Gallbladder and bile duct cancer
Ahmed O. Kaseb, MD  Marc Uemura, MD
Marc Uemura, MD  Melanie B. Thomas, MD, MS
Melanie B. Thomas, MD, MS  Steven A. Curley, MD, FACS
Steven A. Curley, MD, FACS
Overview
Primary gallbladder and bile duct cancers are relatively rare tumors of the gastrointestinal tract. Both are mainly adenocarcinomas that start as abnormal growths within the mucous lining of the gallbladder and bile ducts. Bile duct tumors, or cholangiocarcinomas, are further differentiated by their location within the liver (intrahepatic) or outside the liver (extrahepatic). Both tumor types have distinct pathophysiologies and modes of hepatocarcinogenesis. Gallbladder cancers, for example, are associated with gallstones, chronic choleycystitis, and the porcelain gallbladder. They are commonly found incidentally on cholecystectomy specimens. On the other hand, cholangiocarcinomas have frequent associations with parasitic infections and other diseases causing chronic inflammation in the bile ducts (primary sclerosing cholangitis). While both gallbladder and cholangiocarcinomas can be cured if surgically resected, unresectable or advanced tumors have a poor prognosis and usually require systemic chemotherapy.
Gallbladder cancer
Adenocarcinoma of the gallbladder is the sixth most common digestive system malignancy in the United States. In Western countries such as the United States, where there is a lower incidence of hepatocellular carcinoma (HCC), gallbladder cancer is relatively more common. The American Cancer Society estimates that about 10,910 new cases of gallbladder cancer and bile duct cancer (excluding bile ducts within the liver) would be diagnosed in 2016 in the United States. About 3700 people died of these cancers in 2015. Of these new cases and deaths, about half are due to gallbladder cancer.1 Between 1980 and 1995, mortality rates from gallbladder cancer decreased in the United States, Canada, Australia, and the United Kingdom, while increasing in Japan, Italy, Spain, and Chile.2 Unlike HCC and cholangiocarcinoma, gallbladder carcinoma has a higher incidence in females than in males.2 The preponderance of this cancer in females is even greater in patients <40 years old, with a female-to-male ratio of 20 : 1.3
Gallbladder carcinoma is more common in Southwest Native Americans than in the general American population. Incidence rates for U.S. white males, black males, and Native American males in New Mexico are 0.4, 0.6, and 3.8 cases per 100,000 per year, respectively. The corresponding rates for females are 1.0, 0.8, and 10.3.2 Gallbladder carcinoma has been found in 6% of Southwest Native Americans undergoing biliary tract surgery.4 Gallbladder carcinoma is the second most common gastrointestinal malignancy in this population, and the youngest reported case of gallbladder carcinoma occurred in an 11-year-old Navajo girl.5
Other human populations also have an increased incidence of gallbladder cancer. In Chile, the incidence of gallbladder cancer is rising, and gallbladder cancer is the number one cause of cancer mortality in Chilean women.6 The geographic and population-based variations in the incidence of gallbladder cancer suggest that environmental risk factors, including carcinogens, infectious agents like Salmonella typhi and Helicobacter pylori, and diet have a role in gallbladder tumorigenesis.
Causative factors
There are no apparent associations between gallbladder carcinoma and hepatitis B or C virus infection, cirrhosis, or mycotoxin exposure. Similarly, chemical hepatocarcinogens have not been clearly demonstrated to increase the risk of developing gallbladder carcinoma. However, there are suggestions that workers exposed to carcinogenic substances, such as methylcholanthrene and nitrosamines, have a higher incidence and earlier onset of gallbladder carcinoma when compared with control populations.7 There is a significant association between gallstones and gallbladder carcinoma, with gallstones present in 74–92% of patients with gallbladder carcinoma.8, 9 The risk of developing gallbladder carcinoma increases directly with increasing gallstone size.10 Patients with gallstones 2.0–2.9 cm in diameter have a 2.4 times higher relative risk of developing gallbladder carcinoma, whereas patients with gallstones greater than 3.0 cm in diameter have a 10.1 times higher risk. Patients with long-standing chronic cholecystitis can develop calcification of the gallbladder wall, also known as porcelain gallbladder. It is possible that chronic inflammation and/or infection of the gallbladder increases the risk of developing gallbladder carcinoma because 22% of patients with calcified gallbladders have gallbladder carcinoma.11 Furthermore, pathogenic bacteria are cultured from the gallbladders of patients with gallbladder cancer at a significantly greater frequency than from patients with simple cholelithiasis.12 Cholelithiasis and cholecystitis are more common in females, which may in part explain the higher incidence of gallbladder carcinoma in females.13
