance with luminal inflammatory exudates.
Therapy for C. difficile diarrhea depends on disease severity.35 Standard therapy for C. difficile-associated diarrhea is oral metronidazole or oral vancomycin. Metronidazole at a dose of 500 mg three times daily either orally or intravenously for 10–14 days is as effective as oral vancomycin given at a dose of 125 mg four times daily. The lower dose of vancomycin 125 mg four times a day is as effective as the higher dose of 250 mg four times a day in mild-to-moderate case and is much less expensive. Metronidazole has some advantages over vancomycin including its lower cost and the observation that it can reduce selection of vancomycin-resistant enterococci (VRE). Metronidazole is therefore the initial therapy of choice in nonsevere cases of C. difficile-induced diarrhea. If there is no improvement in 3 days, treatment with vancomycin should be initiated. In patients with severe C. difficile infection and signs of systemic toxicity, the recommended treatment regimen is initial therapy with vancomycin 125 mg orally four times daily, with dose escalation at 48 h intervals up to 500 mg four times daily if patients fail to improve. If patients do not respond to oral vancomycin, the addition of intravenous metronidazole 500 mg every 8 h, or vancomycin retention enemas (0.5–1 g of vancomycin dissolved in 1–2 L of normal saline every 4–12 h), should be considered. The use of antiperistaltic agents is not recommended as they may obscure symptoms and there is evidence that decreased transit time can lead to complications and lengthen the duration of illness. Relapse of CDI is common, occurring in up to 10–25% of all patients with CDI. Relapses usually occur within 1–3 weeks after termination of initial therapy, and are probably caused by failure to eradicate the organism rather than development of antibiotic resistance. These patients are likely to relapse repeatedly. First relapses should be treated with a second 10–14-day course of oral metronidazole or vancomycin. If a patient relapses after taking a second course of antibiotics, different approaches have been suggested, including tapered or pulsed antibiotic therapy, longer duration of treatment (several weeks), and the use of toxin-binding resins such as colestyramine or colestipol hydrochloride alone or in combination with vancomycin. Fidaxomicin is an alternative agent for treatment of an initial recurrence of CDI. For treatment of initial recurrence of CDI, the initial response to therapy with fidaxomicin and vancomycin is comparable, but the likelihood of subsequent recurrence is lower with fidaxomicin than vancomycin (20% vs 36%). If there is a third recurrence, fecal microbiota transplant should be considered. In small series, 2 weeks of vancomycin followed by 2 weeks of rifaximin has proven successful in controlling recurrent disease. A recent study used two neutralizing, human monoclonal antibodies against C. difficile toxins A (CDA1) and B (CDB1) in 101 symptomatic patients who were receiving either metronidazole or vancomycin, and the rate of recurrence of C. difficile infection was significantly lower among patients treated with the monoclonal antibodies.36
Other infectious diarrhea
In the posthematopoietic cell transplant patient, infectious diarrhea is relatively uncommon. Viruses are the most common organisms found (astrovirus, adenovirus, CMV, and rotavirus), followed by nosocomially acquired bacteria (C. difficile and Aeromonas). CMV deserves a special mention as it can cause diarrhea and bleeding because of mucosal ulceration. The diagnosis of CMV is made by endoscopic biopsy (Figure 5); specimens should be sent for immunohistochemistry and viral culture. Infectious diarrhea related to Salmonella, Shigella, and Campylobacter species are very rare in hospitalized transplant patients. Diarrhea related to parasites (Cryptosporidium, Giardia lamblia, and Entamoeba histolytica) is also a rare cause of diarrhea; most of these patients are infected pretransplantation.