Patients with these premalignant lesions may progress to invasive gallbladder carcinoma. Epithelial dysplasia, atypical hyperplasia, and carcinoma in situ have been identified in the gallbladder mucosa of 83%, 13.5%, and 3.5%, respectively, of patients undergoing cholecystectomy for cholelithiasis or cholecystitis.14 Areas of mucosal dysplasia can be observed in >90% of patients with invasive gallbladder carcinoma.15 There is also evidence that adenomatous polyps arising from the gallbladder mucosa are premalignant lesions. A review of 1605 cholecystectomies reported 11 benign adenomas, 7 adenomas with areas of malignant transformation, and 79 invasive gallbladder carcinomas.16 There appears to be an increased expression of epithelial growth factors and proto-oncogene, particularly ras, in the progression from chronic cholecystitis to dysplasia and then to invasive carcinoma.17 In patients with anomalous pancreaticobiliary ductal union, a condition known to be associated with an increased risk of developing gallbladder cancer, chronic inflammation results in hyperplasia of the gallbladder epithelium.18 K-ras mutations were noted in some of these patients with high-grade dysplasia, suggesting that mutations in this proto-oncogene may be an early event in gallbladder mucosal proliferation leading to carcinogenesis. Previous studies performed in patients with invasive gallbladder carcinoma have demonstrated that the majority have abnormal or mutated tumor suppressor (p53 and p16), cell cycle regulation (cyclin E), and apoptosis regulation (Bc1-2) genes, as well as increased expression of angiogenesis factors (VEGF).19, 20 More recently, comprehensive genomic profiling has shown that invasive gallbladder carcinomas have alternations in other proteins necessary for cell cycle regulation (CDKN2B), chromatin remodeling (ARID1A), and in important growth pathways, specifically epidermal growth factor receptor family (ERBB2) and PIK3CA/MTOR.21
Pathology
The gross appearance of gallbladder carcinoma varies, depending on the stage of the disease and extent of spread. Early-stage lesions that have not infiltrated through all layers of the gallbladder wall may be indistinguishable from chronic cholecystitis. Occasionally, a sessile or pedunculated tumor is present and suggests the diagnosis of a gallbladder carcinoma.22 More advanced gallbladder carcinomas are grossly evident by infiltration into the liver or contiguous organs, such as the duodenum or stomach.23
Microscopically, >90% of gallbladder carcinomas are adenocarcinomas, with the remaining cases being adenosquamous, squamous, anaplastic carcinomas, and, rarely, carcinoid tumors or embryonal rhabdomyosarcoma.8, 22 Carcinoma in situ is an early lesion, with the malignant cells involving only the mucosal layer of the gallbladder wall. Gallbladder adenocarcinomas generally have a predominant papillary or tubular arrangement of cells.22 Papillary adenocarcinoma is characterized by an extended stroma covered by columnar cells. The tubular formations of tubular adenocarcinoma may be lined by tall columnar cells or by cuboidal epithelium. Mucin production and signet ring cells can be identified frequently in gallbladder adenocarcinomas.22 More poorly differentiated carcinomas have solid sheets or nests of small, scattered cells infiltrating into the stroma and destroying the normal gallbladder wall architecture. Vascular, lymphatic, and perineural invasion by the carcinoma can be demonstrated frequently.
Advanced locoregional disease usually is present at the time of diagnosis of gallbladder carcinoma. Only 10% of patients with this disease have cancer confined to the gallbladder wall.8 Direct extension of the carcinoma into the gallbladder fossa of the liver is present in 69–83% of patients.23–25 Direct invasion of the liver usually indicates the presence of other regional disease because fewer than 12% of patients with liver involvement have no other sites of regional disease. Direct invasion of the extrahepatic biliary tract occurs in 57% of cases; the duodenum, stomach, or transverse colon is involved in 40%; and the pancreas is involved in 23%. The hepatic artery or portal vein is encased by tumor in 15% of patients. Regional lymph node metastases in the cystic, choledochal, or pancreaticoduodenal lymphatic drainage basins are present in 42–70% of patients.23 More distant lymph node metastases occur along the aorta or inferior vena cava in approximately 25% of cases. Importantly, lymph node metastases can occur in the absence of liver or other contiguous organ involvement by the gallbladder carcinoma.
The pattern of lymph node metastases from gallbladder carcinoma is predictable on the basis of anatomic studies that have identified three pathways of lymphatic drainage of the gallbladder (Figure 1).26 The main pathway is the cholecysto-retropancreatic pathway, with lymphatic vessels on the anterior and posterior surfaces of the gallbladder that converge at a large retroportal lymph node. This principal retroportal lymph node communicates with the choledochal and pancreaticoduodenal lymph nodes. The cholecysto-celiac pathway consists of lymphatics from the anterior and posterior walls of the gallbladder that run to the left in front of the portal vein and then communicate with groups of pancreaticoduodenal lymph nodes or aorticocaval lymph nodes lying near the left renal vein. The final pattern of spread of gallbladder carcinoma is related to vascular invasion. Noncontiguous liver, pulmonary, and bone metastases have been found in 66%, 24%, and 12% of gallbladder carcinoma patients, respectively.23
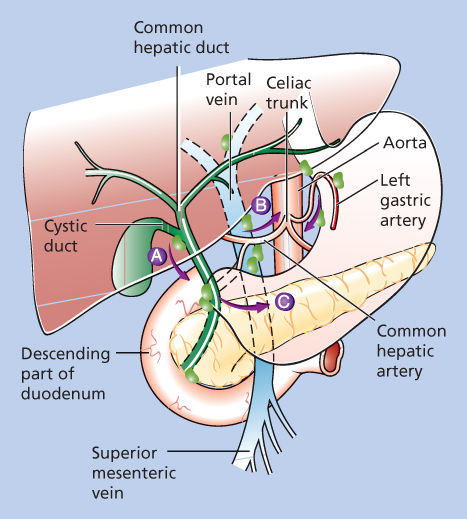
Figure 1 Patterns of lymphatic drainage from the gallbladder. (A) The main pathway of lymphatic drainage and, thus, lymph node metastasis from gallbladder cancer is to the cholecysto-retropancreatic nodes. This pathway drains from the gallbladder to nodes along the cystic duct and common bile duct and then to nodes posterior to the duodenum and pancreatic head. (B) The cholecysto-celiac pathway courses from the gallbladder through the gastrohepatic ligament to celiac nodes. (C) The third lymphatic drainage route is the cholecysto-mesenteric pathway, coursing from the gallbladder posterior to the pancreas to aortocaval lymph nodes.
The staging systems used for gallbladder carcinoma are based on the pathologic characteristics of local invasion by the tumor and lymph node metastases. Before the American Joint Cancer Committee (AJCC) developed a tumor-node metastasis (TNM) staging schema for gallbladder carcinoma, the Nevin and colleagues staging system was used frequently.27 Studies of gallbladder carcinoma performed in Japan generally apply the staging system of the Japanese Society of Biliary Surgery.28 Most recent studies stage patients according to the TNM criteria. Carcinoma in situ corresponds to a T1aN0M0 tumor in the AJCC staging system. The characteristics of these three staging systems are outlined in Table 1.
Table 1 Comparison of the three most commonly used staging systems for gallbladder carcinoma
| Stage | Nevin | JSBS | AJCC-TNM |
| I | Cancer confined to the mucosa | Cancer confined to subserosal layers | T1aN0M0, T1bN0M0 |
| II | Cancer involves the mucosa and muscularis | Direct invasion of the liver and/or bile duct, porta hepatis lymph node metastases | T2N0M0 |
| III | Cancer extends through the serosa (all three layers of the gallbladder wall involved) | More extensive liver invasion by cancer, more extensive regional lymph node metastases (gastrohepatic, retropancreatic) | T1N1M0, T2N1M0, T3AnyNM0 |
| IV | Tumor extends through all three layers of the gallbladder wall with cystic lymph node metastasis | Liver, peritoneal, and/or distant organ metastases | T4AnyNM0, any TAnyNM1 |
| V | Tumor invades the liver by direct extension and/or metastasis to any distant organ | No stage V | No stage V |
Abbreviations: AJCC, American Joint Committee on Cancer; JSBS, Japanese Society of Biliary Surgery; T, primary tumor; Tx, primary tumor cannot be assessed; T1, tumor invades mucosa or muscle layer; T1a, tumor invades mucosa; T1b, tumor invades muscle; T2, tumor invades perimuscular connective tissue, no extension beyond serosa or into liver; T3, tumor invades beyond serosa or into one adjacent organ or both (extension <2 cm into liver); T4, tumor extends >2 cm into liver and/or into two or more adjacent organs (stomach, duodenum, colon, pancreas, omentum, extrahepatic bile ducts); N, regional lymph nodes; Nx, regional lymph nodes cannot be assessed; N0, no regional lymph node metastasis; N1, regional lymph node metastasis; N1a, metastasis in cystic duct, pericholedochal, and/or gastrohepatic lymph nodes; N1b, metastasis in peripancreatic, periduodenal, periportal, celiac, and/or superior mesenteric artery lymph nodes; M, distant metastasis; Mx, presence of distant metastasis cannot be assessed; M0, no distant metastasis; M1, distant metastasis.
Clinical presentation
The most common symptoms and signs in patients with gallbladder carcinoma are nonspecific. Right upper quadrant abdominal pain, which may or may not be exacerbated by eating a fatty meal, is the predominant presenting complaint in 75–97% of patients.2, 8, 29 Right upper quadrant abdominal tenderness is present in a slightly smaller percentage of patients. These symptoms and signs usually are ascribed to cholelithiasis or cholecystitis. Nausea, vomiting, and anorexia are present in 40–64% of patients; clinically evident jaundice is present in 45%; and weight loss greater than 10% of normal body weight is noted in 37–77%.