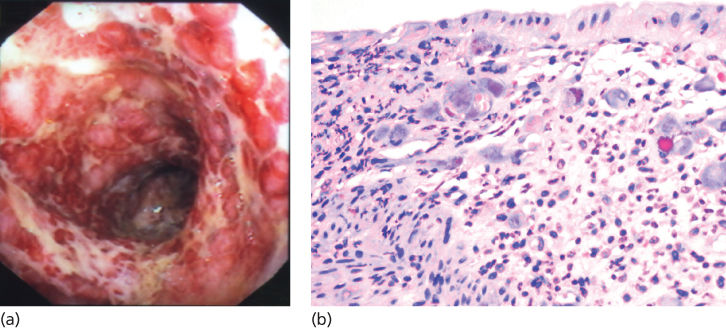
Figure 5 Cytomegalovirus (CMV) colitis. (a) CMV colitis as seen at endoscopy. (b) High-power view of inflamed colonic mucosa demonstrates multiple viral inclusions in stroma cells.
Colitis
Neutropenic enterocolitis
Neutropenic enterocolitis is characterized by fever and right lower quadrant pain in neutropenic patients. It is seen in children and adults with hematologic malignancies, aplastic anemia, and after myelosuppressive therapy for solid malignancies.37–42 Histologic examination of biopsy samples from patients with neutropenic enterocolitis is characterized by a thickened bowel wall, edema, mucosal ulcerations, focal hemorrhage, and mucosal or transmural necrosis. Numerous bacterial and/or fungal organisms have been identified in surgical specimens and peritoneal fluid from patients with neutropenic enterocolitis, A diagnosis of neutropenic enterocolitis is usually established by computerized tomography (CT). Abnormal findings on CT and ultrasound include a fluid-filled, dilated cecum, a right lower quadrant inflammatory mass and pericecal fluid, or inflammatory changes in the pericecal soft tissues Treatment consists of bowel rest, intravenous fluids, and broad-spectrum antibiotics. Cytopenias and coagulopathy associated with oncologic treatment should be corrected as neutropenia contributes to the pathogenesis of the disease, and coagulopathy can be associated with blood loss from mucosal hemorrhage. Recombinant granulocyte colony-stimulating factor (G-CSF) may be used to hasten leukocyte recovery, which contributes to the resolution of neutropenic enterocolitis. Surgery has been recommended for patients with persistent GI bleeding despite correction of cytopenias and coagulopathy, and for patients with perforation or clinical deterioration despite pharmacologic therapy.
Colitis secondary to immune-modulatory, antineoplastic agents
Immune-modulatory agents enhance tumor-directed immune responses through modification of immune checkpoint pathways, T-cell stimulatory pathways, or with adoptive cell therapy.43 However, this approach has also been associated with intestinal adverse effects, in particular with therapeutic agents targeting immune checkpoint pathways (i.e., cytotoxic T-lymphocyte antigen-4 (CTLA-4) and PD-1). CTLA-4 is a key negative regulator of T-cell activation in the adaptive immune response. Transient, antibody-mediated CTLA-4 inhibition has been shown to enhance T-cell response. Ipilimumab and tremelimumab are fully humanized, monoclonal IgG1 antibodies targeted against CTLA-4. While the GI adverse effect profile of tremelimumab was similar to ipilimumab, tremelimumab had no survival benefit over chemotherapy for melanoma in a recent randomized controlled phase III trial.44 CTLA-4 inhibitors have predominantly been used for melanoma although they have also been evaluated in other solid malignancies such as pancreatic carcinoma, prostate carcinoma, renal cell, ovarian, and small-cell lung cancer.45, 46 Given the regulatory role of inhibitors such as CTLA-4 and PD-1 in adaptive immune response, they have a broad spectrum of immune-mediated Adverse Events (imAE) including enterocolitis, hepatitis, dermatitis, hypophysitis, uveitis, and nephritis; enterocolitis is the most common adverse event.47 The majority of immune-related adverse events have been observed during the induction and reinduction periods.46 The development of ipilimumab-associated enterocolitis is independent of the number of cycles, but appears to be dose dependent.47, 48 There is some evidence that the development of enterocolitis might be a positive predictor for tumor response in patients with melanoma and renal cell cancer treated with ipilimumab.47 Enterocolitis most commonly presents with diarrhea but abdominal pain, nausea/vomiting, fever, anal pain, and constipation have been reported as well.5 The majority of patients who develop enterocolitis do so within 21 days following their last dose.47 Overall, up to 33–51% of ipilimumab-treated patients experience diarrhea, including 16% with grade 3 and <1% with grade 4.44–46, 48 Intraluminally, the colon is particularly involved