Although 45% of patients are jaundiced at presentation, 70% of patients present with a serum bilirubin elevated at least two times greater than normal.29 Serum alkaline phosphatase levels are elevated in two-thirds of patients with gallbladder carcinoma. Alanine aminotransferase and aspartate aminotransferase levels are elevated in one-third of patients and are consistent with advanced hepatic invasion and metastases. In these patients with TNM stage III or IV disease, the serum CEA level is elevated in >80% of patients.29 The incidence of elevated serum CEA levels in early-stage disease is not known.
Diagnostic studies
Before ultrasonography and CT (computerized tomography) became widely available, the preoperative diagnosis rate for gallbladder carcinoma was only 8.6–16.3%.2 Ultrasonography is the primary imaging study for symptomatic patients with presumed cholelithiasis or choledocholithiasis. High-resolution ultrasonography is able to detect early and locally advanced gallbladder carcinoma.30 An early tumor as small as 5 mm can be recognized as a polypoid mass projecting into the gallbladder lumen or as a focal thickening of the gallbladder wall.31 In patients with locally advanced gallbladder carcinoma, ultrasonography can demonstrate extrahepatic and intrahepatic bile duct obstruction, porta hepatis lymphadenopathy, direct hepatic extension of tumor, and hepatic metastases. Preoperative ultrasonography may suggest the correct diagnosis in up to 75% of patients with gallbladder carcinoma.31, 32 However, ultrasonography does not accurately detect celiac or paraortic lymphadenopathy or peritoneal tumor dissemination.33 Blood flow studies with color Doppler ultrasonography are also useful because gallbladder cancers have high-velocity arterial flow in 90% of cases, while benign lesions have minimal flow.34 Recent advances in endoscopic ultrasonography, including the use of contrast-enhancing agents, may improve the diagnostic accuracy in assessing the T stage of the gallbladder cancer.35
CT scans are performed less frequently in patients with presumed benign biliary tract disease. However, if gallbladder carcinoma is suspected, CT findings can correctly predict the diagnosis in 88–95% of patients.36, 37 The CT characteristics of gallbladder carcinoma include diffuse or focal gallbladder wall thickness of greater than 0.5 mm in 95% of patients, gallbladder wall contrast enhancement in 95%, intraluminal mass in 90%, direct liver invasion by tumor in 85% (Figure 2), regional lymphadenopathy in 65%, concomitant cholelithiasis in 52%, dilated intrahepatic or extrahepatic bile ducts in 50%, noncontiguous liver metastases in 12%, invasion of contiguous gastrointestinal tract organs in 8%, and intraluminal gallbladder gas in 4%.36 CT can also demonstrate calcification of the gallbladder wall (Figure 3).
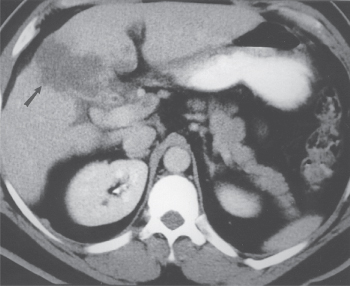
Figure 2 High-resolution, helical CT scan in a patient with gallbladder carcinoma. Direct tumor invasion into the hepatic parenchyma is evident.
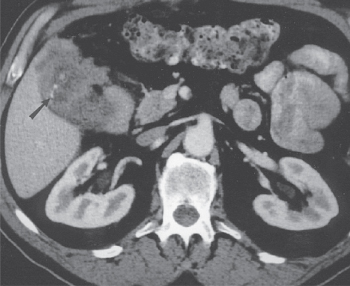
Figure 3 A high-resolution, helical CT scan in another patient with gallbladder cancer. A locally invasive tumor is again noted with areas of calcification (arrow) seen in the thickened gallbladder wall.
Treatment
Resection
The curative resection rates for gallbladder carcinoma range from 10% to 30%.38 The majority of patients are not candidates for curative resection because of extensive locoregional disease, noncontiguous liver metastases, and/or distant metastases. Although it is clear that long-term survival can be achieved in some patients with resectable lesions, the extent of resection remains a controversial issue.
Simple cholecystectomy is an adequate therapy for gallbladder carcinoma confined to the mucosa (T1aN0M0). The 5-year survival rate for patients undergoing simple cholecystectomy for disease confined to the mucosa ranges from 57% to 100%.39, 40 However, employing simple cholecystectomy as the sole therapy for patients with T1aN0M0 tumors is not universally agreed upon. Some authors recommend that extended cholecystectomy (cholecystectomy, wedge resection of the gallbladder fossa including a 3–5 cm margin of normal liver, and a cystic, pericholedochal, gastrohepatic, pancreaticoduodenal, and paraortic lymphadenectomy) be performed to treat patients with these very early-stage lesions.41, 42
These authors recommend that all gallbladders be opened at the time of cholecystectomy for frozen section evaluation of any suspicious areas in the mucosa. If an unsuspected gallbladder carcinoma is diagnosed by frozen section biopsy or if a T1aN0M0 gallbladder carcinoma is diagnosed on final pathology, these authors advocate that an extended cholecystectomy be performed. The bias for this aggressive surgical treatment of T1aN0M0 gallbladder carcinoma is based on the small number of cases of regional lymph node recurrence in patients treated with simple cholecystectomy alone. No rationale is provided for the liver resection because the small number of patients who did fail after simple cholecystectomy developed metastases in the pericholedochal or cystic lymph nodes and not in the liver. Furthermore, the incidence of subsequent lymph node metastases in T1aN0M0 patients was <10% in the small groups of 32 and 36 patients, respectively.41, 42 The incidence of lymph node metastases in 201 patients with gallbladder carcinoma confined to the mucosa was only 2.5% in a study of patients who underwent cholecystectomy and regional lymphadenectomy.39 The mortality rate for extended resection ranges from 2% to 5%, and major postoperative morbidity occurs in 13–40%.39, 40, 43 Therefore, the morbidity and mortality associated with extended cholecystectomy is excessive compared with the potential survival benefit that would occur in <5% of patients with T1aN0M0 lesions.
There is a rationale for performing extended cholecystectomy in patients with T1b tumors or AJCC-TNM stage II and III gallbladder carcinomas (Figure 4). In 165 patients with T1b gallbladder carcinomas, there was a 15.6% incidence of regional lymph node metastasis.39 Of 867 patients with a T2 primary lesion, 56.1% had regional lymph node metastases.39 The 453 patients with T3 tumors had a 74.4% incidence of regional lymph node metastases. The 5-year survival rate following extended cholecystectomy for AJCC stage II and III gallbladder carcinoma ranges from 7.5% to 71%.39, 40, 43, 44 Regional lymph node metastases and/or direct tumor invasion of the hepatic parenchyma are indicators of poor prognosis, with significant reductions in 5-year overall survival rates associated with these pathologic findings.45, 46 Microscopically positive liver resection margins also have a negative impact on survival because these patients had a median survival of 8.9 months compared with 67.2 months for patients with tumor-free margins.45 Preoperative helical CT scans and intraoperative ultrasonography are used to assess the extent of direct invasion into the hepatic parenchyma, which assists with decision making regarding the extent of liver resection necessary to clear all disease. If adequate tumor-negative resection margins are attained, the radicality of liver resection does not affect survival, as attested by similar long-term survival rates following right lobectomy, extended right lobectomy, right trisegmentectomy, and central bisegmentectomy for gallbladder cancer.39, 40, 47–49 T1b patients are classified as stage I in the AJCC system, but, arguably, with a 15.6% incidence of regional lymph node metastases, long-term survival benefit may occur in a significant number of these patients who undergo an extended cholecystectomy (Figure 4).
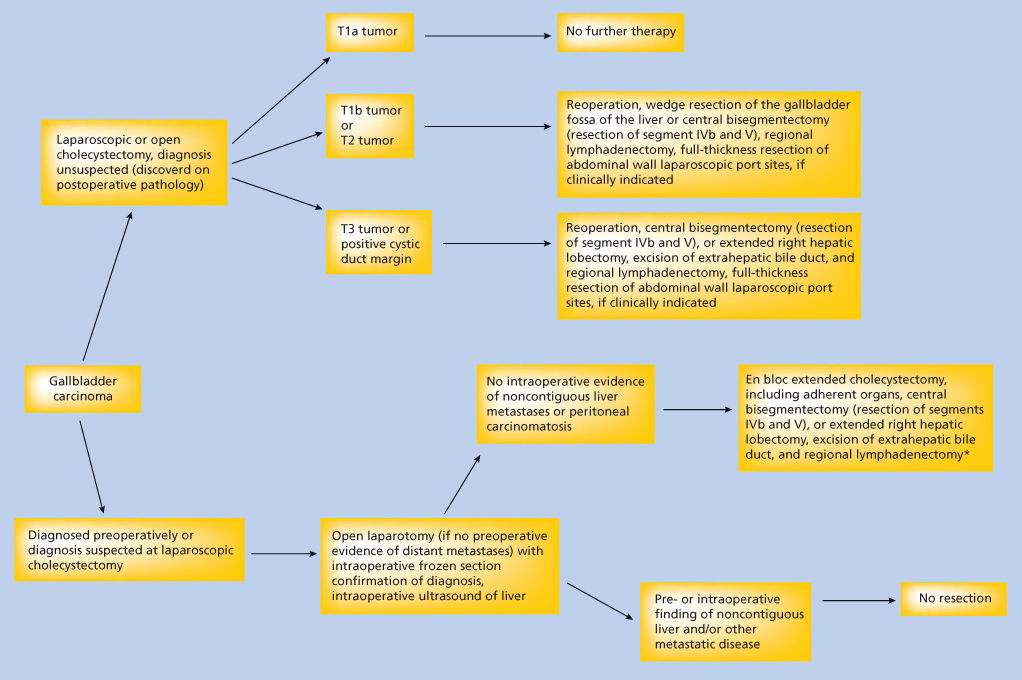
Figure 4 Algorithm to guide surgical decision making for patients with gallbladder cancer. *Regional lymphadenectomy includes complete dissection and removal of the cystic, pericholedochal, pancreaticoduodenal, gastrohepatic, and paraortic lymph nodes.