with up to 8% of patients developing colitis, including 4–5% and 0–1% with grade 3 and 4 colitis.45, 46 Diarrhea is usually watery with few leukocytes and only rarely positive for blood.49 Radiologically, ipilimumab-associated colitis is characterized by mesenteric vessel engorgement, colonic wall thickening, mucosal enhancement, and fluid-filled colonic distension. Colitis can either develop diffusely or as segmental colitis associated with diverticulosis (SCAD). While panenteritis has been described, the colon is the most commonly involved area.49, 50 Endoscopic presentation of ipilimumab-associated colitis is unspecific and severity dependent showing macroscopic findings such as mucosal erythema, friability, edema, and ulcerations.47 In the majority of patients, histologic findings include neutrophilic inflammation with cryptitis and occasional crypt abscesses, or a mixed neutrophilic–lymphocytic inflammatory picture. In few patients, lymphocytic predominant inflammation is seen with increased numbers of CD8+ T-cells in crypts and CD4+ cells in the lamina propria.47, 49 0.7–6.6% of ipilimumab-treated patients develop colonic perforation or require colectomy.5 Mortality of patients developing enterocolitis secondary to ipilimumab treatment has been reported as high as 5%.47 Treatment of ipilimumab-associated adverse events is guided by severity (Table 3), but other etiologies need to be ruled out.51 Mild GI symptoms can be treated symptomatically without discontinuation of ipilimumab; however, frequent reassessment is imperative to identify the development of more severe symptoms or life-threatening complications. In cases of moderate enterocolitis, ipilimumab should be withheld and can be restarted 7 days after significant symptom-improvement or -resolution. If moderate symptoms persist after 7 days, initiation of treatment with systemic corticosteroids is recommended (e.g., 0.5 mg/kg/d prednisone or equivalent) until improvement to mild symptoms or symptom-resolution is observed. Once symptoms are mild and corticosteroid dose has been tapered to ≤7.5 mg daily, ipilimumab can be resumed. Severe enterocolitis and life-threatening complications require permanent discontinuation of ipilimumab. In these cases, systemic corticosteroids should be administered (e.g., 1–2 mg/kg/d prednisone or equivalent) once intestinal perforation is ruled out.51 In corticosteroid-refractory cases, the successful use of infliximab has been reported in a limited number of patients.47, 52, 53 Prophylactic initiation of budesonide concurrent with ipilimumab did not significantly decrease rates of diarrhea or colitis.54, 55 Programmed cell death protein-1 (PD-1) is a receptor expressed on T-cells, which attenuates T-cell activation upon binding of its ligands PD-L1 (B7-H1) and PD-L2 (B7-DC). While PD-L2 is frequently expressed by APC, PD-L1 overexpression has been observed in tumors.43, 56 Nivolumab and lambrizumab are PD-1-targeted, humanized monoclonal IgG4 antibodies currently evaluated in ongoing or planned randomized controlled phase II and III trials.43 In a recent nonrandomized controlled trial, the most common GI adverse event in patients treated with Nivolumab was diarrhea with an incidence of 18%, with grade 3–4 diarrhea in 2%. Other GI adverse events included nausea, vomiting, abdominal pain, and dry mouth observed in 5–8% of patients.15 Rates of GI adverse events were not considerably higher with nivolumab/ipilimumab combination therapy compared to monotherapy.57 In two clinical trials including 173 and 135 patients, lambrizumab-associated diarrhea was observed in only 1–20%.56, 58 Given the novelty of these agents, there is currently insufficient experience in regard to optimal management, but a severity-guided, conservative approach similar to ipilimumab-induced enterocolitis has been proposed.43
Table 3 Management of ipilimumab-induced enterocolitis
| Severity | Definition | Management | Advanced management |
| Mild |
| If progression in symptom severity: See below | |
| Moderate |
|
| If no resolution after 7 days:
|
| If progression to severe symptoms: See below | |||
| Severe or life threatening |
|
|