All authors do not perform an en bloc resection of the extrahepatic bile duct as part of an extended cholecystectomy. Because gallbladder carcinoma is found to invade the extrahepatic bile duct in 57% of cases, with almost all cases occurring in patients with T3 or T4 tumors, an en bloc resection of the proper hepatic and common bile ducts with Roux-en-Y hepaticojejunostomy should be included in an extended cholecystectomy of transmurally invasive tumors. This includes those individuals in whom a clinically unsuspected gallbladder carcinoma is diagnosed pathologically following a simple cholecystectomy with a positive margin at the cystic duct. Gallbladder cancer involving the cystic duct and gallbladder neck frequently grows along the proper hepatic and right bile ducts, necessitating a right or extended right hepatic lobectomy and excision of the extrahepatic ducts to remove all disease.50
Extremely radical operations have been proposed for patients with extensive T3N1M0 or T4N01M0 tumors. This includes hepatopancreatic duodenectomy and abdominal organ cluster transplantation for locally advanced gallbladder carcinoma.39, 51, 52 The operative mortality rate for these radical procedures is at least 15%, with a greater than 90% incidence of major morbidity. Resection of the portal vein and/or hepatic artery with vascular reconstruction frequently is necessary to resect completely all gross malignant disease. The largest report of patients undergoing hepatopancreatic duodenectomy for gallbladder carcinoma is 150 cases from Japan, with a 5-year survival rate of 14%.39 The patients who did not die from intraoperative or postoperative complications all succumbed to recurrent and/or metastatic carcinoma.
It is estimated that 80,000 laparoscopic cholecystectomies are performed each year in the United States. On average, gallbladder carcinoma is diagnosed in 2% of patients undergoing cholecystectomy for presumed benign biliary tract disease. Thus, approximately 1600 patients who annually undergo laparoscopic cholecystectomy could suffer inadvertent dissemination of gallbladder carcinoma.53–56 However, the potential laparoscopic dissemination of tumor cells may not significantly alter the natural history of gallbladder cancer in most patients. A review of our experience at the University of Texas MD Anderson Cancer Center with diagnostic and therapeutic laparoscopy in patients with gastrointestinal malignancies indicated that port site recurrence is a harbinger of widespread metastasis in >95% of patients; thus, it is rarely an isolated site of recurrent malignant disease.57 Furthermore, a report drawn from the National Cancer Data Base between 1989 and 1995 revealed no change in incidence or survival from gallbladder cancer during the time laparoscopic cholecystectomy supplanted open cholecystectomy as the procedure of choice for gallbladder disease presumed benign.58 Nonetheless, because of the large number of cholecystectomies being performed laparoscopically and the small but measurable risk of dissemination of tumor cells, it has been recommended that (1) unless the surgeon feels capable of performing a definitive extended cholecystectomy for gallbladder carcinoma, patients in whom gallbladder carcinoma is suspected preoperatively by clinical or radiologic criteria should be referred without laparoscopy, laparotomy, or percutaneous biopsy, and (2) if gallbladder carcinoma is suspected on visual inspection during an attempted laparoscopic cholecystectomy, either an open definitive operation should be performed or the operation should be terminated without biopsy and the patient referred for appropriate surgical therapy.53 Patients who underwent laparoscopic cholecystectomy and were then found on pathologic analysis to have gallbladder cancer should still be considered for aggressive surgical treatment because long-term disease-free survival will result in a subset of these patients.59
Palliation/chemotherapy
The majority of patients with gallbladder carcinoma are diagnosed at an advanced, unresectable stage of disease. As in patients with hilar bile duct cancer, relief of symptomatic jaundice should be considered. Patients with unresectable gallbladder carcinoma frequently have extensive involvement of the extrahepatic bile duct and may have bulky porta hepatis lymphadenopathy, which makes endoscopic placement of an internal stent difficult. When unresectable gallbladder carcinoma is diagnosed at the time of laparotomy, a surgical biliary bypass, such as an intrahepatic cholangioenteric anastomosis, can be performed and results in significant symptomatic relief in >90% of patients.60 When the diagnosis is made on the basis of radiographic and percutaneous biopsy findings, jaundice can be relieved by placement of percutaneous transhepatic biliary catheters.
In contrast to patients with hilar bile duct carcinoma, in whom gastroduodenal obstruction is a relatively rare event, between 30% and 50% of patients with advanced gallbladder carcinoma will develop a clinically significant element of gastroduodenal obstruction.61 This can be treated surgically with a bypass procedure such as gastrojejunostomy, by endoscopic placement of an expandable metal stent, or by placement of a decompressing gastrostomy tube and feeding jejunostomy tube. A percutaneous endoscopic gastrostomy tube can also be used to decompress the obstructed stomach in patients with advanced disease and limited expected survival time.
Chemotherapy studies that describe the results of chemotherapeutic treatment of unresectable or metastatic gallbladder carcinoma suffer from small numbers of patients and inclusion of patients with hilar bile duct carcinoma. In general, primary treatment options for patients with advanced gallbladder carcinoma include a fluoropyrimidine-based or gemcitabine-based chemotherapy regimen. A study of 53 patients with gallbladder carcinoma who received systemic chemotherapy with 5-fluorouracil (5-FU) or 5-FU plus other chemotherapeutic agents showed objective antitumor responses in 12% or less of the patients in each treatment arm.62 Fluoropyrimidines combined with doxorubicin administered systemically have produced objective response rates of 30–40%.63, 64 A meta-analysis of three randomized studies comparing chemotherapy with gemcitabine alone versus gemcitabine plus platinum agents showed improved median overall survival of 3.8 months (P < 0.001) and median progression-free survival of 3.3 months (P < 0.001) in patients receiving doublet chemotherapy for advanced gallbladder and biliary tract malignancies.65 Complete remission is rare and in most studies, median survival is 11 months or less. However, the literature regarding treatment results with specific regimens is limited because most series are small, and many reports consist of a mix of bile duct cancers, gallbladder cancer, and either pancreatic or hepatocellular cancer. More details about systemic treatment options are explained in the cholangiocarcinoma section.
Radiation therapy
Analysis of the patterns of failure after resection of gallbladder carcinoma revealed that local recurrence was the first and, in a significant number of cases, the only site of failure in more than one-half of patients.66 External beam radiation therapy (EBRT) to a total dose of 45 Gy can produce radiographic evidence of tumor reduction in 20–70% of these tumors and provide temporary relief of jaundice in up to 80% of patients.67–69 In general, EBRT is a palliative treatment. The median survival for locally advanced gallbladder carcinoma patients treated with radiation therapy is approximately 10 months.66–69 Occasional long-term survivors are reported following treatment with higher doses of radiation therapy or with administration of radiation-sensitizing chemotherapeutic agents such as 5-FU during EBRT.66 However, extrahepatic bile duct stricture has been reported in several of the long-term survivors treated with high doses of radiation therapy.70
Multidisciplinary approaches
The majority of patients who undergo an extended cholecystectomy or more radical resection for AJCC stage II, III, or IV gallbladder carcinoma develop tumor recurrence and die as a result of their disease. Nonrandomized studies and case reports have suggested that overall survival can be improved by administering adjuvant radiation therapy and/or chemotherapy after resection of stage II, III, or IV tumors.71–73 Unfortunately, the number of patients who have received postsurgical adjuvant treatment is small, and a variety of treatment regimens have been used. In a nonrandomized study, 9 patients with stage IV gallbladder carcinoma were treated with complete surgical resection alone, while 17 patients were treated with complete resection combined with 20–30 Gy of intraoperative radiation therapy.74 Ten of these 17 patients also received 36.4 Gy of postoperative EBRT. The surgical procedures performed in both groups of patients included extended cholecystectomy and a variety of more radical procedures, including hepatopancreatic duodenectomy. There were no 3-year survivors among the 9 patients treated with resection alone, but there was a 3-year survivorship of 10.1% in the 17 patients treated with resection and radiation therapy. There is a single report of 18 patients treated with preoperative chemoradiation therapy (4500 cGy, 180 cGy/fraction, 5 days/week, continuous intravenous infusion of 5-FU 350 mg/m2/day on days 1–5 and 21–25) prior to a planned resection of known gallbladder cancer.75 Thirteen of the 18 patients underwent resection; one patient refused operation, one patient did not complete preoperative chemoradiation, one patient had disease progression after chemoradiation, and two patients had unresectable disease found at laparotomy. The actuarial 5-year survival rate in the 13 resected patients was 57%.
While most additional literature about postoperative therapy comes from single-institution retrospective reviews, a recently reported nonrandomized phase 2 study evaluated the role of adjuvant chemotherapy and concurrent chemoradiotherapy in 79 patients with a pathologic diagnosis of extrahepatic cholangiocarcinoma or gallbladder carcinoma (but not ampullary cancer) after radical resection, with pathologic stage T2-4 or N1 or positive resection margins.76 Patients received four cycles of gemcitabine (1000 mg/m2 on days 1 and 8) and capecitabine (1500 mg/m2/day on days 1–14) every 21 days followed by concurrent capecitabine (1330 mg/m2/day) and radiotherapy (RT) (45 Gy to regional lymphatics; 54–59.4 Gy to tumor bed). For all patients, the 2-year survival rate was 65%; it was 67% and 60% in R0 and R1 patients, respectively. Median overall survival was 35 months (R0, 34 months; R1, 35 months).
Furthermore, a recent meta-analysis that included 20 studies involving 6712 patients with resected gallbladder or bile duct cancers did show a nonsignificant survival benefit in patients who received adjuvant chemotherapy or chemoradiotherapy, especially in those with lymph-node-positive disease.71 However, owing to limited clinical trial data, the optimal adjuvant treatment strategy for patients who have undergone surgical resection for gallbladder carcinoma has not been established.
Bile duct cancer
Cholangiocarcinomas are malignant tumors that arise from the epithelium of the intrahepatic or extrahepatic bile ducts. In the United States, approximately 3000 patients are diagnosed with cholangiocarcinoma of both the intra- or extrahepatic biliary system annually.77 There is only a slight male preponderance of cases of cholangiocarcinoma. Cholangiocarcinomas can arise at any site in the intra- or extrahepatic biliary system, but perihilar tumors comprise two-thirds of the cases of cholangiocarcinoma (Figure 5).78
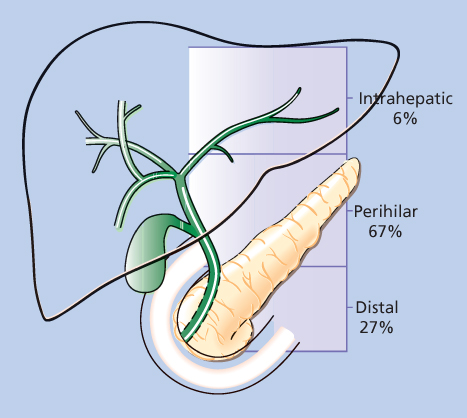
Figure 5 The distribution of 294 cholangiocarcinomas into intrahepatic, perihilar, and distal subgroups.
Source: Aretxabala 1999.75 Reproduced with permission of Elsevier.
Causative factors
There are distinct differences between the factors associated with cholangiocarcinoma and those associated with HCC (Table 2). Only 10–20% of cholangiocarcinomas occur in cirrhotic patients, compared with the 70–90% of HCCs that arise in cirrhotic livers.77, 79, 80 A cohort study in Denmark of 11,605 patients with cirrhosis indicated a 60-fold increased risk of developing hepatocellular cancer and a 10-fold increased risk of cholangiocarcinoma.80 Frequently, the cirrhosis associated with cholangiocarcinomas is a subacute secondary biliary type that results from the neoplastic obstruction of the bile ducts, indicating that, in some cases, cirrhosis in cholangiocarcinoma patients is the result of the tumor rather than its cause.
Table 2 Factors associated with increased risk to develop cholangiocarcinoma versus hepatocellular carcinoma
| Cholangio carcinoma | Hepatocellular carcinoma |
| Liver fluke infection | Cirrhosis |
| Ophisthorchis sinensis | Chronic hepatitis B virus infection |
| Opisthorchis viverrini | Chronic hepatitis C virus infection |
| Congenital/chronic cystic dilation | Aflatoxin B1 ingestion of the bile ducts |
| Choledochal cyst | Chronic ethanol ingestion |
| Caroli disease | Primary biliary cirrhosis |
| Hepatolithiasis | Hemochromatosis |
| Primary sclerosing cholangitis | α-1-Antitrypsin deficiency |
| Ulcerative colitis | Glycogen storage disease |
| Thorotrast exposure | Hypercitrullinemia |
| Cholelithiasis | Porphyrias |
| Asbestos | Hereditary tyrosinemia |
| Dioxin (Agent Orange) | Wilson disease |
| Polychlorinated diphenyls | Hepatotoxin exposure |
| Nitrosamines | Thorotrast |
| Isoniazid | Polyvinyl chloride |
| Methyldopa | Carbon tetrachloride |
Cholangiocarcinoma is more prevalent in Southeast Asia than in other parts of the world. The higher incidence in this geographic region is related to parasitic infection with the liver flukes Opisthorchis sinensis and Opisthorchis viverrini.81, 82 Liver flukes induce hyperplasia, fibrosis, and adenomatous proliferation of human biliary epithelium and are associated with hepatolithiasis. The fluke infestation suggests a direct etiologic role in the subsequent development of cholangiocarcinoma, but this relationship is not established unequivocally.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree